
Suppression of MnSOD by Andrographolide and its Relation to
Oxidative Stress and Viability of Breast Cancer Stem Cells Treated
with Repeated Doxorubicin Administration
Angie Tara Rachman
1a
, Ayu Suraduhita
1b
, Resda Akhra Syahrani
2c
, Melva Louisa
3d
,
Septelia Inawati Wanandi
2,4 e
1
Master’s Programme in Biomedical Science, Faculty of Medicine, Universitas Indonesia
2
Molecular Biology and Proteomics Core Facilities, Indonesian Medical Education and Research Institute (IMERI),
Faculty of Medicine, Universitas Indonesia
3
Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia
4
Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia
Keywords: Breast cancer stem cells, Doxorubicin, Andrographolide, MnSOD, Oxidative stress
Abstract: Cancer stem cells (CSCs) are chemoresistance and could be a preferential target for reversing resistance.
Doxorubicin is one of the most effective chemotherapy agents, but resistance still occurs. Doxorubicin
generates reactive oxygen species (ROS). The increased specific activity of MnSOD has been suggested as
one possible mechanism for breast cancer stem cells (BCSCs) to maintain stemness and survival. The study
performed to determine the effect of andrographolide on oxidative status and its relation to the viability of
BCSCs given repeated doxorubicin. CD24-/CD44+ BCSCs were grown in DMEM/F12 medium with 0,1µM
doxorubicin for 14 days, then treated with a combination of 0,1µM doxorubicin and 0,285 mM
andrographolide until day 22. MnSOD activity was suppressed on day 4 when BCSCs were still sensitive to
doxorubicin treatment. Viability significantly increased on day 20 along with increased MnSOD activity.
Andrographolide restored the sensitivity of BCSCs to doxorubicin, which correlated with MnSOD activity
but not catalase. There was no change in MDA levels in all days of treatment which means BCSCs can
maintain oxidative stress level. Andrographolide supplementation can decrease MnSOD activity but not
catalase and is closely related to decreased cell viability with low sensitivity to doxorubicin.
1 INTRODUCTION
Breast cancer is the most frequently identified cancer
and the leading cause of death from cancer in women
worldwide. (Bray et al., 2018) There are several
treatment options to cure breast cancer, including
mastectomy, chemotherapy, hormone therapy,
radiotherapy, and other therapies. (Sterba et al., 2013)
An anthracycline, doxorubicin, is considered one of
the most effective and most used chemotherapy
agents to treat breast cancer and several other cancer
types. However, resistance to chemotherapy still
a
https://orcid.org/0000-0001-8903-6496
b
https://orcid.org/0000-0001-9465-2357
c
https://orcid.org/0000-0002-4377-9611
d
https://orcid.org/0000-0002-9451-0380
e
https://orcid.org/0000-0002-7963-8853
occurs both intrinsically and resistance that develops
during treatment. (Austreid et al., 2014) One of the
causes of doxorubicin resistance is the reduced
amount of the accumulated drug in the nucleus
resulting in a decrease in DNA damage. Failure to
accumulate drugs is caused by active efflux through
the ATP binding cassette (ABC) transporter family.
The upregulation of this transporter is related to drug
resistance. (Shiraga et al., 2001)
Cancer stem cells (CSCs) represent a
subpopulation of cancer cells with close
characteristics to normal stem cells, namely
pluripotency, self-renewal ability, and differentiation.
28
Rachman, A., Suraduhita, A., Syahrani, R., Louisa, M. and Wanandi, S.
Suppression of MnSOD by Andrographolide and its Relation to Oxidative Stress and Viability of Breast Cancer Stem Cells Treated with Repeated Doxorubicin Administration.
DOI: 10.5220/0010487200280034
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 28-34
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

CSCs can originate from normal cells/progenitors
with mutations or environmental changes and can
also come from normal somatic cells that experience
genetic changes. High CSCs indicate a poor
prognosis because cancer stem cells have a slow rate
of division, better DNA repairability, and a lower
ability to experience apoptosis than cancer cells in
general. The causes of cancer stem cells to be more
resistant to cancer therapies such as chemotherapy
and radiation have been proven in vitro studies using
CD24-/CD44+ cancer stem cells. (Phillips et al.,
2006)he
Oxidative stress is a phenomenon that appears
when an imbalance occurs between reactive oxygen
species (ROS) formation and the capability of cells to
detoxify them. The lipid peroxidation is one of the
results of oxidative stress, which will lead to
malondialdehyde (MDA) formation. MDA is one of
the most reliable markers to determine oxidative
stress. (Giera et al., 2012) Oxidative stress is
remarkably known to play an important role in
damaging lipids, protein, and DNA molecule, alter
signalling pathways and impact cancer progressions.
(Lee et al., 2017) ROS also has a role in stem cell
renewal and differentiation. Breast cancer stem cells
can maintain lower ROS levels than differentiated
cells by increasing antioxidant capacity. The
condition can be an obstacle for treatments that use
oxidative stress like chemotherapy and radiotherapy.
(Gorrini et al., 2013) The conditions doxorubicin was
known to generate ROS, although the other
mechanisms, namely through intercalation of DNA
and poisoning topoisomerase II, constitute their
cytotoxic actions. (Lazo et al., 1998)
Previous studies in our laboratory showed that
doxorubicin could reduce the viability of CD24-
/CD44+ breast cancer stem cells (BCSC) at initial
exposure of doxorubicin MnSOD levels were
maintained, thus high ROS levels. After more
extended doxorubicin administration, the viability of
BCSCs increased, and the MnSOD activity was
significantly high, which caused the ROS levels to
decline. Breast cancer stem cells CD24-/CD44+
maintain survival by increasing the activity of the
MnSOD. (Syahrani et al., 2019)
Andrographolide (ANDRO) is a diterpene lactone
derived from the extract of the plant bitter
(Andrographis paniculata), and it has long been
known for its antioxidant (Xu et a;., 2019), anticancer
(Siripong et al., 1992), immunostimulatory (Puri et
al., 1993), anti-inflammatory (Abu-Ghefreh et al.,
2009), and anti-viral (Manjula et al., 2018) activities.
Andrographolide has demonstrated in promoting
apoptosis in BCSCs by inhibition of anti-apoptotic
protein survivin. (Yunita et al., 2017) Initially,
andrographolide was reported to be a ROS scavenger
(Xu et al., 2019), but natural compounds may
demonstrate both antioxidant and prooxidant
characteristics that depend on the concentrations and
exposure. (Sznarkowska et al., 2017) In a parallel
study, it showed that the administration of
andrographolide could cause cell death in BCSCs.
The study performed to determine the possibility of
andrographolide re-sensitising doxorubicin resistance
due to increased MnSOD activity.
2 MATERIALS AND METHODS
2.1 Cell culture
Breast cancer stem cells CD24-/CD44+ were
obtained from the Department of Biochemistry and
Molecular Biology, Faculty of Medicine Universitas
Indonesia and grown in serum-free Dulbecco’s
Modified Eagle Medium / Nutrient Mixture F-12
(DMEM/F-12 medium) (Gibco, Thermo Fisher
Scientific, Inc. Waltham, MA, USA) supplemented
with 1% penicillin-streptomycin (Gibco, Thermo
Fisher Scientific, Inc. Waltham, MA, USA) and
amphotericin B (Gibco, Thermo Fisher Scientific,
Inc. Waltham, MA, USA). BCSCs were maintained
in 5% CO2 at 37°C. Cells seeded at 1 x 10
5
cells/well
in 12 well plates.
2.2 Doxorubicin and Andrographolide
Treatment
Doxorubicin (Sigma-Aldrich, St. Louis, Missouri,
USA) was prepared in serum-free DMEM/F12
medium at a final concentration of 0,1µM.
Andrographolide prepared in DMSO (Sigma-
Aldrich, St. Louis, Missouri, USA) and diluted in
serum-free DMEM/F-12 medium at a final
concentration of 0,285 mM. Cells were treated with
0,1 µM doxorubicin every two days. After 14 days of
treatment, the cells were treated with a combination
of doxorubicin and andrographolide until day 22.
Counting Cells used by Trypan blue exclusion assay.
Cell viability was determined by dividing the number
of living cells of the treatment group to the number of
living cells of the control group.s
2.3 Protein Isolation
According to the manufacturer's protocol, RIPA
buffer (Thermo Fisher Scientific, Illinois, USA) was
Suppression of MnSOD by Andrographolide and its Relation to Oxidative Stress and Viability of Breast Cancer Stem Cells Treated with
Repeated Doxorubicin Administration
29
used to isolate protein from BCSC CD24-/CD44+.
The supernatant containing protein stored in a new
tube at -80
o
C.
2.4 MnSOD Activity
MnSOD activity of protein samples was determined
using the RANSOD kit (Randox Lab, Crumlin, UK).
Protein samples used for the assay of SOD. To inhibit
Cu / ZnSOD, 5mM sodium cyanide was added.
Absorbance measured at wavelengths of 505 nm on a
spectrophotometer for 30 seconds. Preparation of
percentage inhibition versus standard/protein sample
assay and standard inhibition curve following the
recommended kit manual. The SOD activity values
for protein samples were read based on a curve
divided by the protein concentration.
2.5 Catalase Activity
50 μL of standard/sample were added into a tube
containing 950 μL of H
2
O
2
. The homogenised mixture
read by absorbance at 210 nm. The absorbance
observations were carried out at the first 30 seconds
(00:30) and two minutes after that (02:30) using a
stopwatch. The catalase activity was measured using
the catalase standard curve made in several dilutions,
divided by the protein concentration.
2.6 MDA Levels
The concentration of MDA (malondialdehyde)
formed from lipid peroxidation was measured using
the Wills method. The principle is that MDA will
react with thiobarbituric acid (TBA) at a temperature
of 90
o
C-100
o
C in an acidic atmosphere to form a pink
compound that provides maximum absorbance at a
wavelength of 530 nm. The concentration is
calculated based on the calculations obtained from the
linear regression of the MDA standard curve. The
MDA concentration divided by the protein
concentration.
2.7 Statistical Analysis
The data were analysed with IBM SPSS Statistics 22.
The average probability analysed with a Shapiro-
Wilk test. The data performed as a mean value ±
standard deviation (SD). Statistical evaluation of the
significant differences performed using variance
(ANOVA); the LSD/Tukey test used for multiple
comparisons. The significance level was at p<0.05.
3 RESULTS
3.1 Measurement of MnSOD Specific
Activity
The activity of antioxidant enzyme MnSOD in
doxorubicin treated BCSCs and a combination of
doxorubicin and andrographolide treatment after day
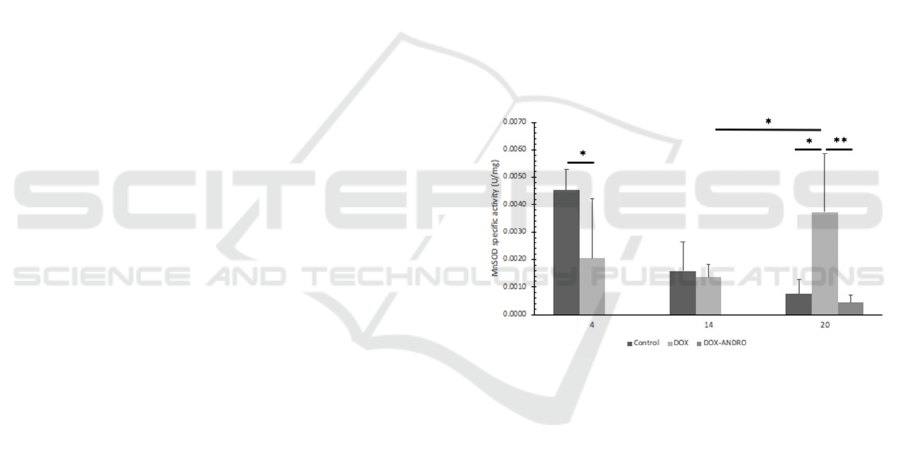
14 to day 22 were assayed. As shown in Figure 1,
BCSCs’ MnSOD activity significantly decreased
compared to control with no treatment on day 4 (p <
0.05). There was no statistical difference between the
MnSOD activity of doxorubicin treated BCSCs
compared to the control group on day 14.
Interestingly, there was a significant increase of
MnSOD activity on day 20 of doxorubicin-induced
BCSCs compared to control on the same day (p <
0.05) and also compared to day 14 of doxorubicin-
induced BCSCs (p < 0.05). Andrographolide was
given on the 14th day and reduced the MnSOD
activity of doxorubicin-induced BCSCs significantly
(p < 0.01).
Figure 1: Effect of doxorubicin and doxorubicin-
andrographolide on BCSCs MnSOD specific activity at
specified times. MnSOD activity was determined using the
RANSOD kit (Randox Lab, Crumlin, UK). Statistical
analysis conducted as described in Materials and Methods.
Significant differences from control values (*, p < 0.05)
(**, p < 0.01) are indicated by the asterisks.
3.2 Measurement of Catalase Specific
Activity
Catalase specific activity was measured based on a
standard H
2
O
2
decomposition curve by measuring the
absorbance of H
2
O
2
with a blank at a concentration of
1: 1000; 1: 2000; 1: 4000; 1: 8000; 1: 16000; 1: 32000
and 1: 64000. The absorbance observations at 210 nm
were carried out at the first 30 seconds (00:30) and
two minutes after that (02:30) using a stopwatch.
Catalase activity compared in doxorubicin treated
BCSCs, doxorubicin-andrographolide treated BCSCs
and no treatment control. There was no difference
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
30
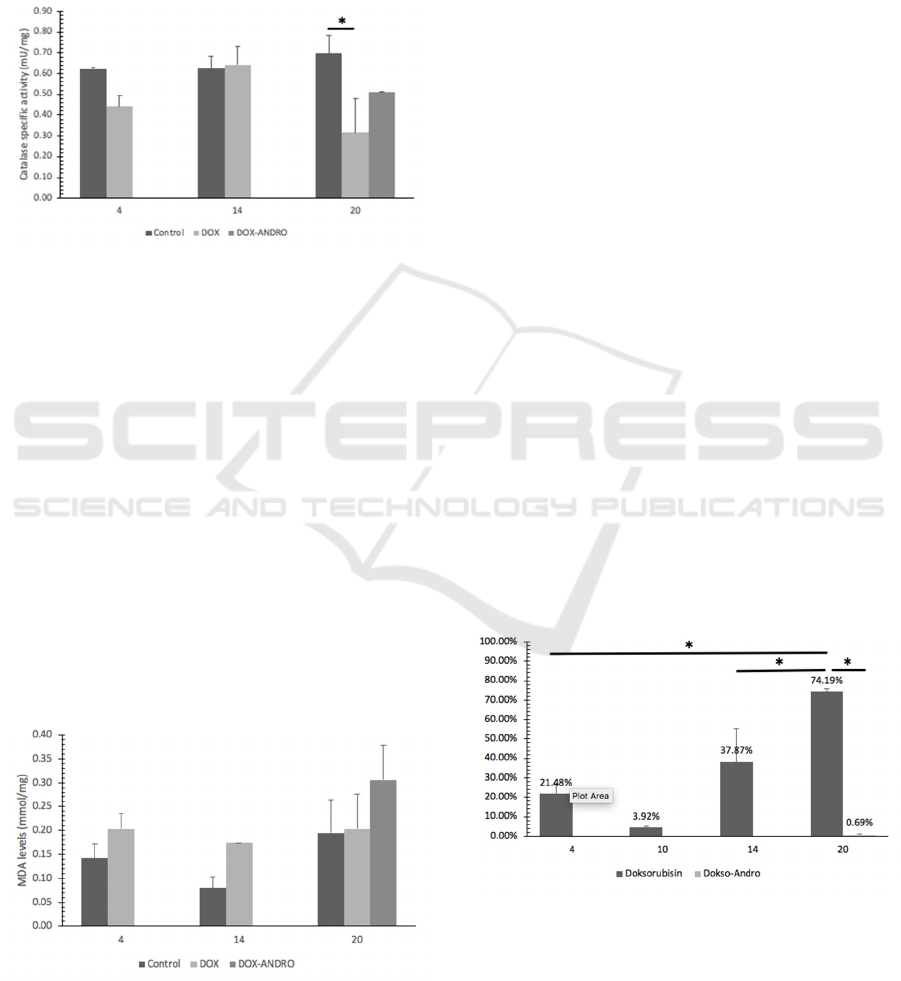
between the catalase activity of doxorubicin-induced
and control BCSCs on day 4 and day 14. There was a
significant decline of doxorubicin-induced BCSCs on
day 20 compared to a control of the same day (p <
0.05). No significant differences observed between
the catalase activity of andrographolide
administration on doxorubicin-induced BCSCs' and
doxorubicin-induced BCSCs.
Figure 2: Effect of doxorubicin and doxorubicin-
andrographolide on BCSCs catalase specific activity at
specified times. Catalase activity was determined using the
catalase standard curve. Statistical analysis conducted as
described in Materials and Methods. Significant differences
from control values (*, p < 0.05) (**, p < 0.01) are indicated
by the asterisks.
3.3 MDA Levels
Oxidative stress is one of the factors that contribute
to the cytotoxicity of doxorubicin. The measurement
of the peroxidation product levels – malondialdehyde
(MDA) was used to calculate the lipid peroxidation
status in BCSCs. Lipid peroxidation was assayed by
the thiobarbituric acid method. As shown in Figure 3,
the MDA levels of BCSCs exposed to doxorubicin
show no difference compared to control.
Andrographolide administration showed a trend of
increased MDA levels on doxorubicin-induced
BCSCs, but the proportion was not significant.
Figure 3: Effect of doxorubicin and doxorubicin-
andrographolide on MDA levels at specified times.
Statistical analysis conducted as described in Materials and
Methods. Significant differences from control values (*, p
< 0.05) (**, p < 0.01) are indicated by the asterisks.
4 DISCUSSION
Doxorubicin is one of the most effective and most
used chemotherapy agents to treat breast cancer and
several other cancer types. One of how doxorubicin
causes cell death is through the mechanism of ROS
formation, increasing oxidative stress. Increasing free
radicals will cause oxidative stress. (Xu et al., 2005)
Syahrani et al. demonstrated that breast cancer stem
cells' sensitivity to doxorubicin decreased when given
long-term exposure. Breast cancer stem cells
(BCSCs) can maintain stemness and survival through
increased antioxidant enzyme MnSOD activity.
(Syahrani et al., 2019)
In a parallel study at the same laboratory,
doxorubicin reduced CD24-/CD44+ breast cancer
stem cells' viability until day ten. The viability
increased on the 14th day, so it indicated the time
when the BCSCs sensitivity decreases. The BCSCs in
this study could not be declared resistant in a study
conducted by Lukyanova et al., a doxorubicin-
resistant variant of breast cancer cells was obtained
by growing cells medium containing doxorubicin
with stratified concentrations over a more extended
period. (Lukyanova et al., 2009) The viability of
CD24-/CD44+ BCSCs continued to increase up to
74.19% on the 20th day. The administration of
andrographolide carried out every other day on day
14 until day 18 caused a decrease in the viability of
breast cancer stem cells exposed to doxorubicin. This
result indicates that andrographolide inhibits the
growth of BCSCs that had reduced sensitivity to
doxorubicin.
Figure 4: Effects of androgapholide on the viability of
doxorubicin-induced BCSCs. BCSCs were treated with 0,1
µM doxorubicin until day 14 then, doxorubicin treated
BCSCS were supplemented with 0,285 mM
andrographolide.
Suppression of MnSOD by Andrographolide and its Relation to Oxidative Stress and Viability of Breast Cancer Stem Cells Treated with
Repeated Doxorubicin Administration
31

CSCs from some types of tumours had lower ROS
levels than differentiated cells. Its indicated that
CSCs could maintain a low ROS level, which might
help them protect themselves from damages caused
by ROS. This low level of ROS was partly caused by
an increase in the production of ROS scavenger
enzymes. (Diehn et al., 2009) Therefore, we analysed
the activity of the antioxidant enzyme. MnSOD was
the most potent antioxidant enzyme because it acts as
the first detoxification enzyme needed to protect cells
from ROS's toxicity produced by metabolism. This
enzyme catalysed the dismutation of potentially
dangerous superoxide anions into hydrogen peroxide
and molecular oxygen. (Oberley, 2005).
Increased expression of MnSOD protein could
lead to resistance to therapy. The mechanism by
which MnSOD protects cells from oxidative damages
was thought by maintaining mitochondrial function
and preventing the reduction in ATP synthesis caused
by oxidants. (Suresh et al., 2003) In the MnSOD
activity analysis using the RanSOD kit, the results of
MnSOD activity from CD24-/CD44+ BCSCs
exposed to doxorubicin on day four significantly
lower compared to the control without treatment. It is
caused by the changes in the balance of oxidative
stress levels, which is one of the doxorubicin's
mechanisms of action. The imbalance between high
levels of oxidative stress and antioxidants could cause
mutations in DNA and damage to genes that produce
antioxidant proteins. (Bagchi et al., 1998) The result
was that BCSCs lose their ability to fight free radicals
due to disruption in antioxidant proteins' production.
Also, MnSOD activity has been widely used to
combat the high amount of ROS, so that the measured
activity was reduced. On day 14, the MnSOD activity
of CD24-/CD44+ BCSCs exposed to doxorubicin
showed no significant difference compared to control.
On day 20, the MnSOD activity of doxorubicin-
induced BCSCs was significantly higher than the
control and the doxorubicin-induced BCSCs on day
4. This research aims to clarify the role of MnSOD in
causing resistance to doxorubicin in BCSCs by
supplementation of androgapholide. The suppression
of MnSOD by andrographolide affected the viability,
sensitising BCSCs to doxorubicin treatment. Another
important finding is that the MDA level after
andrographolide exposure showed a higher trend
compared to doxorubicin alone, although not
significant, the result suggested that there was an
increase in oxidative stress. We suggested that
andrographolide could reduce MnSOD activity and
revive the oxidative stress induced by doxorubicin, so
that viability decreases.
Catalase gives protection against the deleterious
effect against H
2
O
2
and decomposes them to oxygen
and water. Catalase is located in the peroxisomes.
(Kirkman et al., 2007) On day 4 and 14, BCSCs was
sensitised to doxorubicin treatment, and the catalase
level was no different compared to the control
without treatment. Catalase is known to have an
essential role in developing tolerance to oxidative
stress in cells, especially when there is limited
glutathione and decreased GPx activity (Wassmann et
al, 2004) or increasing H
2
O
2
levels. (Yamada et al.,
1991) On day 20, the catalase activity of doxorubicin-
induced BCSCS was decreased significantly
compared to day 14 doxorubicin-induced BCSCS and
control of the same day, but the MDA level remains
stable. The finding of lower catalase activity in this
day might be explained with the regulation of catalase
expression. The catalase core promoter is highly
conserved, allowing efficient binding of transcription
factors to the DNA binding sites and leading to the
positive or negative regulation of catalase expression.
(Nenoi et al., 2001; Glorieux et al., 2015) Epigenetic
changes have been shown to regulate catalase
expression in acute myelogenous leukaemia cells
resistant to doxorubicin, reducing catalase protein
levels compared to the parental cell lines. (Lee et al.,
2012) Besides, catalase expression is also regulated at
the RNA level and post-translational modifications,
causing decreased catalase activity. (Glorieux et al.,
2015) There was no statistical difference in the
catalase activity between doxorubicin-
andrographolide induced BCSCs and doxorubicin-
induced BCSCs on day 20. Based on our results, we
suggested an increase in MnSOD activity, but not
catalase activity leading to a decrease in viability.
Furthermore, increased expression of MnSOD was
associated with a decrease in viability induced by
oxidative stress.
Oxidative stress is one of the factors that
contribute to the cytotoxicity of doxorubicin. ROS
has a short life span, which is not easy to be detected.
(Sanz, 2016) Measurement of lipid peroxidation end
product – malondialdehyde (MDA) was used to
determine lipid peroxidation as a convenient
biomarker for ROS related damage. On day 4, there
was no statistically significant difference between the
MDA levels of BCSCs induced with doxorubicin and
control. After 14 days of exposure to doxorubicin, the
MDA level of BCSCs induced with doxorubicin
shows a higher trend than the control group.
However, when compared to day 4, MDA
concentration was stable.
Similarly, the MDA level of doxorubicin-induced
BCSCs on day 20 was remained stable at the same
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
32

range as day 4 and day 14. A state of redox imbalance
between oxidants and antioxidants is a common
hallmark of cancer resistance to treatment. High ROS
exposure was given to increase oxidants within the
cell and cause ROS-mediated damaged biomolecules
such as DNA and protein. (Ziech et al., 2011) We
suggested that the stable MDA concentration between
MDA treated group showed the ability of BCSCs to
maintain homeostasis and evaded cancer cell death by
developing an antioxidant defence. SOD and catalase
are the best enzymatic antioxidants on scavenging
ROS (Banerjee et al., 2017) suggested that they were
effectively scavenged free radicals and kept the
amount of MDA in doxorubicin-induced BCSCs
balanced. After exposure of andrographolide on day
20, there was a trend that the MDA level of
doxorubicin-induced BCSCs was increasing.
Andrographolide may have prooxidant and
antioxidant characteristics. Previous studies have
shown that andrographolide can trigger intracellular
ROS formation, contributing to apoptosis in cancer
cells. (Banerjee et al., 2017) In this study,
andrographolide did not cause a significant increase
in oxidative stress level, but it could suppress the
antioxidant enzyme that scavenges ROS, MnSOD.
Thus, we demonstrated that andrographolide and
doxorubicin synergistically induced cell death by
MnSOD suppression.
5 CONCLUSIONS
Andrographolide repeated treatment to BCSC can
decrease MnSOD activity but not catalase and
oxidative stress and is closely related to decreased
cell viability with low sensitivity to doxorubicin.
ACKNOWLEDGEMENTS
This research was supported by a grant from the
Penelitian Dasar Unggulan Perguruan Tinggi
(PDUPT).
REFERENCES
Abu-Ghefreh, A.A., Canatan, H., Ezeamuzie, C.I., 2009. In
vitro and in vivo anti-inflammatory effects of
andrographolide. Int. Immunopharmacol, [online],
9,pp. 313–318.
Austreid, E., Lonning, P.E., Eikesdal, H.P., 2014. The
emergence of targeted drugs in breast cancer to prevent
resistance to endocrine treatment and chemotherapy.
Expert Opin Pharmacother, [online], 5, pp. 681–700.
Bagchi, K., Puri, S., 1998. Free radicals and antioxidants in
health and disease. East Mediterranean Health Journal,
[online], 4 (2), pp. 350–60.
Banerjee, A., Banerjee, V., Czinn, S. and Blanchard, T.,
2017. Increased reactive oxygen species levels cause
ER stress and cytotoxicity in andrographolide treated
colon cancer cells. Oncotarget, [online], 8, pp. 26142-
53.
Bray, F., Ferlay, J., Soerjomataram, I., et al., 2018. Global
cancer statistics 2018: GLOBOCAN estimates of
incidence and mortality worldwide for 36 cancers in
185 countries. CA: Cancer J Clin, [online], 68, pp.394.
Diehn, M., Cho, R.W., Lobo, N.A., Kalisky, T., Dorie,
M.J., et al,. 2009. Association of reactive oxygen
species levels and radioresistance in cancer stem cells.
Nature, [online], 458, pp. 780-3.
Giera, M., Lingeman, H., and Niessen, W.M.A., 2012.
Recent advancements in the LC- and GC-based analysis
of malondialdehyde (MDA): a brief overview.
Chromatographia, [online], 75(9), pp. 433–440.
Glorieux, C., Zamocky, M., Sandoval, C.J.M., Verrax, J.,
and Buc Calderon, P., 2015. Regulation of catalase
expression in healthy and cancer cells. Free Radic. Biol.
Med, [online], 87, pp. 84–97.
Gorrini, C., Harris, I.S., Mak, T.W., 2013. Modulation of
oxidative stress as an anticancer strategy. Nat Rev Drug
Discov, [online] 12, pp. 931–947.
Kirkman, H.N. and Gaetani, G.F., 2007. Mammalian
catalase: a venerable enzyme with new mysteries.
Trends in Biochemical Sciences, [online] 32(1), pp. 44–
50.
Lazo, J. S., and Larner, J. M., 1998. Individual antineoplatic
drugs. In Human Pharmacology, Molecular to Clinical
(Brody, T. M., Larner, J., and Minneman, K. P., Eds.),
3rd ed, Mosby, New York: 606.
Lee, J.D., Cai, Q., Shu, X.O., Nechuta, S.J., 2017. The Role
of Biomarkers of Oxidative Stress in Breast Cancer
Risk and Prognosis: A Systematic Review of the
Epidemiologic Literature. J Womens Health (Larchmt),
[online], 26(5), pp. 467-482.
Lee, T.B., Moon, Y.S., Choi, C.H. 2012. Histone H4
deacetylation down-regulates catalase gene expression
in doxorubicin-resistant AML subline. Cell Biol
Toxicol,[online], 28, pp. 11-8.
Lukyanova, N.Y., Rusetskya, N.V., Tregubova, N.A.,
Chekhun, V.F., 2009. Molecular profile and cell cycle
in MCF-7 cells resistant to cisplatin and doxorubicin
Exp Oncol, [online], 31, pp. 87–9.
Manjula, S., Kalaiarasi, C., Pavan, M.S., Hathwar, V.R.,
Kumaradhas, P., 2018. Charge density and electrostatic
potential of hepatitis C anti-viral agent
andrographolide: An experimental and theoretical
study.
Acta Cryst. B, [online], 74, pp. 693–704.
Nenoi, M., Ichimura, S., Mita, K., Yukawa, O., Cartwright,
I.L., 2001. Regulation of the catalase gene promoter by
Sp1, CCAAT-recognizing factors, and a WT1/Egr-
related factor in hydrogen peroxide-resistant HP100
cells. Cancer Res, [online], 61, pp. 5885-94.
Suppression of MnSOD by Andrographolide and its Relation to Oxidative Stress and Viability of Breast Cancer Stem Cells Treated with
Repeated Doxorubicin Administration
33

Oberley, L.W. 2005. Mechanism of the tumor suppressive
effect of MnSOD overexpression. Biomed
Pharmacother, [online], 59, pp. 143–148.
Phillips, T., McBride, W., Pajonk, F., 2006. The response
of CD24(-/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst, [online], 98, pp. 1777-
1785.
Puri, A., Saxena, R., Saxena, R.P., Saxena, K.C.,
Srivastava, V., Tandon, J.S., 1993. Immunostimulant
agents from Andrographis paniculata. J Nat Prod,
[online], 56(7), pp. 995–999.
Sanz, A., 2016. Mitochondrial reactive oxygen species: do
they extend or shorten animal lifespan?. Biochim.
Biophys. Acta, [online], 1857, pp. 1116–1126.
Shiraga, K., Sakaguchi, K., Senoh, T., et al., 2001.
Modulation of doxorubicin sensitivity by cyclosporine
A in hepatocellular carcinoma cells and their
doxorubicin-resistant sublines. J. Gastroenterol.
Hepatol, [online], 16, pp. 460–66.
Siripong, P., Konckathip, B., Preechanukool, K., Picha, P.,
Tunsuwan, K., Taylor, W.C., 1992. Cytotoxic
diterpenoid constituents from Andrographis paniculata
nees leaves. J Sci Soc Thai, [online], 18, pp.187–194.
Sterba, M., Popelova, O., Vavrova, A., et al., 2013.
Oxidative stress, redox signaling, and metal chelation
in anthracycline cardiotoxicity and pharmacological
cardioprotection. Antioxid Redox Signal, [online], 18
(8), pp. 899-929.
Suresh, A., Guedez, L., Moreb, J., Zucali, J., 2003.
Overexpression of manganese superoxide dismutase
promotes survival in cell lines after doxorubicin
treatment. Br J Haematol, [online], 120, pp. 457-63.
Syahrani, R.A., Louisa, M., Wanandi, S.I., 2019.
Sensitivity of Breast Cancer Stem Cells (Cd24-/Cd44+)
to Doxorubicin Is Associated with Oxidative Stress
Status. Int J App Pharm, [online], Vol 11(6), pp. 91-96.
Sznarkowska, A., Kostecka, A., Meller, K. and Bielawski,
K.P., 2017. Inhibition of cancer antioxidant defense by
natural compounds. Oncotarget, [online], 8, pp. 15996–
16016.
Wassmann, S., Wassmann, K., Nickenig, G., 2004.
Modulation of oxidant and antioxidant enzyme
expression and function in vascular cells. Hypertension,
44, pp. 381–386.
Xu, X., Persson, H.L., Richardson, D.H., 2005. Molecular
pharmacology of the interaction of anthracyclines with
iron. Molecular Pharmacology, [online], 68, pp. 261–
71.
Xu, Y., Tang, D., Wang, J., Wei, H., Gao, J., 2019.
Neuroprotection of Andrographolide Against
Microglia-Mediated Inflammatory Injury and
Oxidative Damage in PC12 Neurons. Neurochem Res,
[online], 44(11), pp. 2619-2630.
Yamada, M., Hashinaka, K., Inazawa, J., Abe, T., 1991.
Expression of catalase and myeloperoxidase genes in
hydrogen peroxide-resistant HL-60 cells. DNA Cell
Biol, [online], 10, pp. 735–742 26.
Yunita E, 2017. Efek in vitro andrografolida terhadap
apoptosis jalur intrinsik pada sel punca kanker payudara
manusia yang dipaparkan rotenon: tinjauan ekspresi
caspase-9, caspase-3 dan survivin (thesis). Fakultas
Kedokteran Universitas Indonesia.
Ziech, D., Franco, R., Pappa, A., Panayiotidis, M.I., 2011.
Reactive oxygen species (ROS)–induced genetic and
epigenetic alterations in human carcinogenesis. Mutat
Res. 711, pp. 167-173.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
34
