
Chlorogenic Acid Ameliorates Vascular Remodeling and Perivascular
Fibrosis in Kidney Fibrosis Model in Mice
Gabriella Bamba Ratih Lintin
1a
, Nur Arfian
2 b
, Dwi Cahyani Ratna Sari
2c
, Gina Andyka
Hutasoit
3d
, Mohammad Salman
4e
and Muhammad Mansyur Romi
2f
1
Anatomy Department, Faculty of Medicine Universitas Tadulako, Palu
2
Anatomy Department, Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada, Yogyakarta
3
Patology Anatomy Department, Faculty of Medicine Universitas Tadulako, Palu
4
Histology Department, Faculty of Medicine Universitas Tadulako, Palu
Keywords: Kidney Fibrosis, UUO, CGA, Vascular Remodeling, Perivascular Fibrosis
Abstract: Kidney Fibrosis is the common pathway final of Chronic Kidney Disease, which is characterized by vascular
remodeling and perivascular fibrosis. The Unilateral Ureteral Obstruction (UUO) model is used to cause
kidney fibrosis. Chlorogenic Acid (CGA) is an antioxidant as a renoprotective agent. However, fibrosis
perivascular and vascular remodeling has not analyzed yet. Study has objective to examine the effect of CGA
in vascular aspects, specifically vascular remodeling and perivascular fibrosis in kidney fibrosis. Material and
Methods: This research was a true experimental study. Unilateral Ureteral Obstruction (UUO) was performed
in swiss webster background mice (n=25, 2-3 months old, 20-30 g weight) to induce kidney fibrosis. The mice
were divided into five groups, SO (Sham Operation/control), U7 (UUO day-7), U14 (UUO day-14), UC7
(UUO+CGA day-7), and UC14 (UUO+CGA day-14). CGA 14 mg/kg body weight/day was induced
intraperitoneally. Vascular remodeling based on lumen area, mean wall thickness, and wall/lumen area ratio
(WLAR) and perivascular fibrosis of intrarenal arteries were quantified using Sirius Red staining. Study found
that UUO groups (U7 and U14) had significantly higher vascular remodeling, as shown by lower lumen area,
higher mean wall thickness and higher WLAR, and perivascular fibrosis, as shown by higher area compared
to SO group (p<0.05). On the other hand, CGA groups (UC7 and UC14) revealed lower vascular remodeling,
as shown by higher lumen area, lower WLAR, and perivascular fibrosis, as shown by lower area significantly
compared to UUO group (p<0.05). The mean wall thickness was lower, but the data was not significantly
different. Study conclude that CGA ameliorates kidney fibrosis through vascular remodeling and perivascular
fibrosis.
1 INTRODUCTION
Chronic Kidney Disease (CKD) is one of the world's
public health priorities because of its rapid increase in
the prevalence. Global Burden of Disease study in
2015, renal disease was the 12th leading cause of
death, with 1.1 million deaths worldwide. In
Indonesia, the high prevalence indicated by the
a
https://orcid.org/0000-0002-9791-1200
b
https://orcid.org/0000-0003-1694-2054
c
https://orcid.org/0000-0002-1126-4939
d
https://orcid.org/0000-0002-8043-564X
e
https://orcid.org/0000-0002-1769-6652
f
https://orcid.org/0000-0002-5842-9091
enrollment of more than 15.000 new patients with
CKD in health insurance in 2011, 87% were End-
Stage Renal Disease (ESRD) patients (Perkumpulan
Nefrologi Indonesia, 2011). Decreased kidney
function and scar formation that occurs progressively
will direct CKD to the disease course's final stage,
ESRD (Mutsaers et al, 2015). Kidney fibrosis leads
to the ESRD as a final common pathway to CKD
(Fragiadaki & Mason, 2011). Kidney fibrosis is
Lintin, G., Arfian, N., Ratna Sari, D., Hutasoit, G., Salman, M. and Romi, M.
Chlorogenic Acid Ameliorates Vascular Remodeling and Perivascular Fibrosis in Kidney Fibrosis Model in Mice.
DOI: 10.5220/0010487000150020
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 15-20
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
15

characterized by interstitial fibrosis,
glomerulosclerosis, the formation and activation of
myofibroblasts cells (Duffield, 2014).
Unilateral Ureteral Obstruction (UUO) is the
most used experimental kidney fibrosis model,
especially in studies related to irreversible Acute
Kidney Injury (AKI) and CKD (Ucero et al, 2014).
The changing of hemodynamic in CKD may induce
vascular remodeling, which is influenced by
activation of the renin-angiotensin system (RAS),
endothelin-1 (ET-1), endothelial dysfunction,
oxidative stress and asymmetric dimethylarginine
(ADMA), and the anti-aging molecule Klotho (Briet
& Burns, 2012). Fibrosis also causes vascular
remodeling characterized by interstitial remodeling
and then vascular remodeling, resulting in reduced
blood supply to the kidneys, causing kidney failure
(Efstratiadis et al, 2009). Endothelin-1 (ET-1) and
Endothelial Nitric Oxide Synthase (eNOS), which
catalyzes the production of nitric oxide (NO) has a
significant role in vascular function as
vasoconstriction and vasodilatation agent (Schiffrin,
2012). It is becoming increasingly clear that an
imbalance between these two mediators is a
characteristic of endothelial dysfunction and is
essential in vascular remodelling
(Farris & Colvin,
2012).
Myofibroblasts are biomolecular markers and
terminally differentiated cells found in non-
pathological situations responsible for the synthesis
and accumulation of interstitial extracellular matrix
components during kidney fibrosis as the
pathogenesis of CKD (Farris & Colvin, 2012).
Perivascular fibroblasts and pericytes have
previously been identified as the major contributors
to the fibrosis then myofibroblast population in the
kidney, especially in vascular area
(Kramann &
Humphreys, 2014).
Chlorogenic acid is a phenolic acid with vicinal
hydroxyl groups on aromatic residues derived from
cinnamic acid esterification, including caffeic,
ferulic, and p-coumaric acids with quinic acid.
Much evidence has shown that chlorogenic acid has
many biological characteristics, including
antibacterial, antioxidant, and anticarcinogenic
activities, especially hypoglycemic, hypolipidemic,
and renoprotective effects
(Santana-Gálvez et al,
2017). Chlorogenic acid can improve kidney function
in 5/6 nephrectomy rats effectively due to its anti-
oxidation and inhibiting accumulation of
extracellular matrix
(Lou et al, 2016).
None of the existing studies have provided overall
effects from chlorogenic acid on fibrosis conditions
in several other organs, especially from the molecular
profibrotic and vasoactive substances. Therefore, this
study was conducted to examine CGA's effect on
vascular aspects, specifically vascular remodeling
and perivascular fibrosis in kidney fibrosis.
2 MATERIALS AND METHODS
This research was a true experimental study using a
post-test only controlled group design. Consists of
control and treatment groups. This study has obtained
permission from the Medical and Health Research
Ethics Committee Faculty of Medicine, Universitas
Tadulako, based on the Ethical Clearance certificate
number C.0942/UN28.1.30/KL/2018 on February 26,
2018.
2.1 Unilateral Ureteral Obstruction
Swiss Webster mice (n=25, 2-3 months old, 20-30 g
weight) were used for the experiments. Mice were
housed in the Department of Anatomy, Faculty of
Medicine, Universitas Tadulako in a cage with the
light-dark cycle of 12:12 hour, food and water ad
libitum. Unilateral Ureteral Obstruction (UUO) was
performed to induce kidney fibrosis. Mice were
anesthetized with Sodium Pentobarbital (0.1mL/10 g
weight) injected intraperitoneally. The right flank's
region was opened, and the right ureter was visualized
then double ligated, after that cut between the ligation
sides. Sham operation (SO) control group procedure
was used the same procedure except for ligating and
cutting the ureter, only for visualized.
2.2 Chlorogenic Acid Administration
Chlorogenic acid (Sigma-Aldrich C3878-1G) was
done with intraperitoneally injection with a dose 14
mg/kg body weight/day. Mice were divided into 5
groups. The distribution for each group: sham
operation (SO) group, was injected distilled water
intraperitoneally for 14 days as a control; mice with
UUO was injected distilled water intraperitoneally for
7 days, called group U7; mice with UUO was injected
with chlorogenic acid for 7 days called UC7 group;
mice with UUO was injected distilled water
intraperitoneally for 14 days called group U14; mice
with UUO was injected chlorogenic acid for 14 days
group UC14. Mice were euthanized on days 8 and 15.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
16

2.3 Kidney Harvesting
In this study, kidney harvesting mice were
anesthetized with Sodium Pentobarbital (0.1mL/10 g
weight) injected intraperitoneally after that abdomen
and thorax were opened. Perfusion was done with 0.9
% NaCl from the left ventricle. Right kidney tissues
were harvested, and the one-half side was fixated in
Normal Buffer. Formalin for 24 h, and used for the
paraffin-embedded tissue process.
2.4 Sirius Red Staining
Tissue slides in paraffin disk were cut in 4 mm
thickness. Paraffin sections were deparaffinized with
PBS washing. Tissue slides were given Sirius
Red working solution to the entire surface of tissue
for 1 hour. Afterward, the slides were soaked
sequentially in 100% ethanol and xylene 3 times each,
then mounted and incubated for 24 hours. Vascular
remodeling assessment based on lumen area, mean
wall thickness, wall area, and lumen area
ratio/WLAR, while perivascular fibrosis is based on
the difference between perivascular fibrosis and
intrarenal arteries blood vessel area. Ten to fifteen
intrarenal arteries with <50mm in diameter were
captured and used for quantification using ImageJ
software.
2.5 Statistical Analysis
Data were analyzed using the IBM SPSS
Statistics program. Data normality test was conducted
using the Shapiro-Wilk test. The homogeneity was
conducted using the Levene test, then the numerical
test using the One-Way ANOVA test for normal data
distribution and the Kruskal-Wallis test for abnormal
data distribution.
3 RESULTS
Vascular Remodelling and Perivascular Fibrosis Area
Unilateral Ureteral Obstruction (UUO) has known
can induce vascular remodeling based on the
quantification of Sirius Red staining (Figure 1A).
Chlorogenic acid effects to ameliorate vascular
remodeling that occurs in the condition of kidney
fibrosis. Quantitative analysis of the lumen area
showed lower significantly in the U7 and U14 groups
(p<0.05) compared to the SO group, then higher
significantly in the UC7 and UC14 groups (p<0.05)
compared to the U7 and U14 groups (Figure 1B).
Quantitative analysis of the mean wall thickness
showed higher significantly in the U7 and U14 groups
(p<0.05) compared to the SO group, then based on the
means data of UC7 and UC14 groups were lower
compared to the U7 and U14 groups (Figure 1C).
Quantitative analysis on WLAR showed higher
significantly in the U7 and U14 groups (p<0.05)
compared to the SO group, then based on the means
data of UC7 group was lower compared to the U7
group, whereas in the UC14 group (p<0.05) was
lower significantly compared to the U14 group
(Figure 1D).
Unilateral Ureteral Obstruction (UUO) has known
to induce perivascular fibrosis based on the
quantification of Sirius Red staining (Figure 1A).
Chlorogenic acid ameliorated perivascular fibrosis
that occurs in kidney fibrosis. Quantitative analysis in
the area of perivascular fibrosis showed higher
significantly in the U7 and U14 groups (p<0.05)
compared to the SO group, but based on the means
data of UC7 group was lower compared to the U7
group, whereas in the UC14 group (p<0.05) lower
significantly compared to the U14 group (Figure 1E).
Chlorogenic Acid Ameliorates Vascular Remodeling and Perivascular Fibrosis in Kidney Fibrosis Model in Mice
17
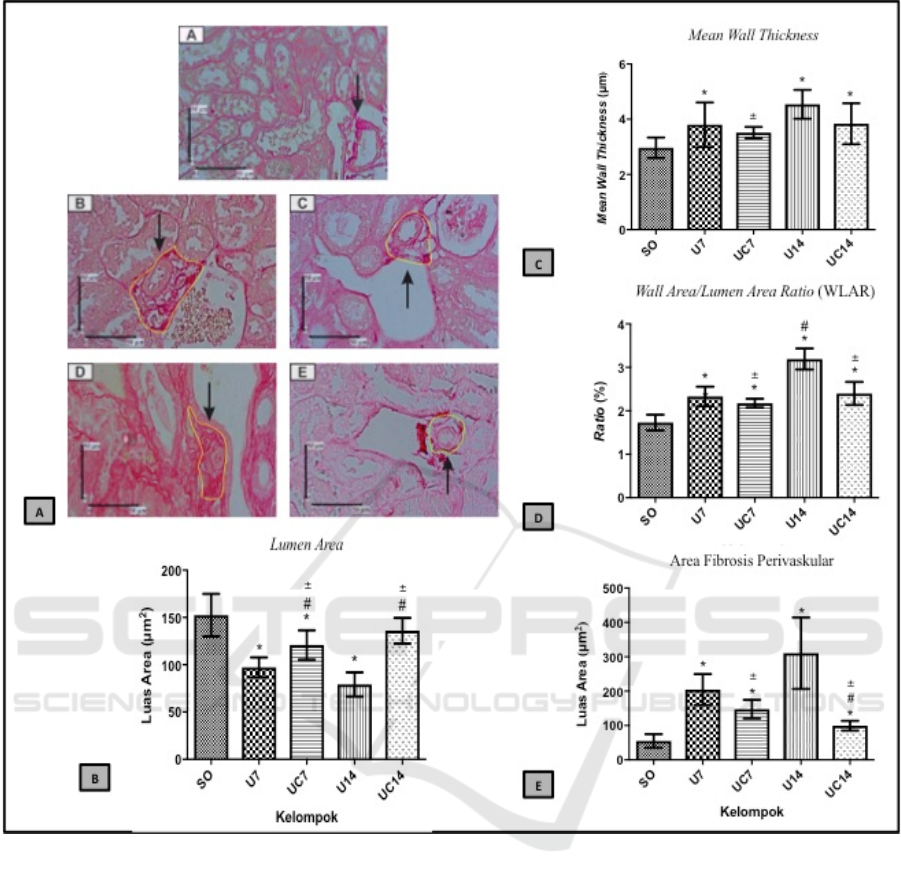
Figure 1. A. Histopatologic view of vessels with vascular remodelling and perivascular fibrosis, A: SO group, B: U7 group,
C: UC7 group, D: U14 group, E: UC14 group. B-E. Results of quantitative analysis of lumen area, mean wall thickness,
WLAR, and perivascular fibrosis. *=p<0.05 vs SO; #=p<0.05 vs U7; =p<0.05 vs U14.
4 DISCUSSION
Unilateral Ureteral Obstruction (UUO) is an
experimental kidney injury model that provides a
representative description of pathological conditions
in CKD because it can trigger kidney fibrosis
characterized by apoptosis, interstitial fibrosis,
glomerulosclerosis, and decrease of kidney mass
(Chevalier et al, 2009). There was an increase of ROS
in UUO due to decreased potential antioxidants,
which activated free radicals bioavailability so that
oxidative stress occurred
(Modaresi et al, 2015).
Oxidative stress, initiated due to an increase in ROS,
was a trigger factor for endothelial dysfunction,
marked by a decrease in NO level, vascular
remodeling, and cellular damage (Craige et al, 2015).
Vascular remodeling is a complex process involving
endothelial cells, smooth muscle cells, and fibroblast
cells. Vascular remodeling represents structural
changes like hypertrophy or hyperplasia of vascular
smooth muscle cells and extracellular matrix
components, which induce changing the artery's
mechanical function. Vascular remodeling can be
observed by assessing lumen area changes, mean wall
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
18

thickness, and the wall area/lumen area ratio
(Tanaka
& Laurindo, 2017).
In this study, the UUO group showed vascular
remodeling; meanwhile, in CGA group showed the
ameliorate of vascular remodeling (Figure 1). There
was an increase in ROS and an oxidation-reduction
reaction, by which c-Jun N-terminal Kinase (JNK)
was made and proliferation and hypertrophy leading
to vascular injury and vascular remodeling in UUO-
induced kidney fibrosis. Reactive Oxygen Species
(ROS) modulated intracellular Ca2+ level as a
primary factor of cellular activity (Görlach et al,
2015). Chlorogenic acid could inhibit the ROS-
modulated Ca2+ influx and restored the viability of
cells and endothelial cells. Other than that,
chlorogenic acid could suppress oxidative stress,
inflammation, apoptosis, and autophagy by
enhancing kidney regeneration (Domitrović et al,
2014). Chlorogenic acid was known to decrease JNK
pathway activation leading to inhibition of apoptosis,
contraction, migration, and inflammation and
reduction of oxidative damage induced by H2O2
(Yu
et al, 2016). We observed increasing vascular
remodeling with lower lumen area, higher mean wall
thickness, and WLAR in UUO might associate with
vasoconstrictor and vasodilator balance might play a
role in regulating vascular remodeling in UUO. Nitric
oxide (NO) plays an essential role in regulating vessel
tonus and remodeling
(Farris & Colvin, 2012).
Kidney vasculature also has a high sensitivity to NO.
NO released in the medulla induces local blood flow
and improves RBF in CKD model (Savard et al,
2012).
Besides affecting vascular remodeling,
chlorogenic acid also played roles in perivascular
fibrosis. We observed higher fibrosis perivascular
area in the UUO group. Meanwhile, CGA might
ameliorate fibrosis perivascular as shown by lower
fibrosis perivascular area in the CGA treated UUO
group (Figure 1). Perivascular fibrosis played active
roles in developing kidney fibrosis, which was
mediated by TGF-B induced myofibroblast
transformation (Kramann & Humphreys, 2014).
Increased ROS caused an imbalance between
oxidation-reduction and modulated the production of
TGF-B through Smad pathway (Liu & Desai, 2015).
Chlorogenic acid had anti-oxidative effects by
decreasing TGF-B gene expression and cytokines
responsible for fibrosis development through miR-21,
which regulated the Smad 7/TGF-B pathway. It
showed that chlorogenic acid was an antifibrosis
agents
(Yang et al, 2017). As a result, CGA treatment
ameliorated vascular remodeling; also reduced
perivascular fibrosis in kidney fibrosis.
5 CONCLUSION
In conclusion, that study highlighted the effect of
chlorogenic acid ameliorated vascular remodeling
based on wider lumen area, thinner mean wall
thickness and lower WLAR; ameliorated perivascular
fibrosis based on the lower area. For a further
research, it is necessary to measure vascular
remodeling using vessel myograph, which could
evaluate endothelial function due to vasoconstriction
and vasodilatation.
ACKNOWLEDGEMENT
The authors are grateful to Wiwit Ananda, Yuyun,
Shyntia, Maulida, and Mulyana in Anatomy
Department, Faculty of Medicine, Public Health, and
Nursing Universitas Gadjah Mada, which has helped
a lot in this research.
REFERENCES
Perkumpulan Nefrologi Indonesia. 4th Report of Indonesia
Renal Registry. 2011; Pernefri, Jakarta.
Mutsaers, H.A.M., Stribos, E.G.D., Glorieux, G.,
Vanholder, R., Olinga, P. Chronic Kidney Disease and
Fibrosis: The Role of Uremic Retention Solutes. Front
Med. 2015; 2(60): 1-7.
Fragiadaki, M., Mason, R.M. Epithelial-Mesenchymal
Transition in Renal Fibrosis - Evidence For and
Against. Int J Exp Path. 2011; 9: 143–150.
Duffield, J.S. Cellular and Molecular Mechanisms in
Kidney Fibrosis. J Clin Invest. 2014; 124(6): 2299-
2306.
Ucero, A.C., Benito-Martin, A., Izquierdo, M.C., Sanchez-
Nino, M.D., Sanz, A., Ramos, A.M., et al. Unilateral
Ureteral Obstruction: Beyond Obstruction. Int Urol
Nephrol. 2014; 46(4): 765–776.
Briet, M., Burns, K.D. Chronic Kidney Disease and
Vascular Remodelling: Molecular Mechanisms and
Clinical Implications. Clin Sci. 2012; 123(7): 399-416.
Efstratiadis, G., Divani, M., Katsioulis, E., Vergoulas, G.
Renal Fibrosis. Hippokratia. 2009; 13(4): 224-228.
Schiffrin, E.L. Immune Modulation of Resistance Artery
Remodelling. Basic Clin Pharmacol Toxicol. 2012;
110(1): 70–72.
Farris, A.B., Colvin, R.B. Renal Interstitial Fibrosis:
Mechanisms and Evaluation In: Current Opinion in
Nephrology and Hypertension. Curr Opin Nephrol
Hypertens. 2012; 21(3): 289-300.
Kramann, R., Humphreys, B.D. Kidney Pericytes: Roles in
Regeneration and Fibrosis. Semin Nephrol. 2014;
34(4): 374–383.
Santana-Gálvez, J., Cisneros-Zelvallos, L., Jcobo-
Chlorogenic Acid Ameliorates Vascular Remodeling and Perivascular Fibrosis in Kidney Fibrosis Model in Mice
19

Velázquez, D.A. Chlorogenic Acid: Recent Advances
on Its Dual Role as a Food Additive and a Nutraceutical
against Metabolic Syndrome. Molecules. 2017; 22(3):
358.
Lou, L., Zhou, J., Liu, Y., Wei, Y., Zhou, J., Deng, J., et al.
Chlorogenic Acid Induces Apoptosis to Inhibit
Inflammatory Proliferation of IL-6-Induces-Fibroblast-
Like Synoviocytes Through The Activation of
JAK/STAT and NF-
K
B Signaling Pathways. Exp Ther
Med. 2016; 11(5): 2054-2060.
Chevalier, R.L., Forbes, M.S., Thornhill, B.A. Ureteral
Obstruction as a Model of Renal Interstitial Fibrosis
and Obstructive Nephropathy. Kidney Int. 2009;
75(11): 1145–1152.
Modaresi, A., Nafar, M., Sahraei, Z. Oxidative Stress in
Chronic Kidney Disease. Iran J Kidney Dis. 2015; 9(3):
165-79.
Craige, S.M., Kant, S., Keaney J.F. Reactive Oxygen
Species in Endothelial Function-From Disease to
Adaptation. Circ J. 2015; 79(6): 1145-1155.
Tanaka, L.Y., Laurindo, F.R.M. Vascular Remodeling: A
Redox-Modulated Mechanism of Vessel Caliber
Regulation. Free Radic Biol Med. 2017; 109: 11–21.)
Görlach, A., Bertram, K., Hudecova, S., Krizanova, O.
Calcium and ROS: A Mutual Interplay. Redox Biol.
2015; 6: 260–271.
Domitrović, R., Cvijanovic, O., Susnic, V., Katalinic, N.
Renoprotective Mechanisms of Chlorogenic Acid in
Cisplatin-Induced Kidney Injury. Toxicology. 2014;
324: 98–107.
Yu, B., Li, J., Guo, B., Fan, H., Zhao, W., Wang, H.Y.
Chlorogenic Acid Analogues from Gynura
Nepalensis Protect H9c2 Cardiomyoblasts Against
H
2
O
2
-Induced Apoptosis. Acta Pharmacol Sin. 2016;
37(11): 1413–1422.
Savard, S., Lavoie, P., Villeneuve, C., Agharazii, M., Lebel,
M., Lariviere, M. eNOS Gene Delivery Prevents
Hypertension and Reduces Renal Failure and Injury in
Rats with Reduced Renal Mass. Nephrol Dial
Transplant. 2012; 27(6): 2182-2190
Liu, R.M., Desai, L.P. Reciprocal Regulation of TGF-β and
Reactive Oxygen Species: A Perverse Cycle for
Fibrosis. Redox Biol. 2015; 6: 565-577
Yang, F., Luo, L., Zhu, Z., Zhou, X., Wang, Y., Xue, J.,
et al. Chologenic Acid Inhibits Liver Fibrosis by
Blocking The miR-21-Regulated TGF-β1/ Smad7
Signaling Pathway in Vitro and in Vivo. Front
Pharmacol. 2017; 8: 929.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
20
