
The Role of Fibroblast Proliferation in Wound Healing by Different
Plants: An Experimental Study
Marisa Riliani
1
a
, Indra Kusuma
2
b
, Abdul Halim
3
c
, Aliya Muhammad
3
d
, Akbar Fitrianto
3
e
,
Ida Bagus Eka Narendra
3
f
1
Department of Anatomy, Faculty of Medicine, Universitas Yarsi, Jakarta, Indonesia
2
Department of Physiology, Faculty of Medicine, Universitas Yarsi, Jakarta, Indonesia
3
Faculty of Medicine, Universitas Yarsi, Jakarta, Indonesia
Keywords: Manihot esculenta, Ageratum conyzoides L., Fibroblast, Wound Healing
Abstract: Wound healing is a process that consisted of inflammation, proliferation, and migration of fibroblasts.
Indigenous people commonly used Manihot esculenta and Ageratum conyzoides L.as bleeding wounds
therapy. This study aimed to investigate these two plant extracts on fibroblast proliferation and migration
capabilities. Skin fibroblasts were cultured with a medium conditioned for each extract with different
concentrations (0.5%, 1%, 2%, 4%), positive and negative control groups. In evaluating proliferation,
fibroblasts were incubated by CCK-8 and measured by spectrophotometer expressed as optical density
(OD). The evaluation of migration was visualized by scratch assay. The distance between each edge of the
scratch was measured using T-scratch software and expressed as the area's closure percentage. There were
significant differences in fibroblast proliferation rate in the groups receiving 0.5% Manihot
esculenta (p=0.00) and 2% Ageratum conyzoides L. (p=0.00). The migration of fibroblasts was no different,
with 0.5% Manihot esculenta (p=0.40) and 2% Ageratum conyzoides L. (p=0.18). Manihot esculenta and
Ageratum conyzoides L. could be considered to be used as agents to accelerate wound healing by increasing
the fibroblast proliferation. Our findings suggest in vivo studies for tissue regeneration.
1 INTRODUCTION
In 2013, skin wound affected 8.2% population in
Indonesia. Metabolic diseases and infections can
influence the healing time; it could make a slow or
even non-wound healing process. Slow or non-
healing wounds affect millions of people worldwide
and result in enormous health care expenditures. The
skin is our outer part of the body, which can protect
us from many environmental stresses. Injured skin
sets into motion a series of repair mechanisms
directed to the injured tissue. Wound healing is a skin
reparative process consist of inflammation,
proliferation, and migration of fibroblasts. The role of
fibroblasts is to synthesize and integrate protein and
a
https://orcid.org/0000-0002-5025-793
b
https://orcid.org/ 0000-0001-9350-431X
c
https://orcid.org/0000-0003-1842-7646
d
https://orcid.org/ 0000-0002-9249-6044
e
https://orcid.org/0000-0002-8553-1550
f
https://orcid.org/ 0000-0001-7800-1335
elastin inside the extracellular matrix from some large
part of the mesenchyme tissue
(Simon, 2020).
Along with proper wound care products, it works
together to repair and replace devitalized tissue.
Many topical drugs are used to create and maintain a
moist environment and provide healing conditions
(Lordani et al., 2018). They are often expensive, and
drugs price increased up to 15% every year
(Blumberg, 2019). Therefore, we need to find
alternative ingredients that easy to find, at a lower
cost and have a shorter healing time. Recently, so
many plants are coming out as tools for therapeutic
application.
For decades, indigenous people have commonly
used the leaves of Manihot esculenta and Ageratum
Riliani, M., Kusuma, I., Halim, A., Muhammad, A., Fitrianto, A. and Eka Narendra, I.
The Role of Fibroblast Proliferation in Wound Healing by Different Plants: An Experimental Study.
DOI: 10.5220/0010486300050009
In Proceedings of the 1st Jenderal Soedirman International Medical Conference in conjunction with the 5th Annual Scientific Meeting (Temilnas) Consortium of Biomedical Science Indonesia
(JIMC 2020), pages 5-9
ISBN: 978-989-758-499-2
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
5

conyzoides L. as therapeutic applications for bleeding
wounds
(Oktaviani et al., 2019). In in
vivo study, Ageratum conyzoides L. increased
cellular proliferation and collagen synthesis
(Arulprakash et al., 2011). Meanwhile, Manihot
esculenta increased the gingival wound healing
process (Nisa et al., 2013). Nevertheless, these plants'
efficacy has never been found to fibroblast cells,
which is very important for the healing process.
Therefore, in this study, we aimed at investigating
fibroblast proliferation and migration capabilities in
vitro in the presence of ethanol extracts of Manihot
esculenta and Ageratum conyzoides L.
2 MATERIALS AND METHODS
2.1 Research Design
The cells were cultured with a medium conditioned
for each extract with different concentrations (0.5%,
1%, 2%, 4%), positive and negative control groups.
For migration assay, cell culture was divided into
four groups; two groups received the most
significantly induced fibroblast proliferation for each
extract; 0.5% Manihot esculenta and 2% Ageratum
conyzoides L., two other groups as positive and
negative control groups.
2.1.1 Time and Place
The experiment was conducted from October to
December 2019 at Laboratorium Penelitian Terpadu
Universitas Yarsi.
2.1.2 Populations and Samples
The preputium skin fibroblast from Biorepository of
Universitas YARSI was routinely cultured in
Dulbecco's modified Eagle's medium (DMEM), low
glucose supplemented with 2 mM L-glutamine and
10% fetal bovine serum (FBS). All cells were grown
in 100 units/mL penicillin, 100 ug/mL streptomycin,
and 0,25 ug/mL amphotericin B. Cells for the
experiments were used at passage 3.
Plant materials of Manihot esculenta and
Ageratum conyzoides L. were collected in October
2019 in the Caringin village, west java. The leaves
were dried at room temperature for five days and then
were cut into fragments of approximately 5 cm. The
fragments were soaked in 70% ethanol and filtered. A
rotatory evaporation machine evaporated the filtrate.
2.1.3 Viability and Proliferation Assay
Fibroblasts were seeded at the concentration of 3000
cells/well in a 96 well plate and allowed to incubate
overnight. After the cells attached to the well surface,
cells were washed in phosphate-buffered saline
(PBS). The cells were cultured with a medium
conditioned for each extract with different
concentrations (0.5%, 1%, 2%, 4%), positive and
negative control groups for 48hr. The culture medium
was discarded every indicated time points and added
10 µl CCK-8 and 90 µL PBS. After 90 minutes of
incubation with CCK-8, absorbance detected at 450
nm using a Tecan microplate reader. The results
expressed in optical density (OD) units as compared
to untreated cells.
2.1.4 Migration Assay
Fibroblasts were seeded at a concentration of 40.000
cells/well in a 12 well plate and incubated until
confluence. A scratch was made using a 10 µl pipette
tip for each well. After removed the media, the cells
were washed in PBS and then added medium
conditioned with 0.5% Manihot esculenta and 2%
Ageratum conyzoides L. The distance between scratch
areas was measured by optical microscopy every two
hours for 48 hours using Nikon advanced research
elements 3.21.00 software and expressed as a
percentage of the area's closure compared to untreated
cells.
2.1.5 Data Analysis
Three technical replicates performed the experiments
for each treatment. All statistical analyses were
performed by comparing 0.5% Manihot esculenta and
2% Ageratum conyzoides L using unpaired Student's
t-test.
3 RESULTS
3.1 Cell Viability and Proliferation
At 48 hours, the extracts of 2% Ageratum conyzoides
L. was the most significantly induced fibroblast
proliferation at 0.22 ± 0.02 (Figure 1) and showed
intact fibroblast morphology (Figure 3, panel D). The
extracts of 0.5% Manihot esculenta was the most
significantly induced fibroblast proliferation at 0.16 ±
0.01 (Figure 2) and showed intact fibroblast
morphology (Figure 4, panel B). DMSO significantly
decreased fibroblast proliferation at 0.01 ± 0.00 and
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
6
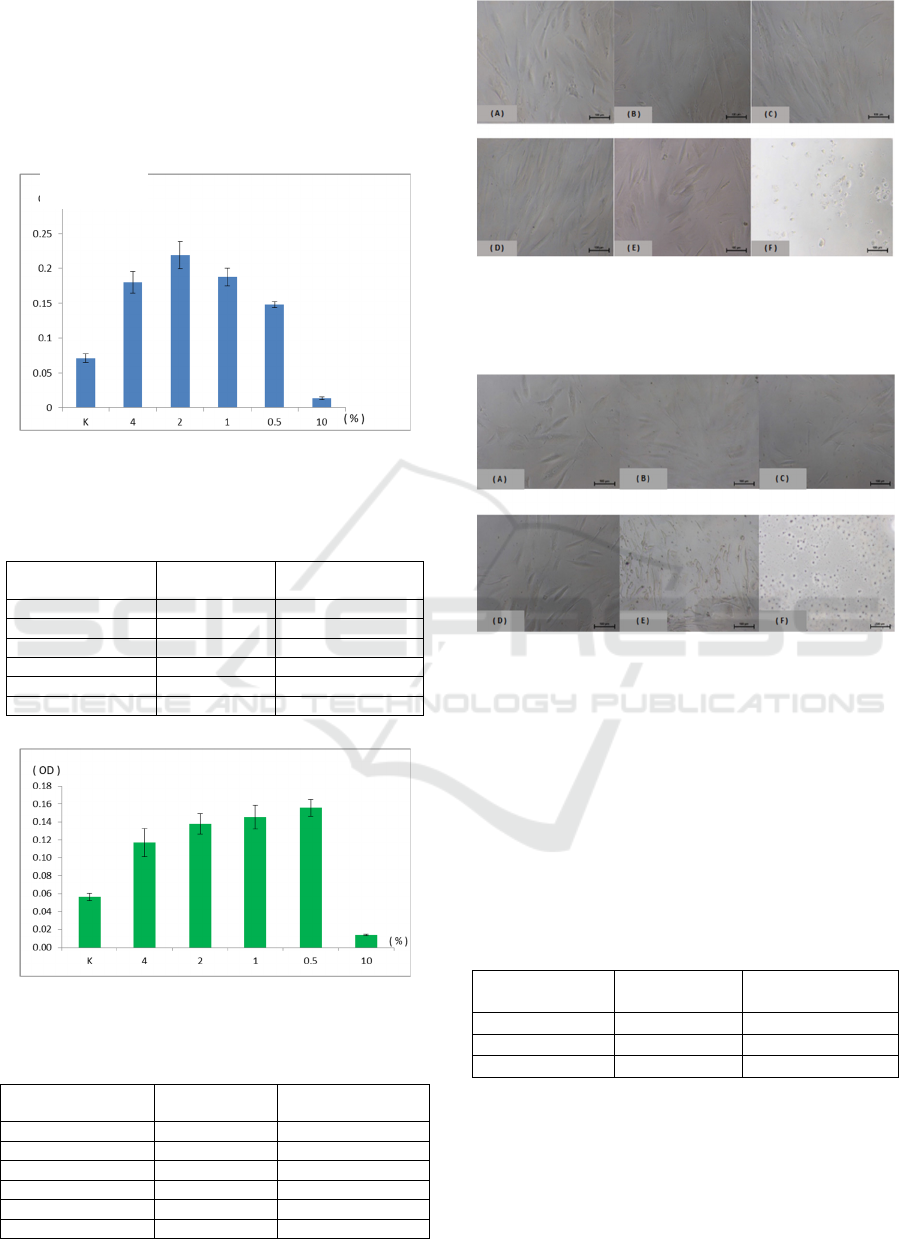
showed damage to fibroblast morphology. The
control group indicated fibroblast proliferation at 0.07
± 0.01 and showed intact fibroblast morphology.
There were significant differences in fibroblast
proliferation rate in 2% Ageratum conyzoides L.
(p=0.00) and 0.5% Manihot esculenta (p=0.00)
compared to the control group.
Figure 1: Fibroblast viability and proliferation in different
concentrations of Ageratum conyzoides L. at 48 hours.
Table 1: T-test for fibroblast proliferation in different
concentrations of Ageratum conyzoides L. at 48 hours.
Concentration
Ageratum
conyzoides L
T-test
0.5% 0.15 0.00
1% 0.19 0.00
2% 0.22 0.00
4% 0.18 0.01
dmso 0.01 0.00
control 0.07
Data were expressed as optical density (OD)
Figure 2: Fibroblast Viability and Proliferation in different
concentrations of Manihot esculenta at 48 hours.
Table 2: T-test for fibroblast proliferation in different
concentrations of Manihot esculenta at 48 hours.
Concentration
Manihot
esculenta
T-test
0.5% 0.16 0.00
1% 0.15 0.00
2% 0.14 0.00
4% 0.12 0.00
dmso 0.01 0.00
control 0.06
Data were expressed as optical density (OD)
Figure 3: Fibroblast Morphology in different concentration
of Ageratum conyzoides L. at 48 hour. (A) control group,
(B) 0.5%, (C) 1%, (D) 2% and (E) 4% extract in complete
medium, (F) 10% DMSO.
Figure 4: Fibroblast Morphology in different concentration
of Manihot esculenta at 48 hour. (A) control group, (B)
0.5%, (C) 1%, (D) 2% and (E) 4% extract in complete
medium, (F) 10% DMSO.
3.2 Cell Migration
At 48 hours, the wound was almost closed for each
group. However, there were no significant differences
in fibroblast migration rate in 2% Ageratum
conyzoides L. (p=0.18) and 0.5% Manihot esculenta
(p=0.40) compared to the control group.
Table 3: Migration of fibroblast.
Control
2% Ageratum
con
y
zoides L
0.5% Manihot
esculenta
61.42 ± 7.88 64.35 ± 6.25 66.65 ± 4.17
31.08 ± 12.29 38.28 ± 8.89 35.36 ± 11.21
0.60 ± 0.15 2.07 ± 2.86 0.43 ± 0.2
Data were expressed as wound closure percentage (%). At
48 hours, 2% Ageratum conyzoides L. at 2.07 ± 2.86, and
0.5% Manihot esculenta at 0.43 ± 0.2 .
(OD)
The Role of Fibroblast Proliferation in Wound Healing by Different Plants: An Experimental Study
7

Figure 5: Analysis of fibroblast migration during wound
closure. Control group at (a): 0hr, (b): 24hr, (c): 48hr. Cells
treated by 2% Ageratum conyzoides L. at (d): 0hr , (e): 24hr ,
(f): 48hr. Cells treated by 0.5% Manihot esculenta at (g):
0hr , (h): 24hr , (i): 48hr.
4 DISCUSSION
Wound healing time can take a year or more to finish.
Some wounds do not heal in a timely and orderly
manner. It is disturbed by various factors such as
infection, tissue hypoxia, necrosis, exudate, and
excess inflammatory cytokines. The wound healing
process has four overlapping stages: homeostasis,
inflammation, proliferation, and migration
(Addis et
al., 2020). While platelets have a role in clot
formation during homeostasis, inflammation cells
debride injured tissue during the inflammation phase.
At the proliferative phase, occur epithelialization,
fibroplasia, and angiogenesis (DesJardins et al.,
2018).
Meanwhile, granulation tissue forms and the
wound begins to contract. In the maturation phase,
collagen forms tight cross-links to other collagen and
with protein molecules. Fibroblasts have a crucial
role in all of these phases, including the deposition of
extracellular matrix (ECM) components, wound
contraction, and new ECM remodeling
(Sumbayak,
2016).
Plants and herbs are active medicinal, are used to
stimulate stem cell proliferation, regeneration, and
rehabilitation in damaged tissue
(Cragg and Newman,
2017). Several studies found that the leaves' active
ingredients mainly activate stem cell regeneration
potential (Maioli et al., 2010). Plant extracts or plant-
derived compounds are preferred because of fewer
side effects and widespread availability
(Agyare et al.,
2014). Moreover, wound healing management can be
elicited by the antioxidant activity of some plant
extracts
(Süntar et al., 2012).
The antioxidant flavonoid of Ageratum
conyzoides L. has anti-inflammatory effects,
especially quercetin, that can inhibit β-glucuronidase,
decrease leukotriene, inhibit histamine, inhibit some
enzymes such as ATPase, phosphodiesterase, and
protein kinases
(Galati, 2008). Meanwhile, the extract
of Manihot esculenta has an anti-inflammatory effect
since having gallic acid, vitamin C, flavonoid,
saponin, tannin, and triterpenoid as antioxidant
compounds
(Oktaviani et al., 2019).
Reactive oxygen species are involved in many
infections, degenerative diseases, cancer, and even
wound healing. Antioxidants enhance the healing of
wounds by reducing the damage caused by oxygen
radicals. Plant-derived antioxidants benefit from their
redox properties, which allow them to act as hydrogen
donors, reducing agents, hydroxyl radicals (OH), or
superoxide radicals (O2) scavengers
(Geethalakshmi
et al., 2013).
These two extracts have toxic components that
may interfere with the healing process since the data
showed no significant difference in fibroblast
migration. Ageratum conyzoides L. contains
pyrrolizidine alkaloids (PAs), which have been
reported to be hepatotoxic, mutagenic, and
carcinogenic
(Bosi et al., 2013). Manihot esculenta
contains toxic agents cyanogenic glycosides, made up
of 95% linamarin and 5% lotaustralin
(Faezah et al.,
2016).
Pyrrolizidine alkaloids are widely distributed in
plants throughout the world. Alkaloids present a
lipophilic character, soluble in apolar organic
solvents and alcohol. Pyrrolizidine alkaloids can
penetrate the nucleus and react with DNA, causing
DNA cross-link and DNA-protein cross-link to elicit
an abnormal function, which will cause damage
(Moreira et al., 2018).
Cyanogenic glycosides are bioactive plant
products derived from amino acids—the primary
biological function is as a plant defense system
against the effects of distinct animals. Acute
poisoning of animals and humans from cyanogenic
consumption can induce rapid and drastic inhibition
of the respiration system in mitochondria
(Vetter,
2017).
Our data showed that the extracts could enhance
wound healing by stimulating the proliferation of
fibroblast. Within this context, the extracts of
Ageratum conyzoides L. and Manihot esculenta could
be used in the future as a topical therapeutic
application to stimulate the wound healing process
and antioxidant responses in damaged skin.
JIMC 2020 - 1’s t Jenderal Soedirman International Medical Conference (JIMC) in conjunction with the Annual Scientific Meeting
(Temilnas) Consortium of Biomedical Science Indonesia (KIBI )
8

5 CONCLUSION
The study of Ageratum conyzoides L and Manihot
esculenta extract's leaves proved it could accelerate
wound healing. The extracts increase fibroblast
proliferation in vitro. Further in vivo study is needed
for tissue regeneration applications.
ACKNOWLEDGEMENTS
We want to thank Herbal Laboratory-Universitas
Yarsi and Yarsi University Foundation for the
support.
REFERENCES
Addis, R., Cruciani, S., Santaniello, S., Bellu, E., Sarais, G.,
Ventura, C., Maioli, M. and Pintore, G., 2020.
Fibroblast Proliferation and Migration in Wound
Healing by Phytochemicals: Evidence for a Novel
Synergic Outcome. International Journal of Medical
Sciences, 17(8), p.1030.
Agyare, C., Adarkwa-Yiadom, M. and Osei-Asante, S.,
2014. Medicinal plants used for treatment of wounds
and skin infections: Assessment of wound healing and
antimicrobial properties of Mallotus oppositifolius and
Momordica charantia.
Arulprakash, K., Murugan, R., Ponrasu, T., Iyappan, K.,
Gayathri, V.S. and Suguna, L., 2012. Efficacy of
Ageratum conyzoides on tissue repair and collagen
formation in rats. Clinical and Experimental
Dermatology: Experimental dermatology, 37(4),
pp.418-424.
Blumberg, Y., 2019. Here's why many prescription drugs in
the US cost so much—and it's not innovation or
improvement. CNBC. Retrieved from:
https://www.cnbc.com/2019/01/10/why-prescription-
drugs-in-the-us-cost-so-much.html
Bosi, C.F., Rosa, D.W., Grougnet, R., Lemonakis, N.,
Halabalaki, M., Skaltsounis, A.L. and Biavatti, M.W.,
2013. Pyrrolizidine alkaloids in medicinal tea of
Ageratum conyzoides. Revista Brasileira de
Farmacognosia, 23(3), pp.425-432.
Cragg, G.M. and Newman, D.J., 2013. Natural products: a
continuing source of novel drug leads. Biochimica et
Biophysica Acta (BBA)-General Subjects, 1830(6),
pp.3670-3695.
desJardins-Park, H.E., Foster, D.S. and Longaker, M.T.,
2018. Fibroblasts and wound healing: an update.
Regenerative medicine, 13(5). Retrieved from
https://www.futuremedicine.com/doi/full/10.2217/rme
-2018-0073
Nur, F.O., Siti, A.H. and Umi, K.Y., 2013. Comparative
evaluation of organic and inorganic fertilizers on total
phenolic, total flavonoid, antioxidant activity and
cyanogenic glycosides in cassava (Manihot
esculenta). African Journal of Biotechnology, 12(18).
Galati, E.M., Miceli, N., Taviano, M.F., Sanogo, R. and
Raneri, E., 2001. Anti-inflammatory and antioxidant
activity of Ageratum conyzoides. Pharmaceutical
biology, 39(5), pp.336-339.
Geethalakshmi, R., Sakravarthi, C., Kritika, T., Arul
Kirubakaran, M. and Sarada, D.V.L., 2013. Evaluation
of antioxidant and wound healing potentials of
Sphaeranthus amaranthoides Burm. f. BioMed research
international, 2013.
Gonzalez, A.C.D.O., Costa, T.F., Andrade, Z.D.A. and
Medrado, A.R.A.P., 2016. Wound healing-A literature
review. Anais brasileiros de dermatologia, 91(5),
pp.614-620.
Lordani, T.V.A., de Lara, C.E., Ferreira, F.B.P., de Souza
Terron Monich, M., Mesquita da Silva, C., Felicetti
Lordani, C.R., Giacomini Bueno, F., Vieira Teixeira,
J.J. and Lonardoni, M.V.C., 2018. Therapeutic effects
of medicinal plants on cutaneous wound healing in
humans: a systematic review. Mediators of
inflammation, 2018.
Maioli, M., Santaniello, S., Montella, A., Bandiera, P.,
Cantoni, S., Cavallini, C., Bianchi, F., Lionetti, V.,
Rizzolio, F., Marchesi, I. and Bagella, L., 2010.
Hyaluronan esters drive Smad gene expression and
signaling enhancing cardiogenesis in mouse embryonic
and human mesenchymal stem cells. PLoS One, 5(11),
p.e15151.
Moreira, R., Pereira, D.M., Valentão, P. and Andrade, P.B.,
2018. Pyrrolizidine alkaloids: chemistry,
pharmacology, toxicology and food
safety. International journal of molecular
sciences, 19(6), p.1668.
Nisa, V.M., 2013. Efek Pemberian Ekstrak Daun Singkong
(Manihot esculenta) Terhadap Proses Penyembuhan
Luka Gingiva Tikus (Rattus norvegicus). Repository
unej. Retrieved from
http://repository.unej.ac.id/bitstream/handle/123456789
/59375/Vina%20M.Nisa.pdf?sequence=1
Oktaviani, D.J., Widiyastuti, S., Maharani, D.A., Amalia,
A.N., Ishak, A.M. and Zuhrotun, A., 2019. Bahan
Alami Penyembuh Luka. Majalah Farmasetika, 4(3),
pp.45-56.
Simon, P.E. 2020Skin wound healing. Medscape. Retrieved
from https://emedicine.medscape.com/article/884594
Sumbayak, E.M., 2015. Fibroblas: Struktur dan Peranannya
dalam Penyembuhan Luka. Jurnal Kedokteran Meditek,
21(57). Retrieved from
http://ejournal.ukrida.ac.id/ojs/index.php/Meditek/articl
e/view/1169
Süntar, I., Akkol, E.K., Nahar, L. and Sarker, S.D., 2012.
Wound healing and antioxidant properties: do they
coexist in plants?. Free Radicals and Antioxidants, 2(2),
pp.1-7.
Vetter, J. 2017. Plant Toxins : Plant Cyanogenic
Glycosides. Dordrecht, The Netherlands: Springer.
The Role of Fibroblast Proliferation in Wound Healing by Different Plants: An Experimental Study
9
