
Comparison of Activity Coefficient in ABE with MEP with
UNIQUAC Equation
Widi Wurjani
1
, Intan Purbasari
2
and Ni Ketut Sari
3
1
Departement of Agrotechnology, Universitas Pembangunan Nasional Veteran Jawa Timur, Indonesia
2
Departement of Computer Science, Universitas Pembangunan Nasional Veteran Jawa Timur, Indonesia
3
Department of Chemical Engineering, Universitas Pembangunan Nasional Veteran Jawa Timur, Indonesia
Keywords: Activity coefficient, ABE ternary system, MEP ternary system, UNIQUAC equations
Abstract: UNIQUAC equations in calculating the activity coefficient Acetone Butanol Ethanol (ABE) ternary system
using the Mat-lab programming language, then verified with a homologous series of alcohol Methanol
Ethanol Propanol (MEP) ternary system, to see if the mixture is an ideal or non-ideal solution. If the activity
coefficient is close to one, then the solution is the ideal solution, whereas if the activity coefficient is more
or less one, then the solution is a non-ideal solution so that in the separation of the solution, the activity
coefficient should not be assumed to be the same as one, the activity coefficient should be calculated the
actual value. The fixed variable consists of the temperature and Antoine parameter, the change variable
consists of the liquid composition and dimensionless time, then obtained the activity coefficient profile
function dimensionless time from the ABE and MEP ternary systems. With UNIQUAC equations obtained
activity coefficients of the ABE ternary system more than once, while MEP ternary system shows the
activity coefficient close to one, it can be concluded that the ABE ternary system is an a-zeotropic ternary
system.
1 INTRODUCTION
Studied by Rayleigh (1902) and then written in the
manual Separation Process Principles by Henley and
Seader (1998). In the chemical industry,
fermentation process is one way to get a chemical
compound with the help of microorganisms helped,
fermentation products enter the next stage of
separation (Sari, 2009).
Research by Rayleigh
(1902) and later research by Henley and Seader
(1998) book in the Principles of Separation Process.
In the chemical industry, the solution separation
process is an important process for collecting pure
components, one of which uses thermodynamic
theory of the coefficient of activity, to determine the
ideal solution (Sari, 2018). In the separation process,
thermodynamic data in the form of equilibrium data
is very dominant in the solution separation process.
One of the correlations of modern thermodynamics
to the equilibrium phase that is not ideal is the
UNIQUAC equation, the approximate balance and
prediction data can only be obtained in the
experimental data of binary systems. The coefficient
model of ternary system activity with UNIQUAC
equations is developed from a binary mixture, and
has the advantage of application in a multi-
component mixed system and requires no additional
parameters. But the disadvantages of not always
succeeding in predicting a mixed multi-component
balance system are not ideal, especially mixtures
that have limited solubility (Renanto, 1997).
Simulation of activities coefficients equation
ternary system has been investigated using rigorous
methods to DAEs models, where the completion of
the model equations numerically using the Euler
method using Mat-Lab language version 6.1 (Sari,
2006). Results of the simulation system binary
system acetone-butanol, acetone-ethanol, ethanol-
butanol and then validated with a binary system of
benzene-toluene.
Along with the development of information
technology, the program may evolve over time using
the programming methods applied lately that
program object-oriented, in addition to easy to be
developed at a time when that will come, the
software uses object-oriented programming methods
this has other benefits, too in 1 software projects can
use a variety of programming languages that support
Wurjani, W., Purbasari, I. and Sari, N.
Comparison of Activity Coefficient in ABE with MEP with UNIQUAC Equation.
DOI: 10.5220/0010369300003051
In Proceedings of the International Conference on Culture Heritage, Education, Sustainable Tourism, and Innovation Technologies (CESIT 2020), pages 605-609
ISBN: 978-989-758-501-2
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
605

sat
i
.P
i
x
P .
i
y
i
γ
object oriented programming, such as C#.Net and
VB.Net.
Simulation of batch distillation of binary systems
using Mat-lab programming language, which results
in the appearance of the graph using a spreadsheet
tool, less effective and efficient (Sari et al., 2007), so
it is necessary for the visualization of object oriented
programming language, in addition to easily be
developed at a time when that will come, have
another advantage is in the software projects can use
a variety of programming language that supports
object-oriented programming, such as C#.Net and
VB.Net (Sari, et al., 2013) .
This research aims to display simulation profile
coefficient activity function dimensionless with
Antoine equation and activity coefficients process
use Mat lab programming.
2 MATERIAL AND METHODS
Basic concepts of Object Oriented Programming
concepts emphasize the following (Aristarchus et al.,
2011): Class: the collection of data definitions and
functions in a unit for a particular purpose. Class is
the basis of modularity and structure in an object-
oriented programming. A class should typically be
recognizable by even a non-programmer domain
associated with the existing problems, and the code
is contained in a class should be (relatively)
autonomous and independent nature (as the code is
used if not using OOP). With modularity, the
structure of a program will be associated with
aspects of the problem to be solved through the
program. This way will simplify the mapping of the
problem to a program or vice versa. Object:
wrapping the data and functions together into a unit
in a computer program, object is the basis of
modularity and structure in an object oriented
computer program. Abstraction: The ability of a
program to bypass aspects of the information
processed by it, namely the ability to focus on the
core. Encapsulation: Ensuring the user of an object
cannot change the state of an object in a way that is
not feasible; just the method in which the object was
given permission to access the situation.
Polymorphism: through sending messages. Does
not depend on calling subroutines, object-oriented
language can send messages; particular method
associated with a message delivery depends on the
specific object in which the beam is sent. For
example, if a bird received "fast motion", he would
move his wings and fly. When a lion received the
same message, he will move his legs and ran. Both
answered a similar message, but in accordance with
the ability of these animals. This is called
polymorphism as a variable in the program single
can hold different types of objects while running the
program, and the text of the same program can call
several different methods at different times in the
same calling.
This is in contrast to functional languages
achieve polymorphism through the use of first-class
functions. By using the OOP in solving a problem
we do not see how to solve a problem is objects but
what can be done solving those problems. For
example, suppose we have a department that has a
manager, secretary, data and other administration
officials. Suppose the manager wants to obtain data
from the administrative manager of the bag does not
have to take it immediately but can be ordered
officers to take administrative bag. In that case, a
manager does not have to know how to take the data,
but the manager can get the data object through
administrative officer. So in order to solve a problem
with collaboration among existing objects because
each object has its own job description.
In making the application is used batch
distillation program makers and the language used to
create the program: Visual Studio 2010: is a
developer of software (Software Maker) issued by
one of the largest computer software company in the
world that is Microsoft. The advantage of this is that
Visual Studio 2010 has been adopted. Net
Framework 4.0 and the many languages that can be
used to create such software, such as C#.Net,
VB.Net, and so forth. Microsoft.NET Framework
(Microsoft Dot Net Framework) or better known as
the dot net is a software framework that runs
primarily on Microsoft's Windows operating system,
this time. NET Framework generally have been
integrated in the standard distribution of Windows
(starting from Windows Server 2003 Windows
versions and newer). The framework provides a
large amount of computer programming libraries
and supports several programming languages and
good interoperability allowing these languages to
serve one another in the development of the system.
At low pressure, the vapor phase so close to the
ideal gas low pressure liquid vapor equilibrium
becomes,
(3)
Equation (1) is also known as the modified
Raoult's equation. The constant of equilibrium
between the vapor phase and liquid phase is defined
as follows:
CESIT 2020 - International Conference on Culture Heritage, Education, Sustainable Tourism, and Innovation Technologies
606
P
sat
i
.P
i
γ
i
x
i
y
i
K
i
C
P log -
i
A
i
B
sat
i
T
i
sat
i
T
i
x T
j
C
sat
1
P log -
j
A
j
B
T
e
T
)T(T
new
beginningnew
m
1j
jj
x
i
x
i
φ
i
i
φ
i
θ
ln
i
q
2
z
i
x
i
φ
ln
m
1j
m
1k
kj
τ
k
θ
ij
τ
j
θ
m
1j
ji
τ
j
θln1
m
j
r
j
x
i
r
i
x
i
φ
1j
m
1j
jj
ii
i
xq
xq
θ
RT
uu
expτ
iiji
ji
1)(r)q(r
2
z
iiii
(2)
Iteration procedure to find the temperature of
which is to seek price bubble saturation temperature
of pure component Ti
sat
on P (Prausnitz, et al., 2001:
Sari and Dira 2017).
(3)
where A, B, C are Antoine constants for species
i, for all initial estimates.
(4)
For i = 1, 2, 3.
Price T as the initial price will be used to
determine the saturated vapor pressure of a
substance to be estimated with the equation T
Antoine, prices were sought by the equation:
(5)
Then look for the error between the new T with
T the beginning with equation (6)
(6)
γ
i
activity coefficients obtained from:
ln
i
= ln
i
C
+ ln
i
R
(7)
ln
i
C
=
(8)
ln
i
R
=q
i
(9)
(10)
where the coordination number z is set equal to
10.
(11)
(12)
The parameters r, q is a constant component of
the molecular structure based purely on molecular
size and external surface area. For each binary
combination in multi-component mixtures, there are
two parameters that can be adjusted r, q:
(13)
jj
=
ii
= 1
(14)
Table 1: Antoine parameters Acetone-Butanol-Ethanol
Source: Prausnitz, 2001
Parameters Antoine
Com
p
onents A B C
Acetone 4.2184 4.6493 5.3365
Butanol 197.01 1395.14 1648.22
Ethanol 228.06 182.739 230.918
To calculate the saturated vapor pressure Antoine
equation is used data Antoine parameters such as
Table 1 (Prausnitz, 2001), where the temperature (T)
in units of K and saturated vapor pressure (P
SAT
) in
units of Bar.
Table 2: Feed composition of ABE
No. Acetone Butanol Ethanol
1 0,8 0,1 0,1
2 0,7 0,2 0,1
3 0,7 0,1 0,2
4 0,6 0,3 0,1
5 0,6 0,1 0,3
6 0,5 0,1 0,4
7 0,4 0,1 0,5
Feed composition (mole fraction)
The composition of the bait is taken 7 runs,
already represents the profile of the activity
coefficient function ABE ternary system
composition such as Table 2 (Sari and Dira, 2018).
Table 3: Feed composition of MEP
Run Methanol Ethanol Propanol
1 0,80 0,10 0,10
2 0,90 0,02 0,08
3 0,70 0,20 0,10
4 0,30 0,60 0,10
5 0,39 0,60 0,01
6 0,50 0,40 0,10
Feed composition (fraction mole)
The composition of the bait is taken 6 runs,
already represents the profile of the activity
coefficient function ABE ternary system
composition such as Table 3 (Sari and Dira, 2018).
Comparison of Activity Coefficient in ABE with MEP with UNIQUAC Equation
607
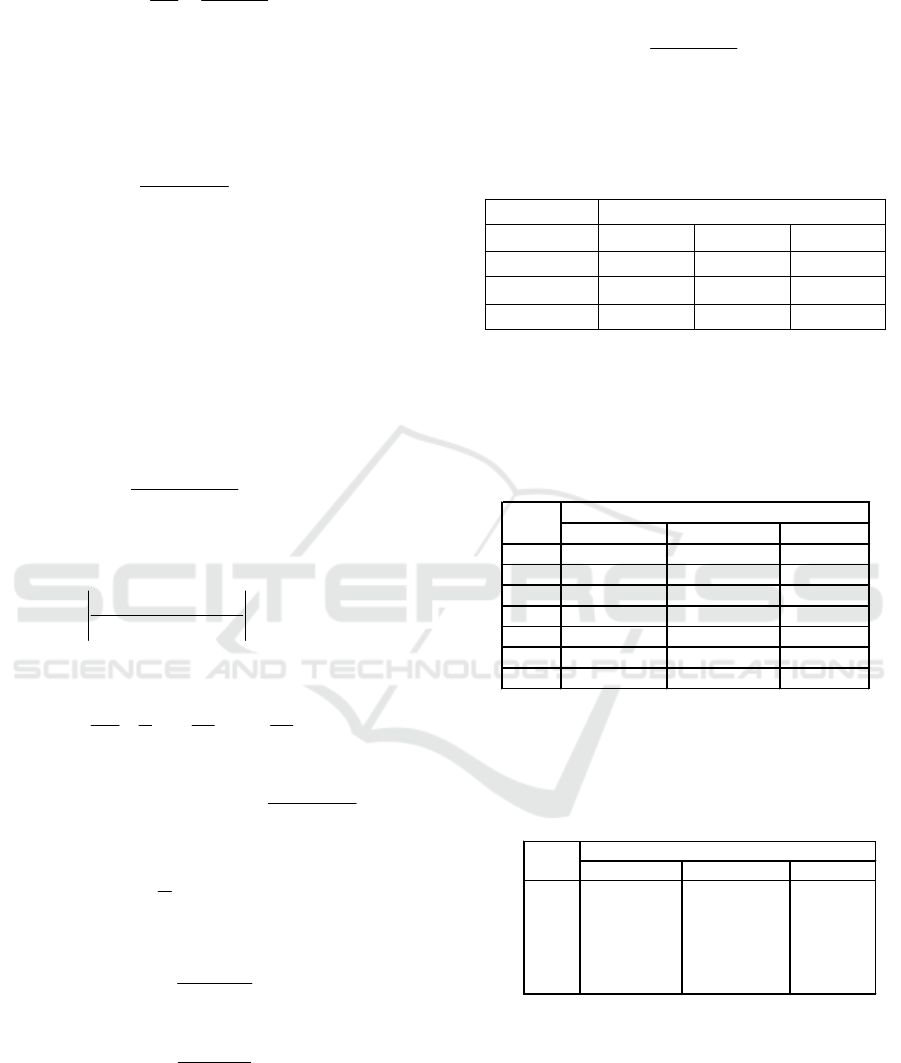
3 RESULTS AND DISCUSSIONS
The temperature profile at the beginning of the
process indicates the propyl decreases to
dimensionless 0.25, as the adaptation process for the
process runs continuously. Temperature increase up
to dimensionless 2.5 for all composition feeds, for
Run 2 and Run 4 shows the highest temperature
achievement, because a large mixture of acetone and
butanol around 0.9, for the composition feed (Run 4)
that is volatile will indicate the highest temperature.
Run 1 Run 5
Run 2
Run 6
Run 3
Run 7
Run 4
Figure 1: Profile temperature ABE versus dimensionless
time
After dimensionless at a value of 3.5 all
composition feeds show a flat profile, a temperature
value with a range of 115 to 120 (
o
C), corresponding
to the mixed temperature of the ABE ternary system,
as shown in Figure 1.
Figure 2: Profile temperature MEP versus dimensionless
time
The temperature profile at the beginning of the
process indicates the propyl decreases to
dimensionless 0.25, as the adaptation process for the
process runs continuously. Temperature increase up
to dimensionless 3.5 for all composition feeds, for
Run 6 shows the highest temperature achievement,
because a large mixture of methanol and ethanol
around 0.9, for the composition feed (Run 4) that is
volatile will indicate the highest temperature. After
dimensionless at a value of 3.5 all composition feeds
show a flat profile, a temperature value with a range
of 78 to 95 (
o
C), corresponding to the mixed
temperature of the MEP ternary system, as shown in
Figure 2.
At the beginning of the process until the activity
coefficient shows a value of 1, Butanol and Ethanol
show a decreased activity coefficient profile,
Acetone shows a rising activity coefficient profile,
all three approaching the ideal solution. At an
activity coefficient value equal to 1.5 to 3.5, the
activity coefficient profile of Butanol and Ethanol
indicates an ideal solution, in which the process of
separation of the mixture of activity coefficients can
be assumed to be equal to 1. At an activity
coefficient value equal to 1.5 to 3.5, the coefficient
profile of Acetone, Butanol and Ethanol activity
coefficient indicates no ideal solution, in which the
process of separation of the mixture of activity
coefficients should be calculated using UNIQUAC
equations, as shown in Figure 3.
Figure 3: Profile activity coefficient of ABE versus
dimensionless time
At the beginning of the process until the activity
coefficient shows a value of 1, Methanol shows a
decreased activity coefficient profile, Ethanol and
Propanol show a rising activity coefficient profile,
50
55
60
65
70
75
80
85
90
95
100
105
110
115
120
0,0 0,5 1,0 1,5 2,0 2,5 3,0 3,5
Temperature (
o
C)
Dimensionless time
CESIT 2020 - International Conference on Culture Heritage, Education, Sustainable Tourism, and Innovation Technologies
608
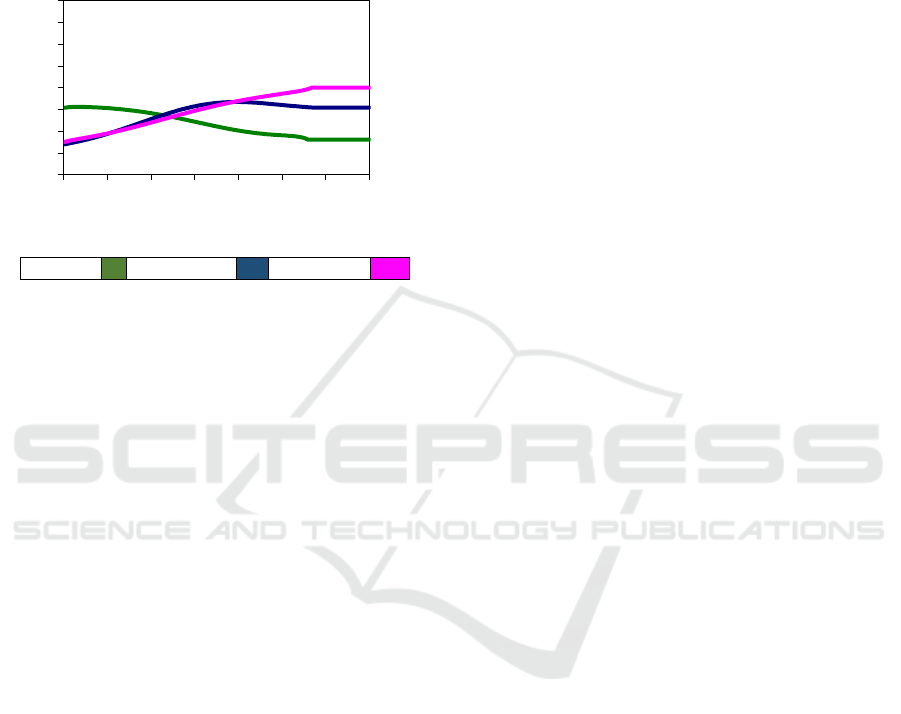
all three approaching the ideal solution. At an
activity coefficient value equal to 1.5 to 3.5, the
coefficient profile of Methanol, Ethanol and
Propanol activity coefficient indicates no ideal
solution, in which the process of separation of the
mixture of activity coefficients should be calculated
using UNIQUAC equations, as shown in Figure 4.
Metanol Ethanol Pro
p
anol
Figure 4: Profile activity coefficient of MEP versus
dimensionless time
4 CONCLUSIONS
ABE temperature profile against dimensionless time
shows the profile corresponding to the boiling point
of each component, the temperature of range from
50 to 118 (
O
C). ABE activity coefficient profile
against dimensionless time (1.5 to 3.5) indicates a
value not equal to 1, ABE is not an ideal solution,
where in the process of separating ABE ternary
system, the activity coefficient should be calculated
using UNIQUAC equation. Verify the coefficient of
ABE activities with the MEP activity coefficient
against dimensionless time (1.5 to 3.5), indicating
the dimensionless time value is not equal to 1, so
that in the process of splitting the MEP ternary
system, the activity coefficient must be calculated
using UNIQUAC equations
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial
support of the Ministry of National Education of the
Republic of Indonesia with the Research-based
Competence Grant, Contract Number:
SPP/8/UN.63.8/LIT/III/2018.
REFERENCES
Aristarkhus A., Abdi I. N., 2011. Simulasi Rancangan
Rumah Asitektur Bali Berbasis Pemrograman
Berorientasi Objek, Skripsi Politeknik Negeri Bali,
Denpasar, Bali, Indonesia.
Handogo, R., and Wibawa G, 1997. Experiments and
Correlations of Vapor-Liquid Equilibria of Acetone-1-
Butanol-Ethanol Ternary Mixture, International
Conference on Fluid and Thermal Energy Conversion,
Yogyakarta, Indonesia.
Henley, E. J. and Seader J. D., 1998. Separation Process
Principles, pp. 586-712, John Wiley & Sons, Inc.,
New York.
Rayleigh, L., 1902. Phil. Mag. [Vi.], No. 4 (23), p. 521.
Sari N. K., Kuswandi, Nonot S., and Renanto Handogo,
2006. Komparasi Peta Kurva Residu Sistem Terner
ABE Dengan Metanol-Etanol-1-Propanol, Jurnal
REAKTOR, Jurusan Teknik Kimia UNDIP
Semarang, Vol. 13, No. 2.
Sari N. K., Kuswandi, Nonot S., and Renanto Handogo,
2007. Pemisahan Sistem Biner Etanol-Air Dan Sistem
Terner ABE Dengan Distilasi Batch Sederhana, Jurnal
INDUSTRI Jurnal Ilmiah Sains dan Teknologi,
Fakultas Teknik Industri ITS Surabaya, Vol. 6, No.5.
Prausnitz, J. M., 2001. The Properties of Gases and
liquids, ed. 5, Mc. Graw-Hill, New York.
Widagdo, S. and Warren D. Seider, 1996. Journal Review
Azeotropic Distillation, AIChE J., Vol. 42, No.1
Sari N. K., Aristarkhus A, and Abdi I. N., 2013. 20th
Regional Symposium on Chemical Engineering (RSCE
2013), Alona Kew White Beach Resort Panglao
Island, Bohol, Philippines 12–13 November 2013.
http://www.dlsu.edu.ph/conferences/rsce/registration_f
ee.asp
Sari N. K., and Ernawati D., 2018. Simulation of Liquid
Vapor Equilibrium in Batch Distillation Process from
Cellulose (Bamboo), Nusantara Science and
Technology Proceedings, 111-117
Sari N. K., and Ernawati D, 2017. Simulation of Activity
Coefficient System Ternary in Acetone Buthanol
Ethanol with Uniquac Equation, conference on
Information Technology and Busines
0,7
0,8
0,9
1,0
1,1
1,2
1,3
1,4
1,5
0,0 0,5 1,0 1,5 2,0 2,5 3,0 3,5
Koefisien Aktifitas
Dimensionless time
Comparison of Activity Coefficient in ABE with MEP with UNIQUAC Equation
609
