
Antiproliferative and Apoptotic Induction of n-Hexane Fraction of
Picria fel-terrae lour. Herbs on T47D Cell Line
Denny Satria
1,4*
, Jansen Silalahi
2
, Ginda Haro
2
, Syafruddin Ilyas
3
1
Department of Pharmaceutical Biology,
2
Department of Pharmaceutical Chemistry,
3
Department of Biology
1,2,
Faculty of Pharmacy,
3
FMIPA, University of Sumatera Utara
4
Faculty of Health Sciences and Pharmacy, Universitas Sari Mutiara Indonesia
Keywords: Antiproliferative, Apoptotic, Picria fel-terrae Lour., herbs, n-hexane
Abstract: A recent research reported that breast cancer is leading to the estimated new cancer cases, and the second
most incidence death cause of women affictioning from cancer. This research aim is to evaluate cytotoxic,
antiproliferative and apoptotic induction activities of n-hexane fraction (nHF) of Picria fel-terrae Lour.
herbs. Cytotoxic activity of nHF was determined with MTT method, cell cycle and apoptotic analysis were
determined with flow cytometry method towards T47D cell line. Cytotoxic activity from nHF with MTT
assay measured as IC
50
was 75.87 ± 0.75 µg/mL, nHF at 15 µg/mL caused accumulation in G
2
-M
(37.47%)
and S phase accumulation (19.41%) and increased early (24.25%) and late apoptosis (4.26%). The results
reveal that nHF of Picria fel-terrae Lour. herbs have antiproliferative and apoptotic induction activities. Our
further study is to isolation anticancer compounds from Picria fel-terrae Lour. herbs.
1 INTRODUCTION
Breast cancer take place when breast cells start to
grow with uncontrollably. Cells could invade nearby
tissues and spread pass through the body. Each kind
of tissue in the breast can form a cancer, but the
cancer generally arises in the milk ducts or glands.
Factors which influence the risk of breast cancer are
reproductive factors (e.g. no children and first
pregnancy at an advanced age), the length of
exposure to hormones, dietary factors and lack of
physical activity, radiation during breast
development, hormone replacement therapy, as well
as congenital genetic factors (Barnett, et. al., 2008).
WHO reported that breast cancer is one of the main
cause of death and the most common incidence of
cancer type amongst women worldwide in 2012
(WHO, 2015).
Poguntano (Picria fel-terrae Lour.) have
been used for treat of colic, diuretic, fever, malaria,
and skin disease (Perry, 1980). The modern
pharmacological assessment indicated that the Picria
fel-terrae Lour. exerts antidiabetic, antioxidant, anti-
inflammatory, anthelmintic, diuretic, antipyretic,
hepatoprotective, cardioprotective, and analgesic
activities (Sitorus, et al., 2014; Dalimunthe, et al.,
2015; Sihotang, et al., 2016;Huang, et al., 1994;
Thuan, et al., 2007; Zhong, et al., 1979; Zou, et al.,
2005; Harfina, et al., 2012; Patilaya and Husori,
2015). Moreover, Picria fel-terrae inhibits hepatitis
B (HB) e-antigen excreted by HepG2 2215 cell
lines, suggesting to have anti-HB virus activity
(Zheng, et al., 2010). It can be developed as co-
chemotherapeutic regimen and inhibit metastasis for
breast cancer by inducing apoptosis, cell cycle
arrest, suppressing cyclin D1 and Bcl-2 expression,
suppressing expression of COX-2 and VEGFR2
based on the recent studies (Satria, et al., 2015;
Lestari, et al., 2013, Harahap, et al., 2018). The aim
of this study was to assess the antiproliferative and
apoptosis induction activities of n-hexane fraction of
Picria fel-terrae Lour. Herbs.
2 MATERIALS AND METHODS
2.1 Plant and Chemicals Material
Fresh herbs of Picria fel-terrae Lour. was collected
from Tiga Lingga village, Dairi regency, Sumatera
Utara province, Indonesia. Picria fel-terrae Lour.
was identified in Research Centre for Biology,
Indonesian Institute of Science, Bogor, and the
voucher specimen was deposited in
190
Satria, D., Silalahi, J., Haro, G. and Ilyas, S.
Antiproliferative and Apoptotic Induction of n-Hexane Fraction of Picria fel-terrae lour. Herbs on T47D Cell Line.
DOI: 10.5220/0008359701900193
In Proceedings of BROMO Conference (BROMO 2018), pages 190-193
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

herbarium. Chemicals used were annexin-V
(BioLegend), distilled water, DMSO (Sigma), [3-
(4,5-dimethylthiazole-2-yl)-2,5-diphenyl tetrazolium
bromide] (MTT) (Sigma), propidium iodide kit
(BioLegend).
2.2 Preparation of n-Hexane Fraction
(nHF)
The air-dried and powdered herbs of Picria fel-
terrae Lour. (1 kg) were repeatedly fractionated by
cold maceration with n-hexane (3x3 d, 7.5 L) at
room temperature with occasional stirring. The
filtrate was collected, and then evaporated under
reduced pressure to give a viscous fraction and then
freeze dried to dry (Satria, et al., 2015; Anggraeni, et
al., 2015; Hasibuan, et al., 2015).
2.3 Cytotoxicity Assay
The cells were treated with nHF. In this test, T47D
cell line was grown in RPMI 1640 medium, medium
containing 10% Fetal Bovine Serum (Gibco), 1%
penicillin-streptomycine (Gibco), and fungizone
0.5% (Gibco) in a flask in a humidified atmosphere
(5% CO
2
) at 37
o
C. The inoculums seeded at
1x10
4
cells/mL at an optimal volume of 0.1 mL per
well. After 24 h incubation, the medium was
discharged and treated by nHF. After incubation 24
h, the cells were incubated with 0.5 mg/mL MTT for
4 h in 37
o
C. Viable cells reacted with MTT to
produce purple formazan crystals. After 4 h, SDS
10% as stopper (Sigma) in 0.01N HCl (Merck) was
added to dissolve the formazan crystals. The cells
were incubated for 24 h in room temperature and
protected from light. After incubation, the cells were
shaken, and absorbance was measured using
microplate reader at λ 595 nm. The data which were
absorbed from each well were converted to
percentage of viable cells
(Hasibuan, et al., 2015;
Satria, et al., 2014).
2.4 Preparation of Cells for
Flowcytometry Analysis
T47D cells (5x10
5
cells/well) were seeded into 6-
well plate and incubated for 24 h. After that, the
cells were treated with nHF and then incubated for
24 h. Both floating and adherent cells were collected
in conical tube using tripsin 0.025%. The cells were
washed thrice with cold PBS and centrifuged 2500
rpm for 5 min. The supernatant was separated, while
the sediment was collected (Satria, et al., 2015;
Anggraeni, et al., 2015).
2.5 Cell Cycle Analysis
Cells were fixed in cold 70% ethanol in PBS at -
20
o
C for 2 h. The cells were washed thrice with cold
PBS and resuspended then centrifuged at 3000 rpm
for 3 min and PI kit (containing PI 40 µg/mL and
RNAse 100 µg/mL) added to sediment and
resuspended and incubated at 37
o
C for 30 min. The
samples were analyzed using FACScan flow
cytometer. Based on DNA content, the percentage of
cells in each of stage in cell cycle (G1, S and G2/M)
were calculated using ModFit Lt. 3.0.s.
2.6 Apoptosis Analysis
Annexin V kit was added to sediment and suspended
and incubated at 37
o
C for 30 min. The samples were
analyzed using FACScan flow cytometer (Harahap,
et al., 2018).
2.7 Statistical Analysis
Data were expressed as mean ± SD with descriptive
analysis. All statistics were analyzed using the SPSS
21 software.
3 RESULTS AND DISCUSSION
3.1 Inhibitory Concentration 50% (IC
50
)
MTT method was used to determine cell viability
after incubation for 24 h. In every treatment nHF
was shown to inhibit cells growth. The IC
50
value of
nHF was 75.87 ± 0.75 µg/mL.The natural product is
suspected to have cytotoxic properties based on their
active compound in Picria fel-terrae Lour.
Triterpenoids/steroids are suspected to be the main
active compound (Yadav, et al., 2012). Triterpenoids
are also considered as one of promising anticancer
drugs (Petronelli, et al., 2009)
3.2 Effect on Cell Cycle and Apoptosis
To assess the activity of nHF to increase cell death
by increasing cell cycle, we concentrated on it for
further studies using flow cytometry method. The
effect of nHF is given in Figure 1. Whereas
treatment of nHF in 15 µg/mL caused cell
accumulation at G
2
-M phase (37.47%) and for
control cell (30.11%). At S phase the accumulation
Antiproliferative and Apoptotic Induction of n-Hexane Fraction of Picria fel-terrae lour. Herbs on T47D Cell Line
191
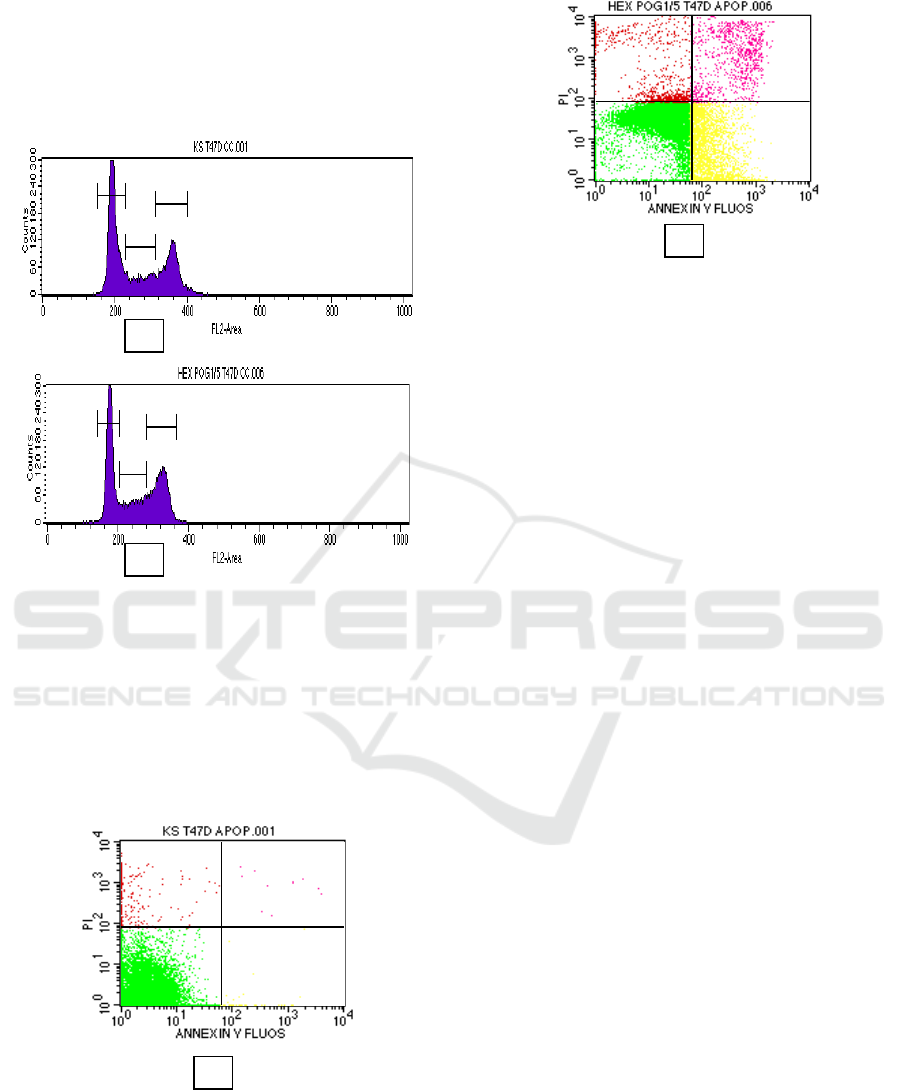
after nHF treatment (19.41%) and for control cell
(16.80%). This fact was to indicate that nHF can
inhibit cell grow that G
2
-M
and S phase. In the cell
cycle analysis, nHF was exhibited higher G
2
-M
and S phase accumulation compared to control cells
(Harahap, et al., 2018; Satria, 2015).
Figure 1. Cell cycle analysis using flowcytometry.
T47D cells were treated by nHF for 24h and stained
using propidium iodide. (a) control cells; (b) nHF 15
µg/mL. nHF exhibited G
2
-M
and S phase.
Evaluation of apoptosis induction was
performed using flowcytometry method with
Annexin-V. as shown in Figure 2.
Figure 2. Apoptosis analysis using flowcytometry.
T47D cells were treated by nHF for 24h and stained
using Annexin-V. (a) control cells; (b) nHF 15
µg/mL.
As shown in Figure 2, the cells in the upper
and lower right quadrants represent late apoptotic/
necrotic and early apoptotic cells, respectively. The
percentage of control and nHF in early apoptotic
0.18% and 24.25%, in late apoptotic/early necrotic
0.06% and 4.26%. In apoptotic study, nHF increased
the cells go through apoptosis in early apoptosis and
late apoptosis if compared to control T47D cell
lines. Apoptosis is a mechanism of programmed cell
death with alterations on morphology, membrane
blebbing and chromatin (Ruddin,et al., 1997).
4 CONCLUSIONS
The results suggest that n-hexane fraction of Picria
fel-terrae Lour. herbs may exhibit an anticancer
activity towards T47D cell lines through cell cycle
inhibition and induction apoptosis.
ACKNOWLEDGEMENTS
We gratefully thank to DRPM Ministry of Research
Technology and High Education, Indonesia through
“Hibah Disertasi Doktor” Research Grant 2018 for
financial support in the study.
Marker
Events
% Gated
% Total
Mean
CV
Median
All
16415
100.00
82.08
259.35
28.41
223.00
GO-G1
8581
52.28
42.91
196.41
6.51
194.00
S-phase
2757
16.80
13.79
273.57
9.08
274.00
G2-M
4942
30.11
24.71
354.60
5.45
356.00
File: KS T47D CC.001
Total Ev ents: 20000
X Parameter: FL2-A FL2-Area (Linear)
Marker
Events
% Gated
% Total
Mean
CV
Median
All
20000
100.00
100.00
295.38
55.50
249.00
M1
845
4.23
4.23
15.29
195.18
0.00
GO-G1
8607
43.04
43.04
196.42
6.53
194.00
S-phase
2800
14.00
14.00
273.64
9.09
274.00
G2-M
5365
26.82
26.82
355.52
5.54
357.00
M5
2478
12.39
12.39
628.52
29.57
570.00
File: KS T47D CC.001
Total Ev ents: 20000
X Parameter: FL2-A FL2-Area (Linear)
M1
GO-G1
S-phase
G2-M
M5
GO-G1
S-phase
G2-M
R1
Marker
Events
% Gated
% Total
Mean
CV
Median
All
15205
100.00
76.02
244.92
26.71
235.00
GO-G1
6584
43.30
32.92
179.52
5.29
179.00
S-phase
2951
19.41
14.75
245.74
8.98
247.00
G2-M
5697
37.47
28.48
319.67
6.09
321.00
File: HEX POG1/5 T47D CC.006
Total Ev ents: 20000
X Parameter: FL2-A FL2-Area (Linear)
R1
Marker
Events
% Gated
% Total
Mean
CV
Median
All
20000
100.00
100.00
293.48
57.43
271.00
M1
987
4.93
4.93
14.43
187.63
1.00
GO-G1
6590
32.95
32.95
179.53
5.30
179.00
S-phase
2968
14.84
14.84
245.76
8.98
247.00
G2-M
6203
31.01
31.01
321.27
6.23
323.00
M5
3351
16.75
16.75
589.53
28.65
546.00
File: HEX POG1/5 T47D CC.006
Total Ev ents: 20000
X Parameter: FL2-A FL2-Area (Linear)
M1
GO-G1
S-phase
G2-M
M5
GO-G1
S-phase
G2-M
File: KS T47D APOP.001
Patient ID: 0417.18
Acquisition Date: 17-Apr-18
Gate: No Gate
Total Events: 20000
Quad Location: 64, 79
Quad
% Gated
% Total
UL
1.34
1.34
UR
0.06
0.06
LL
98.41
98.41
LR
0.18
0.18
File: KS T47D APOP.001
Patient ID: 0417.18
Acquisition Date: 17-Apr-18
Gate: No Gate
Total Events: 20000
Region
% Gated
% Total
R1
98.37
98.37
R2
0.19
0.19
R3
0.06
0.06
R4
1.38
1.38
R2
R3
R4
R1
File: HEX POG1/5 T47D APOP.006
Patient ID: 0417.18
Acquisition Date: 17-Apr-18
Gate: No Gate
Total Events: 20000
Quad Location: 64, 79
Quad
% Gated
% Total
UL
4.88
4.88
UR
4.26
4.26
LL
66.61
66.61
LR
24.25
24.25
File: HEX POG1/5 T47D APOP.006
Patient ID: 0417.18
Acquisition Date: 17-Apr-18
Gate: No Gate
Total Events: 20000
Region
% Gated
% Total
R1
64.70
64.70
R2
25.94
25.94
R3
4.44
4.44
R4
5.15
5.15
R2
R3
R4
R1
a
b
a
b
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
192

REFERENCES
Anggraeni R, Hadisahputra S, Silalahi J, Satria D. 2014.
Combinational effects of ethylacetate extract of
Zanthoxylum acanthopodium DC. with doxorubicin on
T47D breast cancer cells. Int J PharmTech Res, 6,
2032-5.
Barnett GC. 2008. Risk factors for the incidence of breast
cancer: do they affect survival from the disease. Oncol,
26, 3310-16.
Dalimunthe A, Urip H, Rosidah, Pandapotan NM 2015.
Evaluation of diuretic activity of Picria fel-terrae
(Lour.) leaves extracts. Asian J Pharm Clin Resc, 8,
204-5.
Hasibuan PAZ, Jessy C, Denny S 2015. Combination
effect of ethylacetate extracts of Plectranthus
ambonicius (Lour.) Spreng. with doxorubicin againts
T47D breast cancer cells. Int J Pharm Pharm Sci, 7,
155-9.
Harahap U, Hasibuan PAZ, Sitorus P, Arfian N, Satria D.
2018. Antimigration activity of an ethylacetate fraction
of Zanthoxylum acanthopodium DC. fruits in 4T1
breast cancer cells. Asian Pac J Cancer Prev, 19(2),
565-9.
Harfina F, Bahri S, Saragih A. 2012. Pengaruh serbuk
daun puguntano (Curanga fel-terrae Merr.) pada
pasien diabetes mellitus. J Pharm Pharmacol, 2, 112-
8.
Huang Y, Cimanga K, Lasure A, Van Poel B, Pieters L,
Berghe-Vanden D. 1994. Biological activities of
Picria fel-terrae Lour. Pharm World Sci, 16, 18.
Lestari P. 2013. Efek kombinasi ekstrak aktif daun
poguntano (Picria fel-terrae Lour.) dengan
doksorubisin terhadap sel kanker payudara secara in
vitro. Thesis: Medan. Faculty of Pharmacy. University
of Sumatera Utara.
Patilaya P, Dadang IH. 2015. Preliminary study on the
anthelmintic activity of the ethanolic extract of
Indonesian Curanga fel-terrae (Lour.) Merr. Int J
Pharmtech Res, 8, 347-51.
Perry LM. 1980. Medicinal plants of East and Southeast
Asia. London: The MIT Press. pp. 384.
Petronelli A, Pannitteri G, Testa U. 2009. Triterpenoids as
new promising anticancer drugs Antican. Drugs.
20(10):880-892.
Rudin CM, Thompson CB. 1997 Regulation and clinical
relevance of programmed cell death. Annu Rev Med,
48, 267-81.
Satria D, Pandapotan M, Illyas S. 2014. Cytotoxcicity
effect of sea horse (Hippocampus trimaculatus Leach.)
extract and fractions on MCF-7 cell line. Int J
Pharmtech Res, 6, 212-16.
Satria D, Furqan M, Hadisahputra S, Rosidah. 2015.
Combinational effects of ethylacetate extract of Picria
fel-terrae Lour. and doxorubicin on T47D breast
cancer cells. Int J Pharm Pharm Sci, 7, 73-6.
Siegel RL, Miller KD, Jemal A. 2015. Cancer statistics.
CA Cancer J Clin. 65, 5-29.
Sihotang YM, Silalahi, J, Hadisahputra H, Hasibuan PAZ,
Satria D. 2016. Cardioprotective effect of ethylacetate
extract of poguntano (Picria fel-terrae Lour.) against
doxorubicin-induced cardiotoxicity in rats. Int J
Pharm Clin Resc. 8, 466-70.
Sitorus P, Harahap U, Pandapotan M, Barus T. 2014.
Isolation of β-sitosterol from n-hexane of Picria fel-
terrae Lour. leave and study of its antidiabetic effect in
alloxan induced diabetic mice. Int J Pharmtech Res, 6,
137-41.
Thuan ND, Ha Do T, Thuong PT, Na MK, Lee JP. 2007.
A phenylpropanoid glycoside with antioxidant activity
from Picria tel-ferae. Arch Pharm Res, 30, 1062-6.
WHO. 2015. World Cancer Report 2014.
Yadav VR, Sahdeo P, Bokyung S, Ramaswamy K, Bharat
BA. 2010. Targetting inflammatory pathways by
triterpenoids for prevention and treatment of cancer.
Toxins, 2, 2428-66.
Zeng J, Pan X, Yang K, Wei Z, Chen C. 2010. Chin Med
Herald, 7, 27-29.
Zhong SQ, Zhang BN, Huang FX.1979. An anti-tumor
herb Cucao. China: China Traditionally Herb Drugs
Lett. pp. 45-6.
Zou JM, Wang LS, Niu XM, Sun HD, Guo YJ. 2005.
Phenylethanoid glycosides from Picria felterrae Lour.
J Integ Plant Bio, 47, 632-6.
Antiproliferative and Apoptotic Induction of n-Hexane Fraction of Picria fel-terrae lour. Herbs on T47D Cell Line
193
