
Antibacterial Activity of Several Indonesian Endemic Plants
against Staphylococcus epidermidis, Staphylococcus aureus and
Methicillin-resistant Staphylococcus aureus
Ariyani Kiranasari*
1
, Angela Bonita
2
, Elizabeth Melina
2
, Kevin Winston
3
, Naivedh Baht
3
,
Nathania Sutandi
3
, Beti Ernawati Dewi
1
, Ika Ningsih
1
, Fithriyah Sjatha
1
1
Department of Microbiology, Fculty of Medcicine, Universitas Indonesia,Jl. Pegangsaan Timur no. 16 Jakarta
10320,
2
Undergraduate Program, Faculty of Medicine, Universitas Indonesia,,
3
Undergraduate Program,
International Class, Faculty of Medicine, Universitas Indonesia
Keywords: Antibacteria, plant extract, MRSA, Staphylococcus epidermidis
Abstract: In this current era, infectious diseases worldwide is increasing and due to misuse of antibiotics for treating
infections eventually leads to the emergence of antibiotic resistant microbes. As a country with abundance
of natural resources, Indonesia must be the forefront on research in finding new antibacterial candidates
resourced from endemic medicinal plants. The objective of this research is to assesed the activities of
several Indonesian endemic plants extract to inhibit several bacteria in-vitro by microdilutin method to
obtain Minimal Inhibitory Concentration (MIC) and Minimal Bactericidal Concentration (MBC) and agar
diffusion method to obtain inhibition zone. In this study, extract of Syzigium aromaticum, Piper betle and
Aleurites moluccana were show anti bacterial activity against MRSA (Methicillin-resistant Staphylococcus
aureus), Staphylococcus aureus and Staphylococcus epidermidis. On the other hand, extract of Curucuma
longa and Samanea saman didnt show any anti bacterial properties. This study show the potency of several
endemic plants extract to inhibit Staphylococcal bacteria.
.
1 INTRODUCTION
Antimicrobial agents in today medicine play a very
important role in treating infectious disease that was
once fatal and incurable (Katzung B et al, 2012) In
this modern age, there is a rising concern of
antimicrobial resistance due to extensive and
unregulated antimicrobial used in clinical setting by
medical professionals (Fraise A P, 2002; Nikaido H,
2014). Consequently, more and more antimicrobial
resistance is being reported and in addition, it is
discovered that the highest rate of antimicrobial
resistance is located in developing world (Nikaido
H. 2014). Nevertheless, the demand of new novel
effective anti-microbial to combat pathogenic
microorganisms in clinical setting has increased
(WHO, 2002).
Staphylococcus epidermidis is a gram-positive
bacteria belonging to the coagulase-negative
staphylococci group. This bacteria is a normal flora
of human skin but also the most common cause of
infection in the use of medical devices (WHO,
2002). Increased resistance of S. epidermidis to
various antibiotics led to the treatment of
nosocomial infections more difficult (Yuwono H,
2010). Based on a study conducted by Najar-
Peerayeh et al, 2014, 92.2% of the 64 isolates of
S.epidermidis have mecA genes that play a role in
penicillin-binding expression protein (PBP2a) that
decreases the affinity of the beta-lactam antibiotic.
In addition, the ability of S. epidermidis to form
biofilms makes this bacteria able to avoid the
immune system and antibacterial drugs (Solati SM
et al, 2015; Abidi et al, 2015).
Staphylococcus aureus is a bacteria that can cause
various diseases because of the toxin it produces or
direct invasion that damages the tissue (Murray PR
et al, 2013). In early 1940, the infection caused by S.
aureus was successfully treated with penicillin, a
beta-lactam antimicrobial group, which rapidly
replaced by the new resistant strain encodes a
betalactamase enzyme. This new strain is actually
can be resolved through the administration of new
antimicrobial methicillin (Yuwono H, 2010) but in
the early of 1980s, methicillin resistant S. aureus
178
Kiranasari, A., Bonita, A., Melina, E., Winston, K., Baht, N., Sutandi, N., Dewi, B., Ningsih, I. and Sjatha, F.
Antibacterial Activity of Several Indonesian Endemic Plants against Staphylococcus epidermidis, Staphylococcus aureus and Methicillin-resistant Staphylococcus aureus.
DOI: 10.5220/0008359501780182
In Proceedings of BROMO Conference (BROMO 2018), pages 178-182
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

(MRSA) strains spread rapidly and alter the
therapies available for S. aureus infection. In 2003, a
new strain of MRSA caused outbreak of cutaneous
infection and pneumonia.
Compared to other resistant bacteria, MRSA
infection is epidemiologically significant. Studies
conducted by CDC more than half Staphylococci
bacteria ehich caused Hospital Acquired Infections
are resistant to oxacillin (De Angelis G et al, 2010)
and its infection nowadays is an endemic in US
hospitals and communities (Klein E et al, 2007;
Crum NFet al, 2006). Furthermore, MRSA in Cipto
Mangukusumo Hospital, Indonesia also show an
increase from 28.5% in 2009 to 32% in 2010 (Liana
P., 2014).
Several research to find a novel anti Staphyococcal
bacteria from plants is also progressing. Pradhan D
et al, 2013 and Dwivedi Vet al, 2014 reported Piper
betle leaves and leaves extract have antimicrobial,
anti-inflammatory, antioxidant and antiseptic
properties. Specifically, P. betle shown to have
antibacterial activity against S. aureus,
Streptococcus pyogenes, E. coli, Pseudomonas
aeruginosa, Enterococcus fecalis, Klebsiella
pneumoniae, and others. The content of sterols in
betel leaf extract interact with bacterial cell wall,
disturbing its permeability.
Other plant that also potential to be an antibacterial
are bark of candlenut (Aleurites moluccana) which is
used traditionally for the treatment of diarrhea and
thypoid fever (Alimboyoguen AB, et al 2014).
Research shows 3acetyl aleuritolic acid from bark
extract has an antimicrobial activity. Moluccanin
from A. moluccana also has antibacterial including
S. aureus and antiviral activity (Othman AS and
Rasyidah MR, 2010).
Albizia saman (Jacq.) Merr. formerly known as
Samanea saman is having several phytochemical
components which are flavonoids, alkaloids, tannins,
carbohydrates, glycosides, saponins, steroids, and
reducing sugar are widely used as the remedy for
colds, diarrhea, headache, and stomach ache.
According to Perry in 1980, the alcoholic extract of
S. saman is also proven to inhibit the growth of
Mycobacterium tuberculosis (Kirithika T.2013).
The clove plant (Syzygium aromaticum) contain
chemical compounds that provide its aromatic and
antibacterial nature. The active compound being
studied is eugenol, one of many phenolic
compounds. Eugenol has been widely used in dental
care settings, and has been proven as an effective
anesthetic and antiseptic (Cortés-Rojas Det al, 2016;
Neveu Vet al, 2010).
With its promising properties as antibacterial and its
abundancy worldwide, we assessed several
concentration of P. betle leaves extract, A.
moluccana stem bark extract, S. saman extract, C.
longa extract and S. aromaticum flower bud extract
against S. epidermidis, S. aureus and MRSA. As
our result show that several extracts have a good
potency as anti-staphylococcal infection.
2 MATERIAL AND METHODS
2.1 Bacteria, Medium and Extract
S. epidermidis, S. aureus and MRSA bacteria were
grown in nutrient agar. All bacteria were from
Microbiology Department culture collection, Faculty
of Medicine Universitas Indonesia, which identified
using commercial Vitex identification kit and tested
for its resistancy according to CLSI. Broth Brain
Heart Infusion (BHI) medium and Muller Hinton
Agar (MHA) for antibacterial testing and Plate
Count Agar (PCA) were provided by Department of
Microbiology, Faculty of Medicine, Universitas
Indonesia. Extracts of P. betle leaves, A. moluccana
stem bark, S. saman, C.longa and S. aromaticum
flower bud in ethanol were prepared by Medical
Pharmacy Department, Faculty of Medicine,
Universitas Indonesia. Antibiotic ciprofloxacin or
clindamycin was used for positive control.
2.2 Antibacterial Assay
2.2.1 Agar Diffusion Method
An overnight bacteria culture was diluted into 0.9%
NaCl to reach McFarland value of 0.5. Bacterial
suspension was then applied into MHA followed by
creating 7 diffusion wells in the media using blue
tips. Each extract at several concentrations was
applied in to the well which are : P.betle extract at
62,5; 125; 250; 500 and 1000 mg/ml was tested
against S. epidermidis and A.moluccana extract at
50; 100; 200; 400 and 800 µg/ml against MRSA.
With addition for aquadest and antibiotics at 20
µg/ml as negative and positive control respectively.
Plate was then incubated for 16 – 18 hours at 37
o
C.
Observed inhibitory zone was measured using
calipers.
Antibacterial Activity of Several Indonesian Endemic Plants against Staphylococcus epidermidis, Staphylococcus aureus and
Methicillin-resistant Staphylococcus aureus
179

2.2.2 Dilution Method
An overnight bacteria culture were diluted into BHI
media followed by addition of final concentration of
S. saman and C. longa extract at 12.5%, 6,25%,
3,125%, 1.563%, 0.782% and 0.391% meanwhile
for S. aromaticum extract at 0.0488%, 0.0977%,
0.19530%, 0.3906%, 0.7813%, 1.563%, and
3.125% all against MRSA and non-MRSA.
Aquadest and antibiotic at 20 µg/ml were used as
negative and positive control respectively. Culture
were then incubated for 18 – 24 hours at 37
o
C.
MIC value defiened as the smallest extract
concentration that inhibits bacteria growth in BHI
media. Two cultures at higher and lower
concentration of MIC value were smeared at PCA
followed by incubation for 18 – 24 hours at 37
o
C to
obtain number of bacterial colony from the tested
concentration. Lowest extract concentration giving
smaller amount of 30 CFU/ml (colony forming unit)
bacteria defined as MBC value.
3 RESULT AND DISCUSSION
Several plants extract tested in this study are briefly
conclude in table 1. Extract of C. longa, A.
moluccana and S. saman didn’t show any
antibacterial activity against S. aureus and MRSA
by dilution method. Our result is contrary with
several studies which show anti bacterial inhibition
of S. saman and C. longa against several bacteria
including S. aureus and MRSA due to lower extract
concentration that we use in this study (Rita et al,
2013, Thippeswamy et al, 2011, Prasad et al, 2008,
Bengmark et al, 2009 and Moghadamtousi et al,
2014, Othman et al, 2009). Obasi et al, 2011, found
that tannin is one of phytochemical compund found
in S. saman that can inhibit the growth of
microorganism by precipitating the microbial protein
needed for their growth, resulting in protein
deprivation of the microorganism. Experiments
performed by Ibrahim A, 2011 showed A.
moluccana bark extract produced an antimicrobial
effect against Salmonella thyphii and Vibrio
cholerae but not tested against MRSA or other
positive gram cocci bacteria.
Our research show the potency of P. betle leaf
extract formed the inhibitory zone of S. epidermidis
on MHA for all tested concentrations as listed in
table 2. The results were greater than the positive
control of ciprofloxacin which resulted in an average
inhibitory zone diameter of 31.70 ± 0.94. Based on
the Pearson correlation test, it was found that the
concentration of P. betle leaf extract correlated with
moderate (r = 0.642) to the large increase in
inhibition zone diameter of S. epidermidis bacteria
(Tumbelaka AR et al, 2011). Chakraborty, et al,
2011 tested metalloic leaf extract of P. betle against
S. aureus and proved an increase in antibacterial
activity assessed by measurement of inhibitory zone
diameters along with increased concentration of
extract (5 mg/ml, 10 mg/ml, 25 mg/ml, 50 mg/ml,
100 mg/ml).
Our result for S. aromaticum flower bud extract
show anti S. aureus and MRSA with similar MIC
and MBC value at 0.7813% and 0.3906%
respectively. Interestingly, our result show inhibition
and bactericidal value of S. aromaticum extract
against MRSA is lower than S. aureus which may
need further research. We hypothesize the effect of
mecA and change in the cell wall structure of MRSA
increased sensitivity towards eugenol, confirmed
phenolic compund of S. aromaticum (Cortés-Rojas
Det al, 2016; Neveu Vet al, 2010).
A research on the effect of Indian spices on food
borne pathogens showed that an aqueous extract of
S. aromaticum has inhibitory activity against S
aureus at 1% concentration, and complete
bactericidal activity at 3% concentration (Sofia P et
al, 2007). Another research compared aqueous clove
extracts from Sri Lanka and Zanzibar, and its results
suggested that the end-point of antimicrobial activity
against S aureus is at 6.25% (Nzeako BCet al,
2006). These results suggest that multiple external
factors influence the content and potency of the
herb. These factors also influence the inhibitory and
bactericidal effects. In this research, S aureus is
inhibited at 0.7813%, which is much lower to other
results.
4 CONCLUSION
Our study shows that extract from Piper betle and
Syzygium aromaticum were found to have a good
anti Staphylococcal activity which can be further
analyed for purification and bacterial inhibition
mechanism.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
180
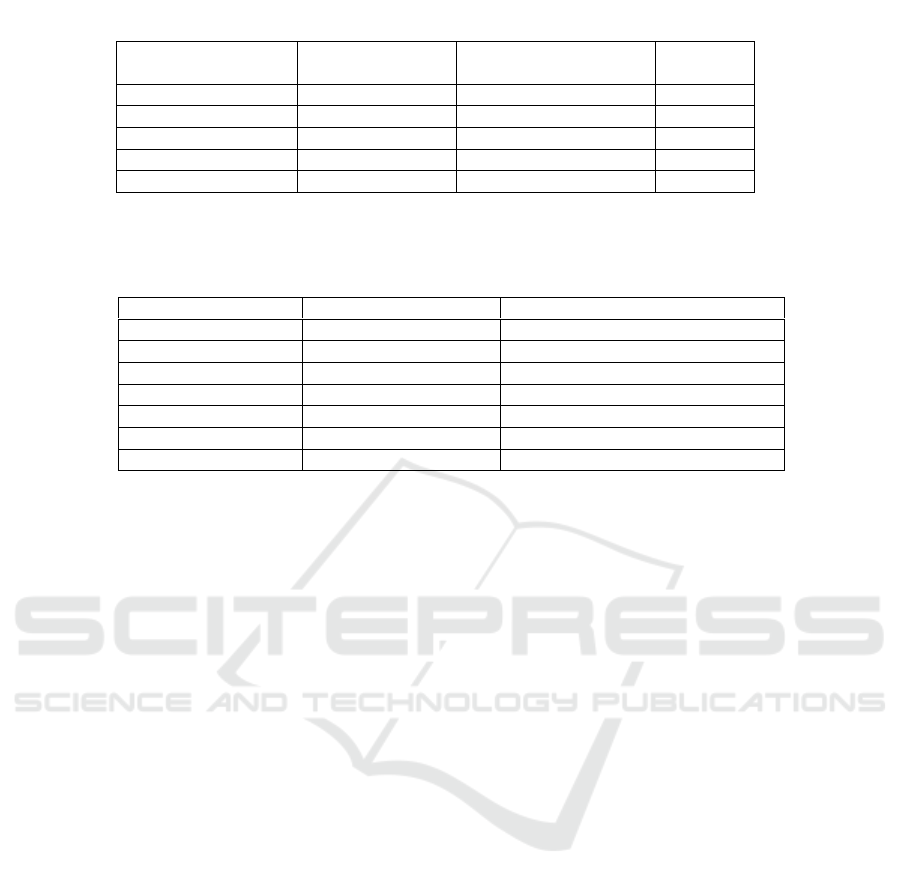
Table 1. Plant extract, antibacterial method and result
Plant extract
Antibacterial
method
Bacterial tested
Inhibition
Curcuma longa
Dilution
S. aureus, MRSA
-
Aleurites moluccana
Diffusion
MRSA
-
Samanea saman
Dilution
S. aureus, MRSA
-
Piper betle
Diffusion
S. epidermidis
+
Syzigium aromaticum
Dilution
S. aureus, MRSA
+
Table 2. Inhibition zone of ciprofloxacin and P.betle leaf extract against S. epidermidis
Treatment
Concentration (mg/ml)
Average inhibition zone (nm) ± SD
Aquadest
-
0
Ciprofloxacin
5
31,70 ± 0,94
P. betle extract
1000
32,38 ± 2,63
P. betle extract
500
30,05 ± 1,38
P. betle extract
250
27,85 ± 1,91
P. betle extract
125
23,80 ± 2,1
P. betle extract
62,5
23,78 ± 0,47
ACKNOWLEDGEMENTS
Publication of this was study supported by Hibah
PITTA UI 2018/2019.
REFERENCES
Abidi SH, Ahmed K, Sherwani SK, Kazmi SU. Synergy
between antibiotics and natural agents results in
increased antimicrobial activity against
Staphylococcus epidermidis. J Infect Dev Ctries. 2015;
9(9): 925-29. doi:10.3855/jidc.5164Katzung B,
Masters S, Trevor A. Basic & Clinical Pharmacology.
12th Ed. New York: Mcgraw-Hill Medical; 2012. 789
P.
Alimboyoguen AB, Castro-Cruz KA, Shen CC, Li WT,
Ragasa CY. Chemical constituents of the bark of
aleurites moluccana L. willd. J. Chem Pharm Res.
2014; 6(5): 1318-1320Fraise A P. Biocide Abuse And
Antimicrobial Resistance--A Cause For Concern? J
Antimicrob Chemother. 2002;49(1):11–2. Available
From:
Http://Jac.Oxfordjournals.Org/Content/49/1/11.Long.
Bengmark S, Mesa M. D, Gil A. Plant-derived health: the
effects of turmeric and curcuminoids. Nutr Hosp. Aula
Médica Ediciones (Grupo Aula Médica S.L.); ;
24(3):273–81.
Chakraborty D, Shah B. Antimicrobial, antioxidative, and
anti-hemolytic activity of Piper betel leaf extracts. Int J
Pharm Pharm Sci. 2011; 3(3): 19299Nikaido H.
Multidrug Resistance In Bacteria. Annu Rev
Biochem;78:119–46.
Cortés-Rojas D, de Souza C, Oliveira W. Clove
(Syzygium aromaticum): a precious spice. Asian
Pacific Journal of Tropical Biomedicine. 2014.
Crum NF, Lee RU, Thornton SA, Stine OC, Wallace MR,
Barrozo C, et al. Fifteen-year study of the changing
epidemiology of methicillin-resistant Staphylococcus
aureus. Am J Med. 2006 Nov;119(11):943–51.
De Angelis G, Murthy A, Beyersmann J, Harbarth S.
Estimating the impact of healthcare-associated
infections on length of stay and costs. Clinical
Microbiology and Infection. 2010;16(12):1729-1735.
Dwivedi V, Tripathi S. Review study on potential activity
of Piper betle. Journal of Pharmacognosy and
Phytochemistry. 2014; 3(4): 93-98.
Ibrahim A. Aktivitas antibakteri tumbuhan prinjak
(aleurites moluccana (L.)) terhadap bakteri salmonella
thyposa dan vibrio cholera. J Trop Pharm Chem. 2011;
1(3): 198.
Klein E, Smith D, Laxminarayan R. hospitalizations and
deaths caused by methicillin-resistant staphylococcus
aureus, United States, 1999–2005. CDC. 2007.
Liana P. Gambaran kuman methicillin-resistant
staphylococcus aureus (MRSA) di laboratorium
mikrobiologi departemen patologi klinik rumah sakit
dr. cipto mangunkusumo (RSCM) periode januari-
desember 2010. J Kedokt dan Kesehat. 2014;(3):171–
5.
Kirithika T. Preliminary phytochemical screening and
antioxidant property of various extracts of Albizia
saman leaves. IJPRBS. 2013;2(1):315-323.
Moghadamtousi SZ, Kadir HA, Hassandarvish P, Tajik H,
Abubakar S, Zandi K. A review on antibacterial,
antiviral, and antifungal activity of curcumin. Biomed
Res Int. 2014 Jan;2014:186864.
Antibacterial Activity of Several Indonesian Endemic Plants against Staphylococcus epidermidis, Staphylococcus aureus and
Methicillin-resistant Staphylococcus aureus
181

Murray PR, Rosenthal KS, Pfaller MA. Medical
microbiology. 7th ed. Philadelphia: Elsevier Saunders;
2013. P. 174-184.
Najar-Peerayeh S, Moghadas AJ, Behmanesh M.
Antibiotic susceptibility and mecA frequency in
Staphylococcus epidermidis, isolated from intensive
care unit patients. Jundishapur J Microbiol. 2014 Aug;
7(8). doi:10.5812/jjm.11188.
Naveen P, et al. Preliminary phytochemical screening and
antimicrobial activity of Samanea saman. JMPR. 2008
: 2(10); 268-270. Available from :
http://www.academicjournals.org/article/article138052
6442.
Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut
L, Mennen L et al. Phenol-Explorer: an online
comprehensive database on polyphenol contents in
foods. Database. 2010;2010(0):bap024-bap024.
Nzeako BC, Al-Kharousi ZSN, Al-Mahrooqui Z.
Antimicrobial Activities of Clove and Thyme Extracts.
Sultan Qaboos University Medical Journal.
2006;6(1):33-39.
Obasi NL, et al. Comparative phytochemical and
antimicrobial screening of some solvent extracts of
Samanea saman (febaceae or mimosaceae) pods.
AJPAC. 2010 ; 4 (9);206-212.
Othman AS, Rasyidah MR. Antibacterial activity of
aleurites moluccana against some clinical isolates.
Research Journal of Biotechnology. 2010; 5(3): 25.
Patel JB, Cockerill FR, Bradford PA, Eliopoulos GM,
Hindler JA, Jenkins SG et al. Performance standards
for antimicrobial susceptibility testing; twenty-fifth
informational supplement. Pennsylvania: Clinical and
Laboratory Standards Institute; 2015. P. 70.
Pradhan D, Suri KA, Pradhan DK, Biswasroy P. Golden
heart of nature Piper betle L. Journal of
Pharmacognosy and Phytovhemistry. 2013; 1(6): 147-
67.
Rupp ME, Fey PD. Rupp ME, Fey PD. Staphylococcus
epidermidis and other coagulase-negative
staphylococci. In: Bennett JE, Dolin R, Blaser MJ,
editors. Mandell, Douglas, and Bennett’s principles
and practice of infectious diseases. 8th ed.
Philadelphia: Elsevier Saunders; 2015.
Sofia P, Prasad R, Vijay V, Srivastava A. Evaluation of
antibacterial activity of Indian spices against common
foodborne pathogens. International Journal of Food
Science & Technology. 2007;42(8):910-915.
Solati SM, Tajbakhsh E, Khamesipour F, Gugnani HC.
Prevalence of virulence genes of biofilm producing
strains of Staphylococcus epidermidis isolated from
clinical samples in Iran. AMB Expr. 2015; 5(47).
doi:10.1186/s13568-015-0134-3.
Thippeswamy S, et al. Antimicrobial evaluation and
phytochemical analysis of a known medicinal plant
Samanea saman (Jacq.) Merr. Against some human
and plant pathogenic bacteria and fungi. Int J Pharm
Pharm Sci. 2011 ; 2 (2);443-452.
Tumbelaka AR, Riono P, Sastroasmoro S, Wirjodiarjo M,
Pudjiastuti P, Firman K. Pemilihan uji hipotesis. In:
Sastroasmoro S, Ismael S, editors Dasar-dasar
metodologi penelitian klinis. 4th ed. Jakarta: CV
Sagung Seto; 2011.
World Health Organization. Preventions of hospital-
acquired infections: a practical guide. 2nd ed. 2002
Yuwono H. Pandemi resistensi antimikroba: belajar dari
MRSA. JKK. 2010; 42(1): 2837-2839.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
182
