
The Effectivity of Butanol Fraction of Calophyllum nodosum as
Antiviral Drug to Dengue Virus Serotype 2 In Vitro
Syifa Salsabila
1
, Nabilla Calista
1
, Hidayati Desti
2,3
, Beti Ernawati Dewi
2,3
1
Undergraduate Student, Medical Faculty Universitas Indonesia
2
Department of Microbiology, Medical Faculty Universitas Indonesia-Cipto Mangunkusumo Hospital Jalan Pegangsaan
Timur no 16, Jakarta, , Indonesia.
3
Infectious Disease and Immunolgy Research Center, Indonesian Medical Education and Research Institute, Jalan Salemba
Raya no 6, Jakarta, Indonesia
Keywords: Antiviral drug, Butanol fraction of Callophylum nodosum, Dengue virus serotype 2
Abstract: Dengue fever still has a high incidence rate especially in Indonesia. Until now, there is no dengue antiviral
therapy found. Researches to develop dengue antiviral from herbal sources had been done. One of the
potential plants as dengue antiviral is Calophyllum nodosum which is known to have antimicrobial activity.
This study to evaluate the antiviral effects of butanol fraction of Calophyllum nodosum on DENV-2 activity
with Huh-7-it cells as host cells and also to evaluate minimal inhibitory concentration. Antiviral capability
was measured by 50% cytotoxic concentration (CC
50
) values and 50% inhibitory concentration (IC
50
)
values. The IC
50
value showed the effect of extract inhibition and was obtained from the focus assay of
DENV after treated with serial concentrations of extract (80, 40, 20, 10, 5 and 2.5 μg/mL). The CC
50
value
showed the effect of cytotoxic extract and resulted from MTT assay using concentrations of 640, 320 , 160,
80, 40, 20, and 10 μg /mL. The selectivity index (SI) value was ratio of CC
50
and IC
50
. The IC
50
, CC
50
and
SI value of butanol fraction of Calophyllum nodosum was 5.6 μg/mL, 1181 μg/mL and 210.9, respectively..
Statistical analysis showed significant differences between control group and treatment group on focus
assay and MTT assay. It can be concluded that the butanol fraction of Calophyllum nodosum had strong
antiviral effect with low cytotoxic effects.
1 INTRODUCTION
Dengue virus (DENV) infection is serious health
problem in the world, including Indonesia. DENV is
transmitted to humans by infected female Aedes
aegypti or Aedes albopictus (Fatima et al., 2011).
There are four serotypes of dengue virus (DENV 1-
4) that manifest with similar symptoms
2,3
DENV
infection cause various clinical manifestation range
from asymptomatic to severe cases such as Dengue
Hemorrhagic Fever (DHF) and Dengue Shock
Syndrome (DSS). Both DHF and DSS can cause
fatal cases and can lead to death of the patients
(WHO, 1997).
When DENV infect to human, only
few hours after infection, tens of thousands of copies
of viral molecules are produced from a single viral
molecule, leading to severe cases to death. Despite
the availability of a dengue vaccine, improvements
in case management to reduce the risk of severe
dengue are still needed.
The prevention of DENV infection and better
treatment have been developing. Prevention usually
directed to the DENV vector control. In other hand,
for DENV patient management usually given
supportive care. DENV infection is self limiting
disease, but there are patients with severe disease
(WHO, 1997). Improvements in case management to
reduce the risk of severe dengue are still needed.
Current approaches are entirely supportive care in
the form of judicious fluid replacement and close
clinical monitoring during the critical phase of
illness (WHO, 1997). Up to now, there is no specific
antiviral drug to DENV even there were association
between higher viremia levels and severe dengue.
Development of antiviral drug to DENV may help
for better treatment of DENV patients. The
development of dengue antiviral drugs is still in
progress. At present, the development of antiviral
drug to DENV medications leads to sources of
herbal medicines (Sohail et al., 2011). The source of
these herbal medicines is widely discovery because
124
Salsabila, S., Calista, N., Desti, H. and Dewi, B.
The Effectivity of Butanol Fraction of Calophyllum nodosum as Antiviral Drug to Dengue Virus Serotype 2 In Vitro.
DOI: 10.5220/0008358501240129
In Proceedings of BROMO Conference (BROMO 2018), pages 124-129
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

of the possibility of having low side effects and
abundant in nature. Genus of Calophyllum have
been widely used as traditional herbal medicines in
the tropic area (Bernabé-Antonio, 2014). Those
plants have phytochemicals such as flavonoids,
xanthones, coumarin, chalcone, benzofuran, and
triterpene. Those phytochemical have antioxidant
and antimicrobial activity (Alkhamaiseh et al.,
2012). Calophyllum plants able to inhibit the activity
of bacteria and fungi (Hanafi et al., 2017). Some
species have also been reported to have bioactivity
against various viruses such as HIV-1 virus and
human leukemia HL-60 (Sánchez et al., 2000).
Calophyllum nodosum species also contain
phytochemicals that have antioxidant and
antimicrobial activity. This phytochemical content is
thought to have antiviral activity against dengue
virus (Hanafi et al., 2017; Sánchez et al., 2000). But
then, the antiviral activity to DENV of Calophyllum
nodusum has not been discovered yet. Therefore,
the purpose of this study is to investigate the
effectivity of leaf extract Calophyllum nodusum in
butanol fraction as antiviral drug to DENV-2.
2 METHODS
The study was done at Department of Microbiology,
Faculty of Medicine, Universitas Indonesia. We
used Huh 7 it-1 cell and DENV serotype 2 strain
New Guinea C. To evaluate antiviral activity of
Calophyllum nodusum, we used previous method
(Saptawati et al., 2017) with slight modification.
The serial dilution of extract at 320 µg/mL, 160
µg/mL, 80 µg/mL, 40 µg/mL, 20 µg/mL and 10
µg/mL were used to determine inhibition of DENV
replication. To determine cytotoxic effect we used
serial dilution of extract at 640, 320 µg/mL, 160
µg/mL, 80 µg/mL, 40 µg/mL, 20 µg/mL and 10
µg/mL. DMSO as a diluent of extract were used as
negative control of antiviral assay. The test were
made in triplicate.
2.1 Determination of half-inhibitory
concentration
A total of 2×104 cells/well were seeded into 96-well
plate and the plate were incubated at 37°C with 5%
CO2. After 24 hours, the cells were infected with
DENV-2 with MOI of 1 FFU/cell. Various
concentration of extracts ranging from 320, 160, 80,
40 20 and 10, µg/mL were added shortly afterwards.
After 2 hours of infection, a mixture of DMEM+2%
FBS and various concentration of extracts were
added with volume of 100 ul/well. The tested of
each concentration were done in triplicate. Treated
with 0.1% of DMSO were used as negative control
of antiviral treatment. Plates were further incubated
at 37°C for 3 days. Next, supernatant of viruses were
harvested and determined the titter by focus assay.
Briefly, 10-fold serial dilution of the supernatant
was inoculated onto Huh-7 it-1 cell monolayer in
triplicate wells. Absorption was carried out at 37
o
C
with 5% CO
2
for 2 hours with agitation at 30
minutes interval. Methylcellulose 1.5% overlay
medium was added to the cell and incubated at 37
o
C
with 5% CO
2
for 3 days. The infected cells were
stained according to previous study with slight
modification (Saptawati et al., 2017).
First, infected
cells were fixed and increased permeable for
immunostaining. After cell washing, human IgG-
anti dengue were added to each well 1/1000 and
incubated at room temperature for 1 hour. For the
secondary antibody. We used 1/1000 antihuman IgG
label HRP. After washed using PBS, substrate for
horseradish peroxidase were added and cells were
observed for its brownish colour. Number of foci
formed in each well including in negative control
well was counted manually under microscope after
staining. Number of foci in each treatment well was
compared to that of negative control well to obtain
percentage of infectivity of each well. The mean
value of percentage of infectivity for each
concentration triplicate was calculated and then
those values were plotted against corresponding
concentration to generate concentration-percentage
of inhibition curve. The half-inhibitory
concentration (IC
50
) was obtained from nonlinear
regression equation of concentration-effect curves.
2.2 Determination of half-cytotoxic
concentration
To determine CC50, we used MTT assay as
describe in our previous study (Saptawati et al.,
2017). MTT assay that quantified the percentage
viability of Huh-7 cells after treated with a certain
concentration of extract compared with DMSO
0.1%) as negative control. In 96 well flat-bottom
plates (Corning, USA), cell were added as much as 2
× 10
4
cells/well and incubated at 37
o
C with 5% CO
2
for 24 hours. Then, the cells were treated with
various concentration of extract ranging from 640,
320, 160, 80, 40, 20, 10 , 5 and 2.5 µg/mL and were
then incubated at 37
o
C with 5% CO
2
. After 48 hours
of incubation, 20μL of 3-(4,5-Dimethylthiazol-2-yl)-
2,5-diphenyltetrazolium bromide (MTT) (Promega)
salt solution was added into each well and incubated
for 4 hours according to the manufacturer’s
The Effectivity of Butanol Fraction of Calophyllum nodosum as Antiviral Drug to Dengue Virus Serotype 2 In Vitro
125

instruction. Theoretical percentage toxicity of each
concentration was determined by dividing the mean
blanked sample optical density (ODs) by the mean
blanked control ODs for each sample. The resulting
percentage toxicity values of each concentration that
was tested in triplicate was calculated for its mean
and standard deviation and then the mean percentage
was plotted to corresponding concentration to
generate concentration-mean percentage of viability
curve. A nonlinear regression equation was derived
from the curve to calculate the half-cytotoxic
concentration (CC
50
) of each extracts.
2.3 Data Analysis
Mean difference of percentage of cytotoxicity and
infectivity between treatments group and negative
control was analysed using One-way ANOVA using
SPSS version 23 with p value less than 0.05
(p<0.05) considered as statistically significant
difference. The value of CC
50
and IC
50
were
determined using simple arithmetical calculation on
regression equations obtained from concentration-
percentage of viability and concentration-percentage
of inhibition. Then, selectivity index for each extract
was derived from the ratio of CC
50
to IC
50.
3 RESULTS
3.1 Percentage of DENV infectivity and
IC
50
value
After treated with extracts, the percentage of DENV
infectivity in Huh 7it-1 was decrease significantly
(Table 1). Addition of extract to DENV-2 at
concentration of 40ug/mL and more, showed no
DENV-2 in the focus assay with significantly
different (Table 1), Decrease of extract
concentration caused an increase of DENV
infectivity. This results showed that butanol fraction
of Calophyllum nodosum had antiviral activity to
DENV-2. The infectivity value was then used to
figure out an exponential regression and then to
determine IC
50
. Based on the equation, the IC
50
value was 5.6 µg/mL (Figure 1.) with R
2
of 0.927.
Table 1 : Percentage of DENV-2 infectivity after treated with various concentration of Calophyllum nodosum
.
Concentration (µg/ml)
Percentage of infectivity
(mean% ± SD)
p Value
80
0.0 ± 0.0
0.034
40
0(0 - 2.9)
0.043
20
3.9 ± 4.5
0.046
10
41.8 ± 20.7
0.046
5
62.2 ± 17.1
0.046
2.5
52.5(37.9 - 52.5)
0.043
DMSO
99.1(99.1 - 102)
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
126
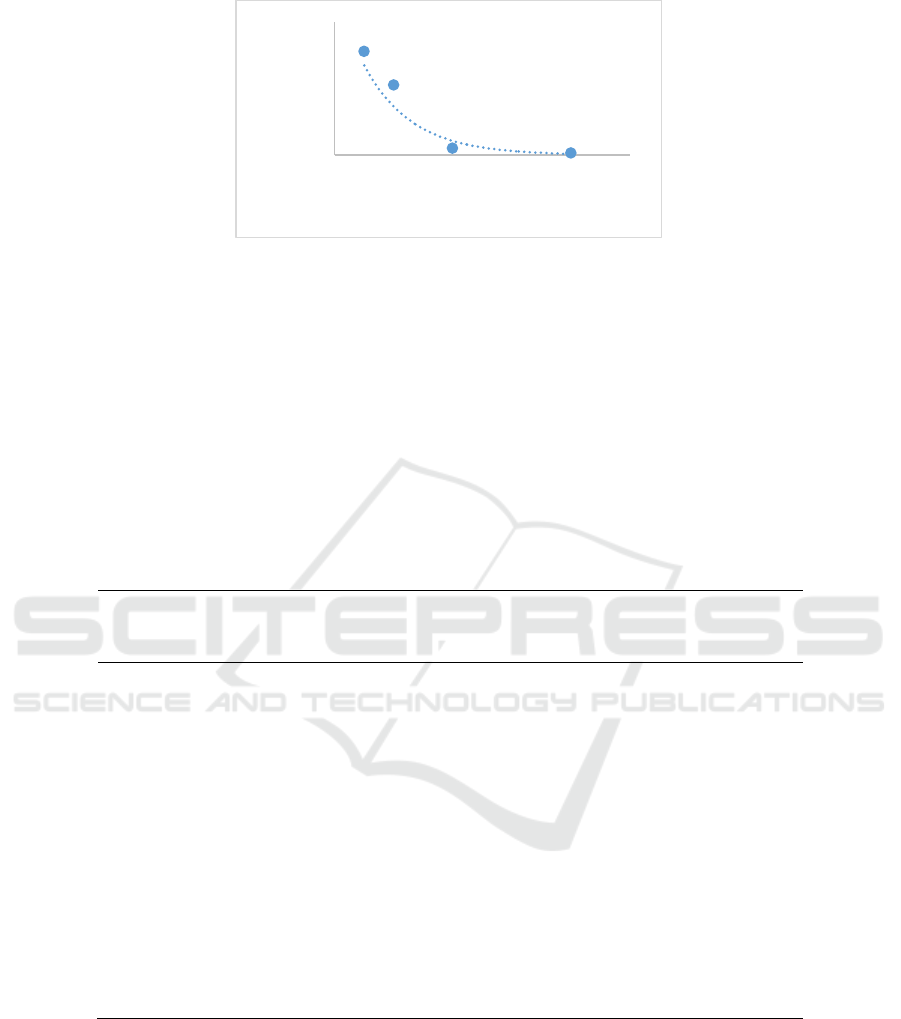
Figure 1: Exponential regression graph of DENV-2 infectivity after treated with serial concentration Calophyllum nodosum
3.2 Cytotoxicity and CC
50
value
The cytotoxicity of butanol fraction of Calophyllum
nodosum was determined by MTT assay. In MTT
assay, the absorbance value of the test well divided
by the absorbance value of the DMSO control, times
100% to determine the cell viability value. After
treated with concentration more than 80 ug/mL, the
viability of cell slightly decreased but no statistically
different (Table 2). From the data, increasing of
concentration of extract caused a decrease in the cell
viability (Table 2). There was an abnormality data at
concentration of 320 µg/mL. Treated with 320
ug/mL of butanol fraction of Calophyllum nodosum
the cell viability decreased rapidly in excess of 640
ug/mL. It may due to a laboratory error. The mean
cell viability values were then translated into a graph
with a linear regression to determine CC
50
(Figure
2). The CC
50
value of butanol fraction of
Calophyllum nodosum was 1,181 ug/mL with R
2
of
0.567.
Table 2 :The percentage of cell viability after treated with various concentration of extract.
y = 99.794e
-0.123x
R² = 0.9275
0.0
20.0
40.0
60.0
80.0
0 10 20 30 40 50
Mean Infectivity
Extract Concentration (µg/mL)
Concentration
(µg/ml)
Percentage of Viability
(mean% ± SD)
p Value
640
82.7 ± 5.2
0.05
320
68.4 ± 5.1
0.05
160
99.7 ± 11.4
0.513
80
94.7 ± 1.1
0.05
40
102.1 ± 1.1
0.275
20
106.5 ± 0.9
0.05
10
104.9 (103.6 - 105.5)
0.05
5
101.5 ± 1.7
0.275
2.5
102.4 ± 8.6
0.827
DMSO
100.0 ± 1.7
The Effectivity of Butanol Fraction of Calophyllum nodosum as Antiviral Drug to Dengue Virus Serotype 2 In Vitro
127
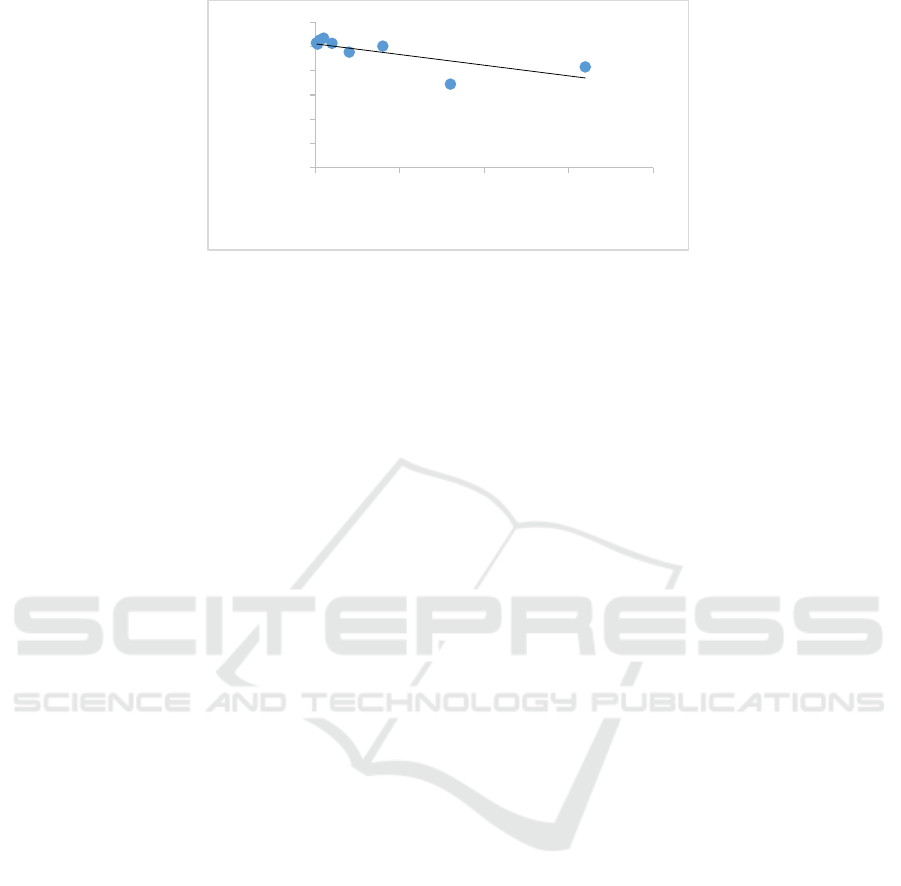
Figure 2. Linear regression graph of concentration-mean percentage of Huh7it-1 cells viability after treated
with Calophyllum nodosum.
3.3 Selectivity Index (SI) value
The selectivity index of butanol fraction of
Calophyllum nodosum was 210.9 based on IC
50
and
CC
50
value.
4 DISCUSSION
Specific antiviral drug to DENV was not avalable
yet. Indonesia has variety of herbal medicine that
can be developed as antiviral drug to DENV.
Calophyllum genus is known to have antimicrobial
properties that can inhibit bacterial, fungal and viral
activity. The phytochemical properties of the
Calophyllum genus are flavonoid, kumarin and
xanthone. Flavonoids have strong antioxidant,
antimicrobial and antiviral activity. Flavonoid from
other plant also contain lots of flavonoids and have
the ability to inhibit viruses including DENV.
11
Several studies on the antiviral effect of
Calophyllum on dengue virus have been carried out
and showed that Calophyllum extract had an
inhibitory effect on dengue virus activity which was
significant with a relatively small cytotoxic effect.
12,13
Similar result was found in this study. Butanol
fraction of Calophyllum nodosum showed antiviral
activity with IC
50
of 5.6 µg/mL.
Host cell viability trend was stay with
numerous test, even we increased the concentration
of the extract (Table 2). From the linear regression,
the R
2
value was 0.567 (Figure 2), this indicate that
no strong correlation between extract concentration
and cell viability. The lowest of R
2
value in this
study may due to no cytotoxic effect at the highest
concentration used in this study. The CC
50
value is
1181.1 µg/mL. We suggested for next cytotoxic
assay to use butanol fraction of Calophyllum
nodosum at concentration more than 1,000 ug/mL.
The development of antiviral drug to treat DENV
infection leads to sources of herbal medicines
5
. The
development of small molecule anti-DENV drugs
has been a slow process. To date, only four small
molecule anti-DENV drugs such as chloroquine ,
celgosivir balapiravir and UV-4B9 already move to
Phase I or Phase II clinical trials.
14
But some of
them with remains unclear out come or
achieved the required safety profile, but did not reduc
e viral load as expected,
15,16
Furthermore, the clinical
trial of the α-glucosidase inhibitor was terminated at
Phase I.
14
Pre-clinical and clinical research into
anti-DENV drugs is still underway, and many
lessons can be learned from the previous studies. In
future, we are bound to overcome the challenges,
and expect our ongoing work to yield a potent anti-
DENV therapy.
In this study, we used DENV-2 NGC. The
promising anti-DENV drugs are anticipated to
inhibit all serotypes of DENV, in the next study we
will use all serotype of DENV. The antiviral drug to
DENV remain challenges. The inhibition of all
serotypes, as well as antibody dependent
enhancement phenomenon observed during DENV
infection, complicates the investigation of anti-
Dengue drugs such as if a patient was re-infected by
a heterotypic virus, the antibodies created previously
would become severe. Moreover, in laboratory
testing, the limited availability of animal models has
hampered of antiviral drug to DENV development.
The value of the selectivity index of Butanol
fraction of Calophyllum nodosum was 210.9. This
value was really high compare with other study such
as Psidium guajava and Carica papaya showed SI
value of 21.28 and 37.25 respectively.
17
It can be
concluded that the butanol fraction of Calophyllum
nodosum has a strong antiviral effect to DENV with
low cytotoxic effects. Further study needed to
determine by which mechanism butanol fraction of
Calophyllum nodosum inhibit DENV replication.
y = -0.0441x + 102.09
R² = 0.5675
0.0
20.0
40.0
60.0
80.0
100.0
120.0
0 200 400 600 800
Mean Viability
Extract Concentration (µg/mL)
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
128

5 CONCLUSION
From this study, we concluded that IC
50,
CC
50 and
SI
value was 5.6 µg/mL, 1181.1 µg/mL, and 210.9.
Butanol fraction of Calophyllum nodosum had
strong antiviral effect against DENV-2 with low
cytotoxic effect. Furthermore, Butanol fraction of
Calophyllum nodosum can be a candidate of
antiviral drug in future.
ACKNOWLEDGEMENT
This study was supported by grant of Publikasi
Terindeks Internasional Untuk Tugas Akhir
Mahasiswa UI (PITTA) 2018 No:
0588/SK/R/UI/2018
REFERENCES
Alkhamaiseh, SI., Taher, M., Ahmad, F., Qaralleh, H.,
Althunibat, OY., Susanti, D., et al. 2012. The
phytochemical content and antimicrobial activities of
Malaysian Calophyllum canum (stem bark). Pak J
Pharm Sci, vol.25, no.3, pp.555-63.
Bernabé-Antonio, A. 2014. Biological Importance of
Phytochemicals from Calophyllum brasiliense
Cambess. Annu Res Rev Biol, vol. 4, no. 10, pp.
1502–17.
Fatima, Z., Idrees, M., Bajwa, MA., Tahir, Z., Ullah, O.,
Zia, MQ., et al. 2011. Serotype and genotype analysis
of dengue virus by sequencing followed by
phylogenetic analysis using samples from three mini
outbreaks-2007-2009 in Pakistan. BMC Microbiol.
Hanafi, MAM. Syatna, FD., Mirawati S., Ratnasari, S.,
Dewi, BE. 2017. Antiviral Effect of Sub Fraction
Cassia alata Leaves Extract to Dengue Virus
Serotype-2 strain New Guinea C in Human Cell Line
Huh-7 it-1 3OP Conf. Series: Earth and Environmental
Science 101.
Ross, TM. 2010. Dengue virus. Clin Lab Med, vol. 30,
pp.149–160.
Sánchez, I., Gómez-Garibay, F., Taboada, J., Ruiz, BH.
2000. Antiviral effect of flavonoids on the dengue
virus. , vol.14, no.2, pp.89-92
Saptawati, L., Febrinasari, R., Yudhani, R., Yono, H.,
Faza, A., Luthfiani, S., et al. 2017. In vitro study of
eight Indonesian plants extracts as anti Dengue virus.
Health Science Journal of Indonesia, vol. 8, no.1.
Sohail, MN., Rasul, F., Karim, A., Kanwal, U., Attitalla,
IH. 2011. Plant as a Source of Natural Antiviral
Agents. Asian J Anim Vet Adv, vol. 6, no. 12, pp.
1125–52.
Wang, E., Ni, H., Xu, R., Barrett, AD., Watowich, SJ.,
Gubler, DJ., et al. 2000. Evolutionary relationships of
endemic/epidemic and sylvatic dengue viruses. J Virol,
vol.74, pp.3227–3234.
WHO. 1997. Dengue haemorrhagic fever: diagnosis,
treatment, prevention and control. Geneva, 2
nd
edition.
The Effectivity of Butanol Fraction of Calophyllum nodosum as Antiviral Drug to Dengue Virus Serotype 2 In Vitro
129
