
Synthesis of Glycerol –– Castor Oil Fatty Acid and Glycerol –– Oleic Acid
Esters, as Emulsifier and Antibacterial Agent, Using Candida rugosa Lipase
Elmira Vania Denada
1
, Sri Handayani
1
Sumi Hudiyono
1
and Siswati Setiasih
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok 16424, West Java,
Indonesia
Keywords: Castor Oil, Glycerol, castor oil fatty acid, ester, oleic, emulsifier, antibacterial, Candida rugosa
Abstract: The research’s objective is to synthesize glycerol – castor oil fatty acid and glycerol oleic acid esters using
Candida rugosa lipase in n-hexane. Molar fatty acid to glycerol ratio that used in esterification varied from
1:1, 1:2, 1:3, and 1:4. The ester products then being analysed by using FTIR. The spectra for glycerol-castor
oil fatty acid ester and glycerol-oleic acid absorption peak at wave number 1735.37 cm
-1
and 1748.25 cm
-1
respectively, which indicate the existence of C=O ester groups. Conversion percentage in esterification was
conducted using titrimetric method. The highest conversion percentage was reached at the molar ratio 1:4
with the value of 92.4% respectively for glycerol-castor oil fatty acid and 86.2% for glycerol oleic ester.
Emulsifier test was performed to observe the stability and the type of emulsion, using ester product as
emulsifier. The properties of the emulsifier in both esters are obtained by type of water in oil. Antimicrobial
assay were also conducted for both esterification product using disc diffusion method against
Propionibacterium acne and Staphylococcus epidermidis. The antimicrobial assay showed that glycerol-
castor oil fatty acid esters has antimicrobial activity, but glycerol-oleic esters did not show the antimicrobial
activity.
1 INTRODUCTION
Castor oil from castor bean plant has many benefits,
such as biodiesel source, plantation commodities,
pharmacology, natural insecticides, and also as basic
materials for moisturizers and cosmetics (Alvarez
and Rodiguez, 2000; Amir and Hartono, 2013;
Santoso et al, 2014). Generally, 89% from all of
fatty acid in castor oil is ricinoleic (Omari et al,
2015). Ricinoleic can be obtained by hydrolysis of
castor oil which involves high pressure and
temperature in the presence of catalyst (Yamamoto
and Fujiwara, 1995). This reaction will produce
glycerol and ricinoleic fatty acid.
Ricinoleic fatty acid from castor oil may gain
higher economic value by converting them into lipid
derivate compounds. Lipids in the form of
monoglycerides or diglycerides are often used as
emulsifiers for cosmetics. Fatty acid monoesters are
one of the compounds from lipid derivates, which
have the highest antibacterial activity against gram
positive and gram negative bacteria (Zhao et al,
2015).
The esterification reaction between fatty acid and
glycerol is capable of producing glycerol derivative
compounds such as monoglycerides, diglycerides,
and triglycerides. The glycerol monoester compound
has significant applications in food, pharmaceutical,
cosmetics, and even detergent industries (Pouilloux
et al, 2000). Mono- and diglycerides may also serve
as emulsifiers. In addition, Kosová, et al (2015) and
Handayani, et al (2018), stated that fatty acid
glycerol ester has antimicrobial activity. In
conventional chemical methods, esterification
processes often involve acid catalysts. However, this
method provides an unwanted by-product (Zaidi et
al 1995), so that esterification using enzymes – such
as lipase – is more commonly used in modern
chemical methods.
Lipase is a hydrolase enzyme. Candida rugosa
lipase often used as catalyst in the reaction of
triglyceride hydrolysis. Candida rugosa lipase can
also be used as a catalyst in esterification reactions.
However, it should be in a certain condition, namely
the small amount of water and the use of organic
solvents (Zaks and Klibanov, 1984).
62
Denada, E., Handayani, S., Hudiyono, S. and Setiasih, S.
Synthesis of Glycerol â
˘
A ¸Sâ
˘
A ¸S Castor Oil Fatty Acid and Glycerol â
˘
A ¸Sâ
˘
A ¸S Oleic Acid Esters, as Emulsifier and Antibacterial Agent, Using Candida rugosa Lipase.
DOI: 10.5220/0008357500620068
In Proceedings of BROMO Conference (BROMO 2018), pages 62-68
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

2 MATERIALS AND METHODS
2.1 Materials
Aquades, Candida rugosa lipase (2.45 U/mg)
from Sigma-Aldrich, 96% ethanol, castor oil,
commercial glycerol, Clindamycin, DMSO 10%,
eosin, hydrogen chloride, n-hexane, nutrient broth,
nutrient agar, oleic acid, phenolphthalein, phosphate
buffer pH 8, potassium hydroxide, sodium
hydroxide, Propionibacterium acnes and
Staphylococcus epidermidis bacteria, are materials
used in this study.
2.2 Methods
2.2.1 Hydrolysis of Castor Oil
Castor oil hydrolysis conducted by mixing 100
grams of the compound with 100 mL KOH 5 M in
ethanol, heated and stirred using hotplate for 2 hours
at 70±2
o
C. The mixture were then allowed to reach
room temperature and added by 50 mL HCl 5 M
while stirring for two hours. The castor oil fatty acid
was obtained by let the mixture stand for 24 hours
until two phased formed. The upper phase were
taken as castor oil-fatty acid.
2.2.2 Esterification Reaction
Esterification delivered by mixing fatty-acid,
glycerol, n-hexane (as a solvent) and Candida
rugosa lipase as biocatalyst. Solvent to substrate
ratio was 1:1 (v:v) while fatty acid to glycerol ratio
were varied among 1:1, 1:2, 1:3, and 1:4 (mol:mol)
and for enzyme use was 5% of the substrate’s total
mass. Horizontal incubator was used to incubate the
mixture. The incubation process was carried out on
18 hours at 200 rpm and 37
o
C. Termination for the
esterification reaction obtained by heating the
mixture at ±80
o
C to denaturate the enzyme. To
separate the mixture, centrifugation process was
executed at 3400 rpm in 15 minutes. Each of the
layers formed were separated and the middle one
was taken as an ester product.
2.2.3 Conversion Percentage Determination
The percentage of conversion determined by
titrimetric method. 0.1 N NaOH employed to titrate
the hexane phase (upper-phase), and
phenolphthalein was involved as an indicator.
2.2.4 Ester Product Characterization using
FTIR
FTIR was used to characterized the ester product,
castor oil, oleic acid, and castor oil-fatty acid.
2.2.5 Emulsifier Test
Oil and water mixed with 0.1 g ester products to
test the emulsion properties. The oil and water
amounts shown on Table 1. The mixture were
shaken using vortex for 30 seconds and the stability
of the emulsion was observed.
Table 1. Oil and Water Amount
Water (mL)
Oil (drops)
Variation
One
2
10
Water (drops)
Oil (mL)
Variation
Two
10
2
2.2.6 Emulsion Type Determination
A drop of emulsion and eosin respectively were
mixed on an object glass. Then the mixture was
observed under the microscope to define the type of
emulsion, whether oil in water (o/w) or water in oil
(w/o).
2.2.7 Antimicrobial Activity Assay
Clindamycin which used as positive control are
involved in Antimicrobial activity assay. The
activity was performed with disc diffusion method
towards castor oil, castor oil-fatty acid, ester
products, glycerol, n-hexane and DMSO as solvent,
and oleic acid. In a sterile petri dish, 20 mL nutrient
agar mixed aseptically with 2 x 10
2
μL aliquot of
Propionibacterium acnes suspension which its cell
density is 1 x 10
8
cells/mL. The medium were then
allowed to harden. Then, the top of the medium
placed by paper disc and 400 x 10
-2
μL sample was
dropped to it. Incubation for the medium was held at
37
o
C for 24 hours. The microbial activity calculated
based on the diameter of the clear zone on the
medium. This assay were also done for
Staphylococcus epidermidis.
Synthesis of Glycerol â
˘
A¸Sâ
˘
A¸S Castor Oil Fatty Acid and Glycerol â
˘
A¸Sâ
˘
A¸S Oleic Acid Esters, as Emulsifier and Antibacterial Agent, Using
Candida rugosa Lipase
63
3. RESULT AND DISCUSSION
3.1 Castor Oil Hydrolysis
The catalyst used for hydrolysis reaction was
potassium hydroxide. Ethanol, as a semi polar
compound, used for this reaction to be an
intermediary of potassium hydroxide and
triglycerides which leads the reaction to be occurred.
The mixture was then added by HCl to acidify and
form the fatty acid. Yield percentage obtained was
87.5%.
3.2 Esterification
This reaction was catalysed by Candida rugosa
lipase in organic solvent with small amount of water.
Although Candida rugosa as hydrolase enzyme, it is
often used as a catalyst in hydrolysis reaction. In this
case it can also be used as a catalyst in the
esterification reaction with requirement of organic
solvent and small amounts of water (Zaks and
Klibanov, 1984). In this study, n-hexane was used as
solvent in the enzymatic esterification. n-hexane, the
non-polar compound, is capable to dissolve fatty
acid. Phosphate buffer pH 8 was used to optimize
Candida rugosa lipase and dissolve glycerol.
Candida rugosa lipase has a low activity when the
pH used is less than 6.0 or more than pH 8.0
(Öztürk, 2001).
The molar ratio variation of fatty acid:glycerol
were intended to observed the optimum formation of
ester and also to keep the reaction equilibrium in the
direction of the ester. The highest ester product
produced by using molar ratio 1:4. The ester formed
supposed to be mono- or diglycerides since the
amount of fatty acid used was lower than the
glycerol.
3.3 Conversion Percentage Determination
The three layers from centrifugation process on
esterification were separated and the top layer was
used to determine the conversion percentage.
Figure 1 describe the conversion percentage from
all of variations (mol:mol). The highest percentage
was reached at the molar ratio 1:4 (fatty
acid:glycerol) with value of 92.4% for ester
glycerol-castor oil fatty acid and 86.2% for ester
glycerol oleic acid.
Figure 1. The Curve of Variation of Mol vs Percentage of
Convertion
3.4 Ester Products Characterization by FTIR
Figure 2 and Figure 3 below show the IR
spectrum of castor oil, castor oil-fatty acid, oleic
acid and ester products.
[VALUE]%
[VALUE]%
[VALUE]%
[VALUE]%
[VALUE]%
[VALUE]%
[VALUE]%
[VALUE]%
1 : 1 1 : 2 1 : 3 1 : 4
Ester Glycerol Oleic
Ester Glycerol Castor Oil - Fatty Acil
a)
b)
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
64

Figure 2. FTIR Spectrum of a) Castor Oil, b) Castor Oil-
fatty acid, c) Oleic Aid
FTIR spectrum above shows the presence of CH
sp
2
vinyl group which indicates the presence of
double bonds in castor oil and castor oil-fatty acid
hydrolysis. In addition, there is also a C=O
functional group detected in castor oil, but its wave
number shifts when the castor oil has been
hydrolysed to fatty acids. The C=O functional group
in castor oil is identified as C=O carboxylate at wave
number 712.97 cm
-1
(Figure 2a). Then on the castor
oil-fatty acid, the functional group identified as C=O
ester at wave number 1745.65 cm
-1
(Figure 2b). Oleic
acid has a typical functional group, specifically C=O
carboxylate (714.61 cm
-1
) and CH sp
2
vinyl
(3009.97 cm
-1
) (Figure 2c). CH sp
2
vinyl group
indicates the presence of double bond of the
compound. As well known, oleic acid has a double
bond on C
9
and C
10
.
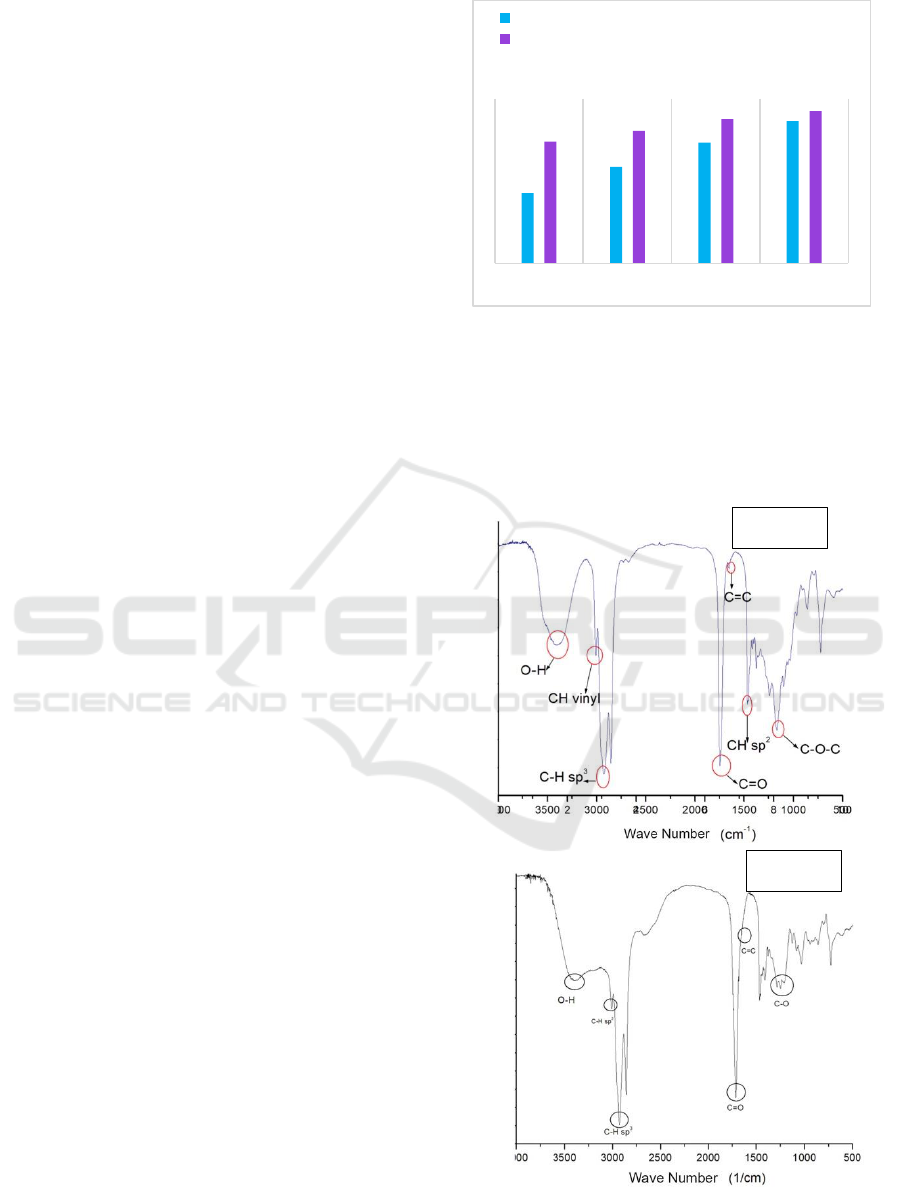
Figure 3. a) Ester Glycerol Oleic, b) Ester Glycerol-Castor
Oil Fatty Acid
Figure 3a. proves that oleic acid has been
esterified, since the spectrum states the presence of
C=O ester groups at wave number 1748.25 cm
-1
. In
addition, there are also OH groups (3319.63 cm
-1
)
whose peaks look widened. The presence of a
widened OH group caused by the hydroxyl group
present in glycerol is not fully esterified.
Furthermore, the absorption band of the carboxylate
group is still visible, so it can be said the ester
glycerol oleic still contain oleic acid.
Figure 3b. shows the absorption band of the OH
functional group of the compound (3410.07 cm
-1
).
The carbon chain of the ester compound is shown by
the functional group CH sp
3
(2923.53 cm
-1
). The
identical group of esters, obtained as the C=O ester
group (1735.37 cm
-1
) has been shown. It can be said
that the castor oil-fatty acid results have been
esterified.
Those functional groups as existed in Silverstein
et al (1981), the spectrum of CH sp
2
vinyl is in the
range 3010 - 3100 cm
-1
, whereas C=O ester is in the
range 1735 - 1750 cm
-1
and C=O carboxylic acid at
1700-1725 cm
-1
, also OH is at 3200 - 3400 cm
-1
, and
CH sp
3
at 2850 - 3000 cm
-1
.
3.5 Emulsifier Test
Result of the emulsifier test indicates that the
ester glycerol oleic and ester glycerol-castor oil fatty
acid have emulsifier properties.
The emulsifier test showed that ester glycerol-
castor oil fatty acid and ester glycerol oleic have
emulsifier properties. The emulsion of ester glycerol
-castor oil fatty acid more stable than the emulsion
of ester glycerol oleic (Figure 4). The emulsions
stable up to 36 hours for ester glycerol-castor oil
fatty acid and 24 hours for ester glycerol oleic.
b)
c)
a)
Synthesis of Glycerol â
˘
A¸Sâ
˘
A¸S Castor Oil Fatty Acid and Glycerol â
˘
A¸Sâ
˘
A¸S Oleic Acid Esters, as Emulsifier and Antibacterial Agent, Using
Candida rugosa Lipase
65

Figure 4. Emulsion test results; a) Variation Two of Ester
Glycerol-Castor Oil Fatty acid, b) Variation One of Ester
Glycerol-Castor Oil Fatty Acid, c) Variation One of Ester
Glycerol-Oleic, d) Variation Two of Ester Glycerol-Oleic.
3.6 Emulsifier Type Determination
Figure 5. Emulsion type of; a) Ester Glycerol-Castor Oil
Fatty Acid, b) Ester Glycerol Oleic
Emulsion’s type was determined through
observation under a microscope. Eosin was added
as a water-soluble dye because of its polar nature. So
that the water phase will change colour to red. The
presence of eosin makes it easy to observe the type
of emulsion because the water phase and the oil
phase will have different colours.
The ester glycerol casto oil-fatty acid and ester
glycerol oleic have a water-in-oil emulsion type
(w/o) (Figure. 5). The red droplets are water, and the
yellow environment is oil.
3.7 Antibacterial Activity Assay
Propionibacterium acnes and Staphylococcus
epidermidis were used in this study. Ester glycerol-
castor oil fatty acid as well as ester glycerol oleic,
were varied in 20%, 40%, 60%, and 80% (w/w).
This variation were used to determine the best
concentration of esters that can inhibit bacterial
growth. Table 2 shows the classification of
antimicrobial effectiveness substances (Greenwood,
1995). Table 3 shows the antimicrobial activity.
Table 2. Classification of Antimicrobial Effectiveness
Inhibition Zone
Diameter
Response of Growth
Barriers
> 20 mm
Strong
16 – 19 mm
Average
10 – 15 mm
Poor
< 10 mm
Ineffective
Table 3. Inhibition Zone of Various Compounds in Antibacterial Activity Assay
Sample
Inhibition Zone Diameter (mm)
Classification
P. acnes
S. epidermidis
Ester Glycerol-Castor Oil Fatty Acid 20%
-
-
No Activity
Ester Glycerol-Castor Oil Fatty Acid 40%
7
9
Ineffective
Ester Glycerol-Castor Oil Fatty Acid 60%
9
9
Ineffective
Ester Glycerol-Castor Oil Fatty Acid 80%
12
10
Poor
Ester Glycerol Oleic 20%
-
-
No Activity
Ester Glycerol Oleic 40%
-
-
No Activity
Ester Glycerol Oleic 60%
-
-
No Activity
Ester Glycerol Oleic 80%
-
-
No Activity
Castor Oil Fatty Acid 50%
12
14
Poor
Castor Oil Fatty Acid 100%
13
15
Poor
Oleic Fatty Acid
-
-
No Activity
Castor Oil 100%
-
-
No Activity
n-hexane 100%
-
-
No Activity
Glycerol 100%
-
-
No Activity
DMSO 100%
-
-
No Activity
Clindamycyn 0.5%
18
15
Average
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
66

The data above shows that there was no
antimicrobial activity from oleic acid and esters
glycerol oleic against Staphylococcus epidermidis
and Propionibacterium acnes. The absence of
antimicrobial activity also found in glycerol, n-
hexane, and DMSO. It clearly shows that the solvent
used in this study has no antimicrobial activity.
In general, the increases of concentration in test
compounds were able to produce larger inhibition
zone diameters for both types of bacteria (Table 3).
Ester glycerol-castor oil fatty acid has the highest
value at 80% concentration by producing 12 mm
inhibition zone for Propionibacterium acnes
bacteria. In the same concentration, the ester is
capable of providing 10 mm inhibition zone for
Staphylococcus epidermidis bacteria. Thus, it can be
said that ester glycerol-castor oil fatty acid has a
stronger inhibitory effect against P. acnes.
The inhibition zone can also be found in castor
oil-fatty acid. The highest diameter at 100%
concentration with 13 mm and 15 mm, respectively
for P. acnes and S. epidermidis. Castor oil fatty acid
and ester glycerol-castor oil fatty acid are in the
same classification of inhibitory resistance, which is
poor. Inhibition zones of clindamycin are 18 mm
for P. acnes and 15 mm for S. epidermidis. This
researched showed that ester glycerol-castor oil fatty
acid have the activity for antimicrobial agent.
4 CONCLUSIONS
Glycerol – castor oil fatty acid and glycerol –
oleic esters were successfully synthesized using
Candida rugosa lipase as catalyst. Both ester
products can be used as emulsifier for water in oil
(w/o) emulsion type. Only glycerol-castor oil fatty
acid ester has antimicrobial activity.
ACKNOWLEDGEMENTS
This researched was funded Hibah Kompetensi
Publikasi Internastional Terindeks Untuk Tugas
Akhir Mahasiswa (PITTA), Universitas Indonesia
2018.
REFERENCES
Alvarez, Antonio M R., Maria L G Rodiguez., 2000.
Lipids in Pharmaceutical and Cosmetic
Preparation. Grasas y Aceites, 51 (1-2), 74 – 96.
doi: 10.3989/gya.2000.v51.i1-2.409
Amir, Andi M., Joko Hartono., 2013. Toksisitas
Insektisida Nabati Minyak Biji Jarak Kepyar
(Ricinus communis L.) Terhadap Thrips
Selenothrips rubrocinctus Giard. Seminar
Nasional Inovasi Teknologi Pertanian. Balai
Penelitian Tanaman Pemanis dan Serat Malang,
Malang.
Greenwood., 1995. Antibiotics Susceptibility
(Sensitivity Test), Antimicrobial and
Chemotheraphy. USA: McGraw Hill Company.
Handayani, S., Putri, A. T., Setiasih, S., &
Hudiyono, S., 2018. Enzymatic Synthesis of
Glyserol-Coconut Oil Fatty Acid and Glycerol-
Decanoic Acis Ester as Emulsifier and
Antimicrobial Agents Using Candida rugosa
Lipase EC 3.1.1.3. IOP Conference Series:
Materials Science and Engineering,299, 012019.
Kosová, M., Hrádková, I., Mátlová, V., Kadlec, D.,
Šmidrkal, J., & Filip, V., 2015. Antimicrobial
effect of 4-hydroxybenzoic acid ester with
glycerol. Journal of Clinical Pharmacy and
Therapeutics,40(4), 436-440.
Santoso, Bambang B., et al., 2014. Hasil dan Kadar
Minyak Jarak Kepyar pada Berbagai Umur
Pemangkasan Batang Utama. J. Agron. Indonesia
42 (3) : 233 -249
Omari, Athumani., et al., 2015. Fatty Acid Profile
and Physio-Chemical Parameters of Casor Oils
in Tanzania. Green and Sustainable Chemistry 5:
154-163
Öztürk, Banu., 2001. Immobilization of Lipase from
Candida rugosa on Hydrophobic and
Hydrophilic Support. Izmir Institute of
Technology, Turkey.
Pouilloux, Y., Métayer, S., & Barrault, J., 2000.
Synthesis of glycerol monooctadecanoate from
octadecanoic acid and glycerol. Influence of
solvent on the catalytic properties of basic
oxides. Comptes Rendus De LAcadémie Des
Sciences - Series IIC - Chemistry,3(7), 589-594.
Silverstein, R.M.; Bassler, G.C.; and Morrill,
T.C., 1981. Spectrometric Identification of
Organic Compounds. 4th ed. New York: John
Wiley and Sons. QD272.S6 S55
Yamamoto, K., & Fujiwara, N. (1995). The
Hydrolysis of Castor Oil Using a Lipase from
Pseudomonas sp. f-B-24: Positional and
Substrate Specificity of the Enzyme and
Optimum Reaction Conditions. Bioscience,
Biotechnology, and Biochemistry,59(7), 1262-
1266. doi:10.1271/bbb.59.1262
Synthesis of Glycerol â
˘
A¸Sâ
˘
A¸S Castor Oil Fatty Acid and Glycerol â
˘
A¸Sâ
˘
A¸S Oleic Acid Esters, as Emulsifier and Antibacterial Agent, Using
Candida rugosa Lipase
67

Zaidi, A., Gainer, J. L., & Carta, G. (1995). Fatty
acid esterification using nylon-immobilized
lipase. Biotechnology and Bioengineering,48(6),
601-605.
Zaks, A., Klibanov, A. M., 1984. Enzymatic
Catalysis in organic Media at 100
o
C. Science
224:1249-1251.
Zhao, Lei., et al., 2015. In Vitro Antibacterial
Activities and Mechanism of Sugar Fatty Acid
Esters Against Five Food-related Bacteria.
Journal of Food Chemistry 187: 370-377
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
68
