
Enzymatic Esterification Ethyl Ester Fatty Acid from Hydrolyzed
Castor Oil and Its Oxidation Product as Emulsifier and
Antimicrobial Compound Using Candida rugosa Lipase E.C.3.1.1.3
Annisa Khairani
1
, Sumi Hudiyono
1
and Sri Handayani
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok 16424, West
Java, Indonesia
Keywords: Castor oil, fatty acid ethyl ester, oxidized fatty acid ethyl ester, lipase, emulsifier, antimicrobial
Abstract: Aim of this study was to synthesis fatty acid ethyl ester compound of hydrolyzed castor oil and its oxidation
product using Candida rugosa lipase. Both of the esterification products were expected to have
antimicrobial activity against Staphylococcus epidermidis and Propionibacterium acnes and may act as
emulsifiers. Optimization of esterification reactions was done by varying the mole ratio between fatty acids
and ethanol, ie 1:1, 1:2, 1:3, and 1:4. The esterification products were then characterized using FTIR.
Conversion percentage was determined by titrimetric method, to calculate the amount of fatty acids that was
converted to ester. The ester product was tested its ability as emulsifier by emulsifier test. The esterification
products were also examined their antimicrobial activity using disc diffusion method. The highest
conversion percentage for fatty acid ethyl ester and its oxidation product were 76 % and 72% respectively.
Characterization using FTIR for both ester showed that the absorption band of C=O ester functional group
at wave number 1731.27 cm
-1
and 1732.15 cm
-1
respectively. The emulsifier test showed that both esters
have ability to stabilize emulsion up to 24 hours for water-in-oil emulsion (w/o) type. Antimicrobial assay
showed that both esters have antimicrobial activity against both bacteria.
1 INTRODUCTION
Indonesia is a tropical country that presents a wide
range of beneficial biological plants that can grow in
it. One of the oil-producing plants that benefit the
wider community is Ricinus communis L. Ricinus
communis L. is an oil-producing plant (castor oil)
which contains triglyceride from a variety of fatty
acids which largest content is risinoleic acid, i.e 85-
95% of the total fatty acids (Gunstone et al.,2007).
Risinoleic acid is a long-chain fatty acid having 18
carbons which the ester form is known to have
strong antibacterial activity against Gram-positive
bacteria (Desbois and Lawlor, 2013) and used as
emulsifier in cosmetic industry (Cavalcante et al.,
2009).
Ricinoleic acid can be obtained by castor oil
hydrolysis in an alkaline solution. Ricinoleic acid
can also be esterified with ethanol (Hykkerud and
Marchetti, 2016). To achieve the equilibrium of an
esterification reaction that is so slow, catalyst is
required to accelerate it. Lipase was used in
enzymatic esterification using ethylene glycol at low
temperature and pressure (Chand et al., 1997).
In this research, the esterification between
ethanol and fatty acid obtained from the hydrolysis
of castor oil used Candida rugosa Lipase as catalyst.
Esterification also conducted between ethanol and
oxidized castor oil fatty acid. The ester products
were then examined as emulsifier and antibacterial
compound.
2 MATERIALS AND METHODS
2.1 Chemicals
The materials used in this study were ethanol, KOH,
ethanol 96%, concentrated HCl, NaOH 0.5 N,
aquades, Na
2
SO
4
anhydrous, n-hexane, phosphate
buffer pH 8, KI 15%, thiosulfate 0.01 N, amilum 1
%, KMnO
4
1M, phenolphthalein indicator, eosin
indicator, clindamycin antibiotics, sterile aquades,
DMSO, nutrient broth and nutrient agar,
Khairani, A., Hudiyono, S. and Handayani, S.
Enzymatic Esterification Ethyl Ester Fatty Acid from Hydrolyzed Castor Oil and Its Oxidation Product as Emulsifier and Antimicrobial Compound Using Candida rugosa Lipase E.C.3.1.1.3.
DOI: 10.5220/0008357200430049
In Proceedings of BROMO Conference (BROMO 2018), pages 43-49
ISBN: 978-989-758-347-6
Copyright
c
2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
43

Staphylococcus epidermidis and Propionibacterium
acnes cultures (obtained from Biochemistry Lab
Department of Chemistry, Universitas Indonesia).
Candida rugosa Lipase obtained from Sigma-
Aldrich.
2.2 Hydrolysis of Castor Oil
To get hydrolized castor oil fatty acid 100 g of
castor oil and 100ml KOH 5 M solution in 96%
were mixed. The mixture was heated in oil bath for 1
hour at 70
o
C with magnetic stirrer and was cooled
at room temperature. HCl 5M was added until pH 5-
4 (± 55 mL). The mixture was then allowed to stand
for 24 hours and will formed 2 phases. The upper
phase (organic phase) were separated and called
hydrolized fatty acids.
2.2.1 Oxidation of Fatty Acids
To 10 ml of hidrolyzed fatty acid 5 mL NaOH 0.5 M
and 2 mL of KMnO
4
was added and stir for 90 min
at 25
o
C. The solution was then left for 24 hours,
then filtered. The filtrate was added by 4 mL of
sulfuric acid. The Na
2
SO
4
anhydrate was added to
the organic phase of oxidized sample, and decanted.
2.2.2 Iod Numbers Test
Before and after oxidized, 0.3-0.4 g fatty acid was
mixed with 10 mL of chloroform and 10 mL of
Hanus solution. The solution was stored for 30
minutes in dark place. Furthermore, 10 mL of 15%
KI solution and 100 mL of aquadest were added.
The solution was titrated using 0.1 N sodium sulfate
solution to a yellow colour. The solution was added
1-2 mL of 1 % amilum solution and re-titrated with
0.1 N sodium sulfate solution until the colour turned
clear (Goud, 2006).
2.3 Esterification
2.3.1 Synthesis of Ethyl Ester Hydrolyzed
Castor Oil Fatty Acid and Its
Oxidation Products Using Candida
Rugosa Lipase
To get ester products fatty acid and the n-hexane,
Candida rugosa lipase as catalyst and ethanol were
mixed. Before mixing Candida rugosa was
dissolved with pH 8 phosphate buffer solution. The
mol ratio of fatty acid to etanol (respectively)
varians were 1: 1, 1: 2, 1: 3 , and 1: 4 (mol/mol). The
amount of solvent were used are 1:1 (v/v substrate in
each ratio). The 5% of the substrate total mass was
used as enzyme mass (w/w of each substrate ratio).
The incubation was conducted using horizontal
incubator shaker at 250 rpm and 37 °C for 18 hours.
To terminate the reaction, the mixture was heated in
a water bath at temperature of ± 80
o
C for 1-3
minutes. The same treatment was applied for
oxidized fatty acid ethyl ester.
2.3.2 Conversion Percentage Determination
Conversion percentage was calculated by using
titration method. Titrations were performed on an
organic phase (upper phase) which is a residual fatty
acid dissolved in n-hexane. 1 mL the organic phase
that has been separated after the centrifugation
process is transferred into a 10 mL measuring flask
and adjusted its volume with n-hexane. Then as
much as 1 mL aliquot was titrated with 0.1 N NaOH.
2.3.3 Identification Product Using FTIR
Esterification products, hydrolyzed castor oil fatty
acid, and oxidized fatty acids were identified using
FTIR.
2.4 Emulsifier Test and Determination
of Emulsion Type
Emulsifier test was carried by mixing water and oil
with a certain ratio according to Table 1. A total of
0.1 g of fatty acid ester were added into each
mixture, then shaken using a vortex for 30 seconds
to form an emulsion. Then the stability emulsion
was observed.
Table 1: Oil and Water Composition for Emulsions
Emulsion
Type
Water (ml)
Oil (drop)
Type 1
1
1
1
1
1
2
4
6
8
10
Water(drop)
Oil (ml)
Type 2
2
4
6
8
10
1
1
1
1
1
To determine the emulsion type, a drop of
emulsion and eosin was mixed on object glass. The
observation of emulsion type was performed under
microscope to determine an oil-in-water (o / w) oil
or water in oil (w / o) emulsion type.
2.5 Antimicrobial Activity Assay
Disc diffusion method was used as antimicrobial
activity assay. Aliquot 200 μL of P.acnes and
S.epidirmidis suspension with cell density 1x10
8
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
44
cells/mL was mixed with 20 mL nutriet agar in
sterile petri dish aseptically. The media was then
allowed to harden. Sterile disc paper with diameter
of 6 mm was placed on top of the medium and
dropped by 4 μL on sample. The incubation was
performed at 37 °C for 24 hours. Clindamycin
(0.5%) was used as positive control while DMSO as
negative control. The clear area around disc paper
was measured to determine antimicrobial activity.
The assay was performed on ethyl ester fatty acid
product with concentrations of 20%, 40%, 60%, and
80%.
3 RESULT AND DISCUSSION
3.1 Hydrolysis of Castor Oil
The hydrolysis process in this study was carried out
using an alkaline as catalyst in order to obtain fatty
acids from castor oil. The hydrolysis reaction with
an alkaline catalyst is irreversible, thereby resulting
in a higher fatty acid yield than the reversible acid
catalyst (Rifqy, 2016). To obtain free fatty acids, the
resulting soap was added by HCl. The fatty acid
obtained from this process was about 83.5%. The
hidrolized castor oil fatty acid was identified using
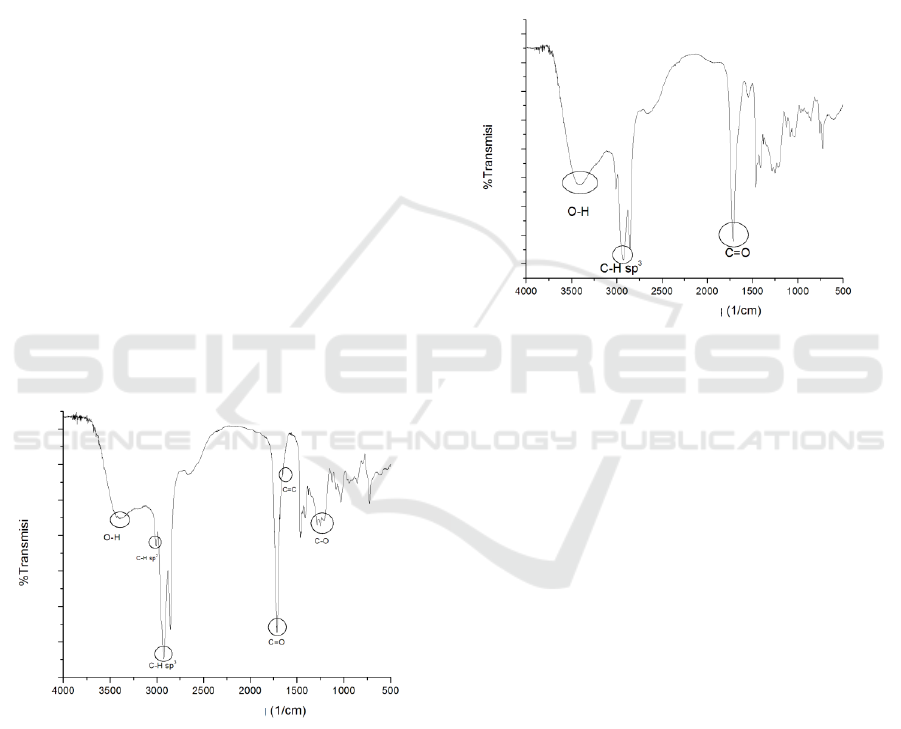
FTIR and the spectrum can be seen in Figure 1.
Figure 1: Castor Oil Hydrolyzed Fatty Acid Spectrum
The figure showed absorbsion at wave numbers
1725-1700 cm
-1
which indicate C=O carbonyl
uptake bands of carboxylic groups after hydrolysis
process. At wave number 1800-1650 cm
-1
appears
the absorption band for C=C accompanied by the
appearance of absorption band CH sp
2
vinyl at wave
number 3150-3000 cm
-1
(Janice, 2011).
3.1.1 Oxidation of Fatty Acids
The fatty acids obtained from castor oil
hydrolized have a double bond (C=C) which may
undergo a oxidation reaction. The oxidation process
was performed to increase the hydroxyl group into
the molecule by breaking the double bond (Pierre,
1994). The oxidation reaction using KMnO
4
at an
alkaline condition provides the oxidation product of
a diol compound (Marlina, 2004). The spectrum of
oxidized can be seen in Figure 2.
Figure 2: Oxidized Fatty Acid Spectrum
The loss of the absorption band for C=C and the
CH sp
2
absorption bands indicated that the fatty
acids had been oxidized to diols . The presence of
hydroxyl group of diols were shown with sharper
and wider absorbing bands for O-H.
The determination of the iodine is performed to
prove that the fatty acid had been oxidized. In this
study showed that iodine number on hydrolyzed
fatty acid is 10 mg/g while the oxidized fatty acids
of 1.5 mg/g. It indicated that the oil has lost its
C=C and the oxidation was successful.
3.2 Esterification
Candida rugosa Lipase can catalyze the
esterification reaction under a slight water condition,
called essential water. Enzyme need water to
perform its catalytic activity called essential water
(Zaks and Klibanov, 1988). The essential water in
this study was obtained from pH 8 phosphate buffer.
The use of alcohol in larger quantities may induce a
reaction towards ester formation (Pandey et al.,
1999). In order to optimize the esterification
reaction, the reaction equilibrium is shifted to the
right direction to the formation of ester. In this
research, ethanol was used in excess amount. The
Wavenumbers
Wavenumber
s
Enzymatic Esterification Ethyl Ester Fatty Acid from Hydrolyzed Castor Oil and Its Oxidation Product as Emulsifier and Antimicrobial
Compound Using Candida rugosa Lipase E.C.3.1.1.3
45
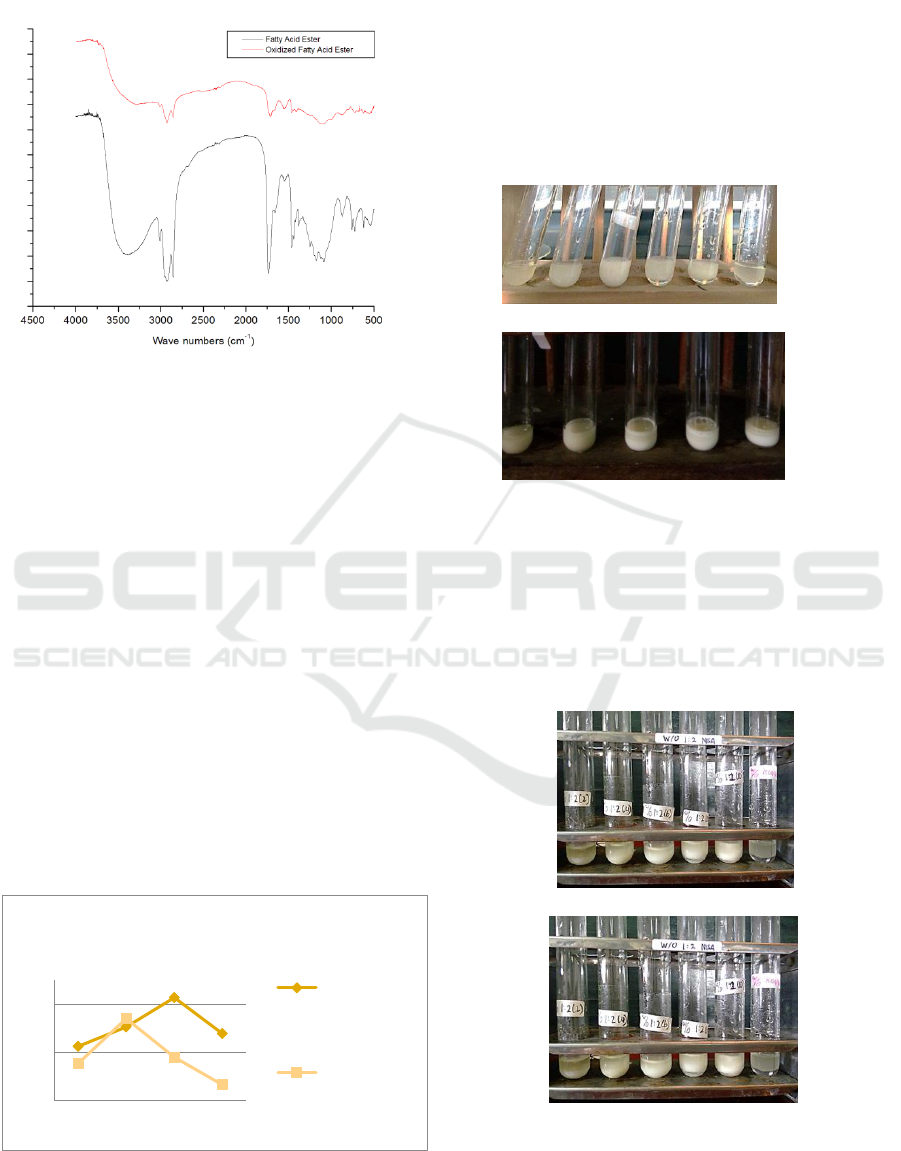
ester was identified using FTIR and the spectrum
can be seen in Figure 3.
Figure 3: Esters Spectrum
The figure shows the presence of the C = O ester
absorption bands at the wave numbers 1735-1750
cm
-1
for both types of esters. This bands shows a
shift to greater wave numbers than fatty acid and
oxidized fatty acid form, this wave number is also
the typical wave number for C=O groups of ester.
The differences between these two spectrum are the
absence of C=C and C-H vinyl absorbtion bands for
oxidized fatty acid esters that appear at wave
numbers of 1652.48 cm
-1
and 3009.73 cm
-1
(Janice,
2011).
3.2.1 Conversion Percentage
For the castor oil hydrolyzed fatty acids has the
highest convertion value at 1:3 composition with
conversion percentage value of 76.31% and the
oxidized fatty acid has the highest conversion
percentage value at 1:2 composition with the value
of 72% (Figure 4).
Figure 4: Konversion Percentage Ester Fatty Acid
3.3 Emulsifier Test
The ester product has a polar group on one side and
a non-polar group on the other side, allowing the
ester to have properties as an emulsifier. Emulsifier
test for castor oil hydrolyzed fatty acid did not
produce emulsifier properties, whereas for its
oxidation product it gives properties as emulsifier
after 24h. This emulsifiers can be seen in Figure 5.
(a)
(b)
Figure 5 : Emulsifiers, (a) Castor Oil Hidrolized Fatty
Acid ; (b) oxidized fatty acid
In this study, these two fatty acid esters provide
properties as emulsifiers but for the oxidized fatty
acid ethyl ester provide more stable emulsion for
48h. This emulsifier test can be seen in Figure 6.
(a)
(b)
Figure 6: Emulsifiers ester, (a) Castor Oil Hidrolized Fatty
Acid ; (b) oxidized fatty acid
66.22
70.26
76.31
68.9
62.7
72
64
58.3
55
65
75
1;1 1;2 1;3 1;4
%Konversion
Hidrolyzed
Castor Oil
Fatty Acid
Oxidized
Fatty Acid
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
46

This indicated that ester of oxidized fatty acid
has stronger ability as emulsifier, due to addition of
hydroxyl groups to the fatty acid structure that
increase its polarity. The increasing polarity is able
to reduce the surface tension between 2 types of
polar and non-polar solutions (in this case water and
oil) (Arbianti, et al., 2009).
The type of emulsion was determined
qualitatively under microscope observation. Figure 7
shows microscopic photograph of emulsion. The red
part is eosin dissolved in water while the yellow part
is the oil phase. In this study, the esters has water in
oil emulsion type.
Figure 7: The Observed Emulsion Type with Microscope
3.4 Antimicrobial Activity Assay
Antimicrobial activity is shown by clear areas
around paper disc that indicate the inhibition of
microorganism growth by antimicrobial agents
(Pratiwi, 2008). The stronger the antibacterial
activity of the compound, the greater the diameter of
clear zone. Based on this research, it was shown that
the oxidized fatty acid, the oxidized fatty acid etyl
ester at concentration of 40% and 80% had the
highest antibacterial activity. All the data of
antibacterial activity assay can be seen in Table 3.
Table 2 show effectivity parameters for
antimicrobial compound.
Table 2: Effectivity parameters for antimicrobial
compound (Greenwood, 1995)
Diameter of clear
zone
Classification
> 20mm
Strong
16-20 mm
Medium
10-15 mm
Weak
< 10 mm
No Effective
The ability of a monoglyceride as an
antimicrobial agent is related to the level of
solubility of the compound in water so that it can
dissolve in an aqueous environment, and its
hydrophobicity properties so as to interact with the
cell membrane structure composed of lipid bilayer.
The longer the chain of C atoms, the more non polar
the compound, so the solubility in the water
decreases (Widiyarti et al, 2009). The oxidized fatty
acid has a polar group at the diol group when it
oxidizes the C=C chain. Oxidized fatty acids and its
ester product showed greater activity than its fatty
acid form because they have a stronger polar side
and a longer non-polar side. The antimicrobial
activity assay can be seen in Figure 8.
(a) (b)
Figure 8: Antimicrobial, (a) Staphylococcus epidermidis;
(b) Propionibacterium acnes
Enzymatic Esterification Ethyl Ester Fatty Acid from Hydrolyzed Castor Oil and Its Oxidation Product as Emulsifier and Antimicrobial
Compound Using Candida rugosa Lipase E.C.3.1.1.3
47

Table 3: Inhibition Zone
Sample
Inhibition Zone (mm)
Classification
P. acnes
S. epidirmidis
Ethyl Ester Hidrolyzed Castor Oil
Fatty Acid (20%)
3
10
Weak
Ethyl Ester Hidrolyzed Castor Oil
Fatty Acid (40%)
X
7
No Effective
Ethyl Ester Hidrolyzed Castor Oil
Fatty Acid (60%)
X
8
No Effective
Ethyl Ester Hidrolyzed Castor Oil
Fatty Acid (80%)
X
X
No Activity
Oxidized Fatty Acid Ethyl Ester
(20%)
12
13
Weak
Oxidized Fatty Acid Ethyl Ester
(40%)
17
13
Medium
Oxidized Fatty Acid Ethyl Ester
(60%)
14
12
Weak
Oxidized Fatty Acid Ethyl Ester
(80%)
12
17
Medium
Oxidized Fatty Acid
15
17
Medium
Ethanol
11
14
Weak
Hidrolyzed Castor Oil Fatty Acid
(50%)
12
14
Weak
Hidrolyzed Castor Oil Fatty Acid
(100%)
13
15
Weak
Klindamisin (500ppm)
11
20
High
N- Heksane
X
X
No Activity
Castor Oil (50%)
X
X
No Activity
Castor Oil (100%)
X
X
No Activity
DMSO
X
X
No Activity
4 CONCLUSIONS
The synthesis of etyl ester castor oil hydrolyzed
fatty acid and its oxidation product using Candida
rugosa lipase was successfully performed. It was
shown by the presence of C=O ester group band of
FTIR spectra. Etyl ester castor oil hydrolyzed fatty
acid and its oxidation product have activity as
emulsifier with water in oil emulsion type. Oxidized
fatty acid ethyl ester at concentration of 40% has the
highest antimicrobial activity against
Propionibacterium acne bacteria and oxidized fatty
acid ethyl ester at 80% has the highest antimicrobial
activity againts Staphylococcus epidermidis bacteria.
ACKNOWLEDGEMENTS
This work was funded by Hibah Kompeteni
Publikasi Internasional Terindeks Untuk Tugas
Akhir Mahasiswa (PITTA), Universitas Indonesia
2018
REFERENCES
Arbianti, R., Utami, T dkk. (2009). Transesterifikasi
Parsial Minyak Kelapa Sawit dengan Etanol pada
Pembuatan Digliserida sebagai Agen Pengemulsi.
Jurnal Teknik Kimia Indonesia, Vol. 8 No. 1 April
2009, 33-37
Cavalcante, K.S.B. et al., (2009). Optimization of
tranesterification of castor oil with ethanol using
central composite rotatable design
Chand, Subhash et al . (1997). Lipase-catalyzed
esterification of ethylene glycol to mono- and diesters.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
48

The effect of process parameters on reaction rate and
product distribution. J . Enzyme and microbial
Technology Elsevier
Desbois, A., Lawlor, K. (2013). Antibacterial Activity of
Long-Chain Polyunsaturated Fatty Acids against
Propionibacterium acnes and Staphylococcus aureus.
Mar. Drugs 2013, 11, 4544-4557;
doi:10.3390/md11114544
Goud, V.V et al., (2006). Epoxidation of Karanja
(pongamia glabra) Oil by H
2
O
2
, J. of the American Oil
Chemists’ Society
Greenwood. (1995). Antibiotic susceptibility (sensitivity)
test, antimicrobial and chemotherapy. USA: Mc
Graw Hill Company.
Gunstone, F.D., Harwood, J.L., Dijkstra, A.J., (2007). The
Lipid handbook, third ed. CRC Press,Boca Raton
Hykerud, A., Marchetti, J.M., (2017). Esterification of
oleic acid with ethanol in the presence of Amberlyst
15. J. of Supercritical Fluids 126 (2017) 25-36
Janice. (2011). Organic Chemistry Third Edition. The
McGraw-Hill Companies Inc, New York
Marlina, N dkk. (2004). Pengaruh Konsentrasi Oksidator
pada Proses Hidroksilasi Minyak Jarak (Castor Oil)
Dengan atau Tanpa Proteksi Gugus Hidroksi. PROC.
ITB Sains & Tek. Vol. 36 A, No. 1, 2004, 33-43
Pandey A, Benjamin S, Soccol CR, Nigam P, Krieger N,
Soccol VT. (1999). Review: the realm of microbial
lipases in biotechnology. Biotechnol Appl Biochem
Pierre L., (1994). Organic Reaction, Simplicity, and Logic,
Wiley and Son, Singapore (1994).
Pratiwi S.T. (2008). Mikrobiologi Farmasi.Penerbit
Erlangga.Jakarta
Widiyarti, Galuh; Hanafi, Muhammad; Soewarso,
Wahyudi.(2009). Kajian Awal Sintesis Monolaurin
sebagai Antibakteri Staphylococcus aureus. Indo. J.
Chem., 2009, 9 (1), 99 - 106
Zaks, Aleksey; Klibanov, Alexander.(1988). Enzyme-
catalyzed processes in nonaqueous solvents. Vol. 263,
pp. 3194-3201, 1988. USA
Enzymatic Esterification Ethyl Ester Fatty Acid from Hydrolyzed Castor Oil and Its Oxidation Product as Emulsifier and Antimicrobial
Compound Using Candida rugosa Lipase E.C.3.1.1.3
49
