
The Effects of Additional Treatment of Activated Coal on the
Increase of Special Characteristics of Gas Diffused Cathods of
Aluminum-air Electrochemical Generator
Elena Alexandrovna Kiseleva
*
, Andrey Zinov’yevich Zhuk, Kleymenov Boris Vladimirovich and
Victor Petrovich Zakharov
Joint Institute for High Temperatures of the Russian Academy of Sciences, Izhorskaya st.13 Bd. 2, Moscow, Russia 125412
Keywords: Carbon materials, iron-cobalt-nitrogen-containing pyrocatalyst
Abstract: The possibility of a significant increase in the specific characteristics due to the treatment of carbon
materials with ammonia is shown in the work. In the course of the work, techniques were developed for
processing a carbon material with aqueous ammonia and pure ammonia, a procedure for the synthesis of an
iron-cobalt-nitrogen-containing catalyst. It has been established that the additional processing of carbon
materials with ammonia improves the characteristics of the experimental samples due to the deposition of
nitrogen-containing functional groups on the surface: for activated carbon from coal raw materials brand
UAF treated at 900 ° C, a current density of 0.041 mA / cm
2
is achieved at a polarization of -0.3 V. It is
shown that the application of iron- cobalt-nitrogen-containing pyrocatalyst synthesized on a activated
carbon brand UAF well catalyzes the oxygen reduction reaction.
1 INTRODUCTION
Currently, much attention is paid to solving the
problems of creating environmentally friendly
autonomous current sources with high energy
intensity for use in various portable electronic
devices, in transport and in electric power industry,
which is caused by environmental pollution by using
hydrocarbon fuels, whose share in large cities is over
90% (Zhuk et al., 2012).
Metal-air energy sources based on aluminum are
promising because they have a high theoretical and
feasible specific energy intensity (250-400 Wh / kg),
low cost, they are environmentally friendly and fire-
proof. Currently, the specific power of air-aluminum
elements is limited by the specific power of the air
electrode (gas diffusion cathode). When discharging
air-aluminum elements with an alkaline electrolyte
at a working temperature of 60 ° C, the current
density at the aluminum anode can reach 1000 mA /
cm
2
, while the current density at the gas diffusion air
cathode is several times lower (150-300 mA / cm
2
).
The most critical and complex component of an Al-
air element is a gas diffusion cathode, which is
responsible for the reduction of air oxygen, and
determines the specific power and working life of
the current source.
The reduction of oxygen can be carried out in
two parallel ways (Bidault et al., 2009; Cheng and
Chen, 2012):
a) by a four-electron reaction to a hydroxide ion:
O
2
+ 2H
2
O + 4e- = 4OH
-
E
0
O
2
/ OH- = + 0.401 V,
b) by a two-electron reaction to hydrogen
peroxide:
O
2
+ H
2
O + 2e- = HO
2
-
+ OH
-
E
0
= -0.076 V
The resulting hydrogen peroxide, depending on
the properties of the catalyst, is then either reduced
to OH
-
or decomposed into oxygen and water.
In order to intensify the process of oxygen
reduction, it is carried out in a porous,
hydrophobized electrode. The efficiency of such an
electrode is determined both by the activity of the
catalyst used and the porous structure formed during
the hydrophobization process, which should have an
optimal ratio of hydrophobic and hydrophilic pores
(Chervin et al., 2012).
In addition to the catalytically active layer, the
GDС, as a rule, contains a hydrophobic layer, the
task of which is to prevent the electrolyte from
getting wet on the back side of the cathode and to
34
Kiseleva, E., Zhuk, A., Vladimirovich, K. and Zakharov, V.
The Effects of Additional Treatment of Activated Coal on the Increase of Special Characteristics of Gas Diffused Cathods of Aluminum-air Electrochemical Generator.
DOI: 10.5220/0008185000340037
In The Second International Conference on Materials Chemistry and Environmental Protection (MEEP 2018), pages 34-37
ISBN: 978-989-758-360-5
Copyright
c
2019 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

ensure diffusion of air to the active layer.
In this paper, various methods of increasing the
electrochemical activity of activated carbons used as
catalysts for the reduction of oxygen on gas
diffusion cathodes are investigated. One of such
methods is the additional treatment of activated
carbon with activated carbon from coal raw
materials brand UAF (UAF) with ammonia in order
to saturate the carbon surface with nitrogen-
containing functional groups. Another method is the
synthesis of a nitrogen-containing catalyst based on
ethylenediamine and salts of transition metal (Co,
Fe) treated with ammonia, followed by pyrolysis of
the resulting product. Figure 1 presents a
hypothetical scheme of an iron-cobalt-nitrogen-
containing pyrocatalyst.
Figure 1: Estimated scheme of iron-cobalt-nitrogen-
containing pyrocatalyst.
2 EXPERIMENTAL TECHNIQUE
A three-layer gas diffusion cathode consists of a
nickel grid, a barrier layer based on acetylene black
and an active layer based on: 1- UAF coal, 2- UAF
coal with additional treatment in ammonia at 350 °C
for 3 hours, 3- UAF coal with additional treatment in
an atmosphere of ammonia at 350 ºС for 3 hours
with the addition of the catalyst 15% TMPPCo/
Vulcan XC72.
The catalyst was synthesized by pyrolysis of
TMPPCo (tetra- (p-methoxyphenyl) -porphyrin
cobalt), adsorbed on Vulcan XC72, at 800 °C in
argon atmosphere for 1 hour. The active layer was
obtained by calendering a mixture of catalyst/
fluoroplastic (9: 1 by weight), pressing with a nickel
mesh and a barrier layer, having in its composition
35% fluoroplastic.
The test of the cathode was carried out in a three-
electrode cell. A silver chloride (Ag | AgCl)
electrode was used as a reference electrode, and 8 M
NaOH as an electrolyte. Air purified from CO
2
was
supplied to the cathode. Polarization measurements
were performed by the potentiodynamic method, the
potential sweep rate was 1 mV / s. The potential
transient was measured by the galvanostatic method
at a current density of 200 mA / cm
2
. The values of
the potentials are given in the scale of the normal
hydrogen electrode with regard to pH and
temperature. The test of the laboratory layout of the
Al-air element was carried out in a special cell
(Figure 2).
Figure 2: Electrochemical cell diagram: 1-working
electrode, 2-reference electrode, 3-thermometer, 4-
auxiliary electrode, 5-warm water supply tube, 6-heater, 7-
warm water drain tube, 8-capacitance with electrolyte.
The anode material was Al-In alloy (A99+0.45%
In), which is more stable in alkaline electrolyte than
Al. The composition Na
2
SnO
3
sodium stannate (0.1
M) was introduced with the electrolyte to reduce
chemical corrosion of the alloy. Heated to 60º C, the
electrolyte was continuously circulated through the
working space of the cell, whose width was 3 mm.
The geometric surface of the electrodes was 8 cm
2
.
3 RESULTS AND DISCUSSION
At the first stage, the dependence of polarization
curves for raw coal UAF and coal UAF treated with
water vapor and ammonia without catalyst and with
a supported catalyst was investigated (Table 1).
The Effects of Additional Treatment of Activated Coal on the Increase of Special Characteristics of Gas Diffused Cathods of Aluminum-air
Electrochemical Generator
35
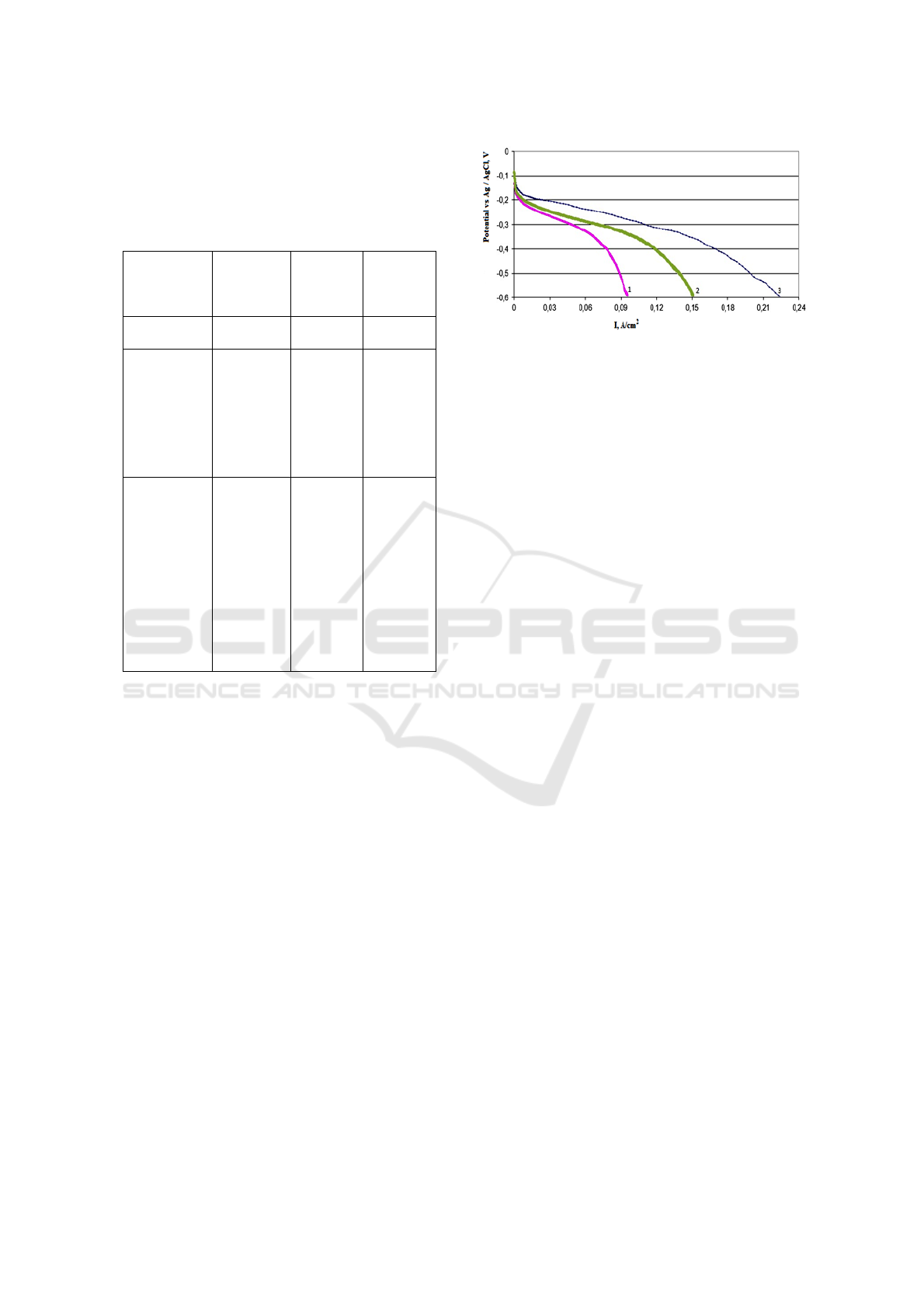
Table 1: Data of open-circuit potential and current density
at different polarization for UAF coal, UAF coal
additionally treated in the atmosphere of ammonia and
coal UAF with additional treatment in the atmosphere of
ammonia with the addition of a catalyst 15% TMPPCo/
Vulcan XC72.
Active layer
material
Open-circuit
potential, V
Current
density at
0.3 V
(A /cm
2
)
Current
density at
0.4 V
(A /cm
2
)
activated
carbon UAF
-0,159
0,025
0,078
UAF treated
with aqueous
ammonia at
350 ° C for 3
hours (UAF in
NH
3
+ H
2
O 350
° C for 3 hours)
-0,131
0,048
0,074
UAF is treated
with aqueous
ammonia at
350 ° C for 3
hours +
pyrolysis of the
catalyst at 800
° C (UAF in
NH
3
+ H
2
O
350 ° C for 3
hours, at a 800
° C for 1 hour)
-0,111
0,076
0,108
Additional treatment with ammonia and
pyrolysis of the catalyst has a positive effect on the
characteristics of the open-circuit potential. For
UAF, the current-free potential is -0.159 V, and for
UAF it is treated with aqueous ammonia at 350 ° C
for 3 hours and the iron-cobalt-nitrogen-containing
catalyst pyrolyzed on it at 800 ° C is -0.111 V.
With the additional treatment of carbon material
with UAF with ammonia at 350 ° C for 3 hours, the
values of current density increase by 0.023 A / cm
2
,
as compared to untreated UAF.
The addition of a synthesized catalyst with
pyrolysis at 800 ° C to the treated coal in an
ammonia atmosphere also has a positive effect on
the current density values.
Figure 3 shows the polarization curves of air
cathodes.
Figure 3: Polarization curves of air cathodes from: 1- UAF
coal, 2- UAF coal with additional treatment in ammonia at
350 ° C for 3 hours, 3- UAF coal with additional treatment
in an atmosphere of ammonia at 350 ºС for 3 hours with
the addition of the catalyst 15% TMPPCo / Vulcan XC72.
Synthesis of catalytically active materials
produced specifically for electrocatalysis oxygen
reaction is carried out in two principally different
methods. First, the most commonly used method is
based on modifying the carbon or other carrier
precursors of various types, including metals and
nitrogen, followed by pyrolysis or without it. The
literature describes many techniques of this method
from simple precursors coadsorption before the
synthesis in pairs (Wood et al., 2008) or plasma (Olson
et al., 2013). However, regardless of the synthesis
procedure for the first method of catalytically inert
carrier is retained, but with doped surface having a
pronounced catalytic activity. The second method is
a meaningful synthesis of substantially new
catalytically active material uglepodobnogo
(Charreteur et al., 2008; Wu et al., 2011) modified by
the atoms (N, Co, Fe, etc.) Which may be included
in the alleged active centers. After introduction of
the particulate carbon in (Charreteur et al., 2008) or ion
exchange (Lefevre and Dodelet, 2012) is carried
material necessary precursor pyrolysis and deep
activation system in ammonia atmosphere to a
weight loss of 60 ÷ 80%. This method allows the
synthesis of the catalyst system with a high volume
concentration of active centers, which we got as a
result of our research work.
4 CONCLUSIONS
1. In the course of the work, methods of processing
carbon material in an ammonia atmosphere, a
method of synthesis of a pyrocatalyst, were
developed.
2. Experimental samples of air gas diffusion
cathodes were made from the obtained carbon
MEEP 2018 - The Second International Conference on Materials Chemistry and Environmental Protection
36

materials, and galvanostatic and galvano-dynamic
characteristics of experimental samples of cathodes
were investigated.
3. The analysis and comparison of the results
obtained for various groups of carbon materials
studied was carried out in order to determine the best
characteristics for gas diffusion cathodes.
ACKNOWLEDGEMENTS
The study was supported by Program no. 56 of the
Presidium of the Russian Academy of Sciences
“Fundamental principles of breakthrough
technologies in the national security interests.”
REFERENCES
Bidault, F., Brett, D.J.L., Middleton, P.H., Brandon, N.P.,
2009. J. Power Sources. V. 187. P. 39-48.
Charreteur, F., Ruggeri, S., Jaouen, F., Dodelet, J.P. 2008.
Electrochim. Acta. V. 53. P. 6881-6889.
Cheng, F., Chen, J., 2012. Chem. Soc. Rev. V. 41. P.
2172-2192.
Chervin, C.N., Long, J.W., Brandell, N.L. et al., 2012. J.
Power Sources. V. 207. P. 191-198.
Lefevre, M., Dodelet, J.-P., 2012. ECS Trans. J.-P. V. 45
(2). P. 35-44.
Olson, T.S., Dameron, A.A., Wood, K., Pylpenko, S.,
Hurst, K.E., Christensen, S., Bult, J.B., Ginley, D.S.,
O’Hayre, R., Dinh, H., Gennett, T. 2013. J.
Electrochem. Soc. V. 160 (4). P. F389-F394.
Wood, T.E., Tan, Z., Schmoeckel ,A.K., O’Neill D.,
Atanasoski, R. 2008. J. Power Sources. V. 178. P.
510-516.
Wu, L., Nabae, Y., Kuroki, S., Kakimoto, M., Miyata, S.,
2011. ECS Trans. V. 41 (1). P. 2313-2323.
Zhuk, A.Z., Kleimenov, B.V., Fortov, V.E., Sheindlin,
A.E., 2012. Electric car on aluminum fuel. Nauka,
p.171.
The Effects of Additional Treatment of Activated Coal on the Increase of Special Characteristics of Gas Diffused Cathods of Aluminum-air
Electrochemical Generator
37
