
Osteogenic Responses of Murine Pre-osteoblastic Cells to
Anodized TiO
2
Surfaces
X H Piao
1
, E R Na
2
, J W Moon
2
and Y J Kim
2, *
1
Department of Dentistry, Affiliated Hospital of Yanbian University, Yanji, Jilin,
China
2
Department of Periodontology, School of Dentistry, Chonnam National University,
33 Yongbong-ro, Buk-gu, Gwangju 61186, Republic of Korea
Corresponding author’s e-mail: Y J Kim,youngjun@chonnam.ac.kr
Abstract. Anodic oxidation of titanium surfaces by electrochemical method is one of
methods for enhancement of osseointegration. This study was to evaluate the surface
characteristics and cell response of titanium samples modified by different treatment
conditions. The samples were divided into 4 groups. Group I was anodized TiO
2
film using a
constant voltage, 270 V for 30 seconds. Group II was anodized TiO
2
film using a constant
voltage, 270 V for 60 seconds. Group III was anodized TiO
2
film using a constant voltage,
270 V for 90 seconds. The results were as follows; XRD analysis showed that the crystallinity
of anodic oxide film was composed of anatase and rutile. With increasing time for
anodization, the intensity of the TiO
2
peaks for anatase phase decreased, while TiO
2
peak for
rutile phase increased. In MTT assay, there was no significant difference in the response of
fetal rat calvarial cells to anodized titanium surfaces with different treatment conditions. The
group II and III showed higher ALP activity levels compared with control and group I
(p<0.01). These results suggest that anodized TiO
2
surfaces treated at 60 and 90 seconds
should promote cellular activity of osteoblasts compared with machined Ti surface.
1. Introduction
Several techniques have been used to produce micro-rough Ti surfaces for promoting bone ingrowth
and fixation between implants and bone. Among them surface blasting, acid-etching and combination
of both are widely used methods to modify surface topography. In addition to surface topography,
surface chemistry is also important for peri-implant bone apposition. Thin native oxide films formed
on Ti surface spontaneously (1.5-10 nm), and titanium dioxide (TiO
2
) forms a direct bond to bone
tissues. However, the layer of naturally formed film is too thin to prohibit toxic metal ions such as
aluminum and vanadium being released in the human body and inducing possible cytotoxic effect
and neurological disorders [1]. Surface modifications are, therefore, indispensable for titanium and
its alloys to form a thick surface oxide layer for enhanced corrosion and a bioactive layer for
apposition and growth of bone cells. Anodic oxidation of titanium surfaces by electrochemical
method is one of the methods for solving above problems [2, 3]. After anodic oxidation, titanium-
based metals form bone-like apatite in simulated body fluid (SBF) which has ion concentrations
442
Piao, X., Na, E., Moon, J. and Kim, Y.
Osteogenic Responses of Murine Pre-osteoblastic Cells to Anodized TiO2 Surfaces.
In Proceedings of the International Workshop on Materials, Chemistry and Engineering (IWMCE 2018), pages 442-449
ISBN: 978-989-758-346-9
Copyright © 2018 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

nearly equal to human body fluid. This phenomenon also occurs on the surfaces of bioactive glass
and glass ceramics.
Research showed that high degree of bone contact and bone formation was achieved with
anodized titanium surface [4]. Saldana et al. reported that the ability of human osteoblasts to
differentiate when cultured on thermally oxidized titanium alloy [5]. The characteristics of anodized
Ti surface varied according to the anodizing conditions such as duration time and electrolytic
compositions used. And anodized Ti surface characteristics, such as surface morphology and
thickness, may affect many cellular responses such as cell adhesion, morphology, proliferation, and
differentiation. However, there is no comprehensive study with regard to interaction of osteoblasts
with anodized titanium with different treatment conditions. Thus, the purpose of this study was to
evaluate the surface characteristics and cell response of titanium samples modified by different
treatment conditions.
2. Materials and methods
2.1. Preparation and characterization of Ti Disks
2.1.1. Fabrication of anodized titanium surfaces. All specimens were kindly provided by the school
of Materials Science and Engineering, Chonnam National University. Briefly, all commercially pure
titanium (grade II, cp-Ti) disks were formed into disks 12 or 25 mm diameter and 1 mm thickness.
These disks were ultrasonically degreased in acetone and ethanol for 10 minutes each, with deionized
water rinsing between applications of each solvent.
The samples were then divided into 4 groups. Group I was anodized TiO
2
film using a constant
voltage, 270 V for 30 seconds. The disks were anodized using pulse power (650 Hz). The electrolyte
solution contained 0.15 M calcium acetate and 0.02 M calcium glycerophosphate. Group II was
anodized TiO
2
film using a constant voltage, 270 V for 60 seconds. Group III was anodized TiO
2
film
using a constant voltage, 270 V for 90 seconds. The control was non-treated machined titanium
surface. The surface morphology of anodized Ti disks and their cross-sections were observed by
scanning electron microscopy (SEM; S-4700, Hitachi, Japan). The surfaces of anodized Ti disks were
examined with x-ray diffractometer (XRD; DMAX/1200, Rigaku, Japan).
2.1.2. Evaluation of corrosion resistance
The samples for corrosion test were embedded in a room temperature curing epoxy resin leaving an
exposure area of 10 × 1 mm
2
. The control and test groups were exposed to the electrolyte. The
electrolyte used was a phosphate buffered saline (PBS) at a room temperature. A three-electrode cell
set-up was used with a saturated calomel electrode (SCE), a platinum wire as reference, and a counter
electrode. A potentiodynamic polarization scan using a frequency response analyzer (Gamry model
EIS 300, USA) coupled to a potentiostat PCI4/300, was acquired following 7 days of immersion in
PBS.
2.2. Cell culture and cell analysis of cell response
2.2.1. Cell cultureof fetal rat calvarial cells. Osteoblast-enriched cell preparations were obtained
from Sprague-Dawley 21 day fetal calvaria by sequential collagenase digestion. The periosteum from
newborn calvaria was removed and bone tissue was cut into small pieces with scissors. The pieces of
calvarial bone were then digested with the mixture of enzyme containing of 0.5% type II collagenase
(Type II; Invitrogen, USA) in phosphate buffered saline at 37°C. During sequential digestion period
of 15 minutes, the cells from the 3rd to the 5th digestion were pooled and filtered with 200μM plastic
meshed screen and plated in 75 mm tissue culture plastic. Cells were cultured in BGJb media (Life
Osteogenic Responses of Murine Pre-osteoblastic Cells to Anodized TiO2 Surfaces
443

Technologies, USA) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 mg/㎖
penicillin, and 100 mg/㎖ streptomycin at 37°C humidified atmosphere of 5% CO
2
-95% air.
2.2.2. Cell viability test. Cells were cultured on machined Ti and three anodized TiO
2
surfaces in 12-
well plates at a density of 1☓ 10
5
cells/㎖ with BGJb medium. At day 3, cell proliferation was
assessed by MTT assay (CellTiter 96 AQueous, Promega, USA). Fomazan accumulation was
quantified by absorbance at 490 nm by an enzyme-linked immunoabsorbant assay (ELISA) plate
reader (microplate manager, BioRad, USA) and analyzed. All experiments were carried out in
triplicate.
2.2.3. Alkaline Phosphatase (ALP) activity. For this purpose, fetal rat calvarial cells were seeded on
machined Ti and three anodized TiO
2
surfaces in 12-well plates at a density of 1☓10
5
cells/㎖ with
BGJb medium, containing 10% FBS. Determination of ALP activity was performed at day 7. Briefly,
cells were lysed in Triton 0.1% (Triton X-100) in PBS, then frozen at -2°C and thawed. One hundred
microliter of cell lysates was mixed with 200 ㎕ of 10 mM p-nitrophenol phosphate and 100 ㎕ of
1.5 M 2-amino-2-methyl-1-propanol buffer, and then incubated for 30 minutes at 37°C. ALP activity
was measured by absorbance reading at 405 nm by ELISA reader. All experiments were carried out
in triplicate.
2.3. Statistical analysis
An analysis of variance followed by Duncan's test was used to assess the data regarding surface
roughness, cell proliferation and ALP activity. Statistical significance was defined as p<0.01(SPSS
20.0, SPCC Inc., USA).
3. Results and discussion
3.1. Surface characterization and roughness test
Because the mechanical characteristics of anodized TiO
2
films are depends on its treatment
conditions, the anodization time and voltage were very important. Based on the preliminary study, it
was established the anodization voltage of 270 V and time for fabrication of anodized TiO
2
films
went up to 90 seconds. The surface roughness of anodized TiO
2
surfaces was 0.25 ㎛, 0.28 ㎛, 0.34
㎛, respectively. The surface roughness had a tendency to increase when the anodizing time was
increased. The surface roughness of control was 0.27 ㎛. There was no significant difference among
groups. Figure 1 showed SEM images of anodized Ti surfaces. The anodic oxide films showed many
overlapping micropores and microprojections. The anodic films were relatively uniform in the
thickness. The thickness of anodic oxide films was 1.32 ㎛, 1.82 ㎛, and 2.54 ㎛ respectively. The
size and thickness of micropores increased as the anodizing time was increased. XRD analysis
showed that the crystallinity of anodic oxide film was composed of anatase and rutile. Figure 2 was
shown that the intensity of the TiO
2
peaks for anatase phase decreased, while TiO
2
peak for rutile
phase increased as increasing time for anodization. Therefore, as the anodizing time was increased,
TiO
2
film was composed of rutile phase rather than anatase.
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
444
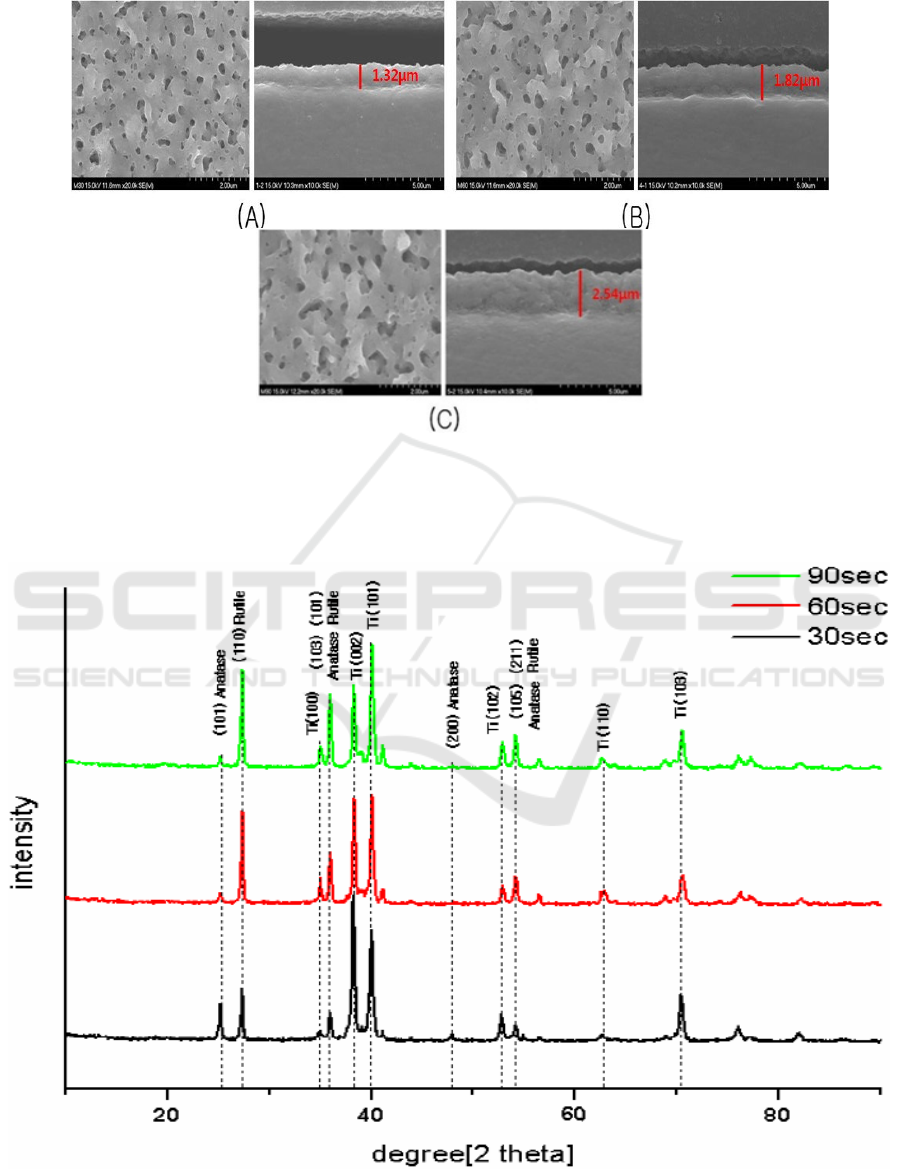
Figure 1. Overview and cross-sectional view of SEM images of anodized TiO
2
films with different
treatment conditions. The surface roughness of anodized TiO2 increased 0.25 ㎛ and 0.34 ㎛,
However, there was no significant difference among groups
(A) Group I (B) Group II (C) Group III.
Figure 2. X-Ray diffraction patterns of anodized titanium in different treatment conditions.
Osteogenic Responses of Murine Pre-osteoblastic Cells to Anodized TiO2 Surfaces
445
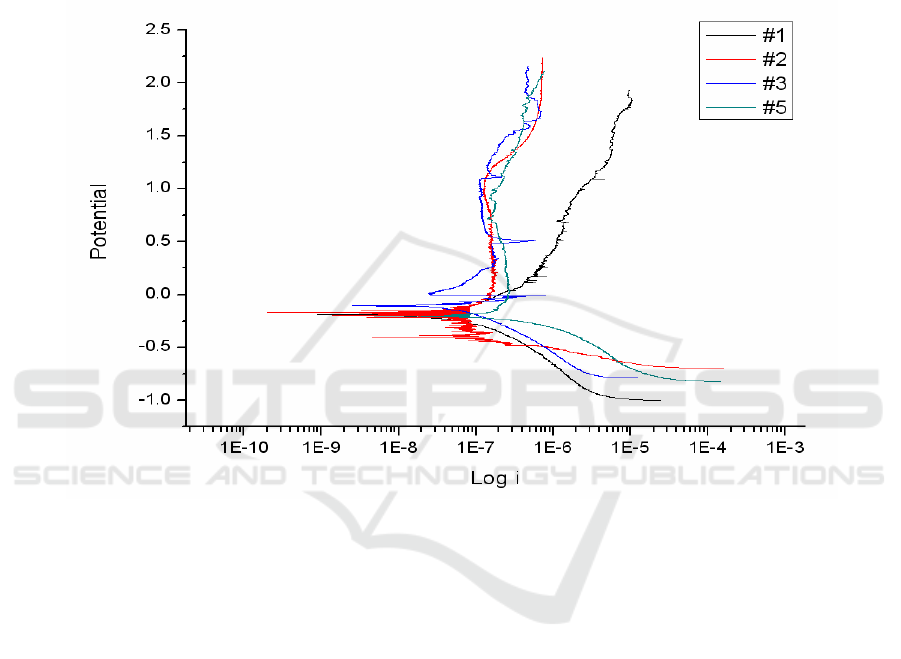
3.2. Evaluation of corrosion resistance
In order to evaluate the corrosion protection by anodized TiO
2
films, potentiodynamic polarization
test was performed. The polarization curves for anodized TiO
2
films were shown in Figure 3. The
corrosion protection of TiO
2
films was not increased by anodization. By comparison with the
anodized TiO
2
films, there was no difference among different treatment conditions. Although
corrosion protection of three test groups was not enhanced by anodization, both the control and test
groups formed stable passive layer on titanium surfaces. Thus, the result of corrosion test of titanium
substrate proved the protective role of anodized TiO
2
films in this study.
Figure 3. Potentiodynamic polarization curves for anodized titanium in different treatment
conditions. #1 the control, #2 Group I, #3 Group II, #5 Group III.
3.3. SEM images of cells on anodized Ti surfaces
Under SEM, cells adhered and grew well on the surfaces of all groups. No difference in cell
morphology was observed in anodized Ti surfaces without regard to anodizing time. The cells
spreaded extensively and totally flattened on all anodized Ti surfaces. They were in polygonal shapes
and individual cells were flat in appearance (Figure 4). SEM study have provided some insight into
the cell response to surface chemistry and morphology. In this study, it was shown that cells had
spread extensively and flattened on anodized TiO
2
surfaces. The absence of significant
morphological modification with different treatment conditions was indicated that anodized TiO
2
films were cytocompatibile. These appearances of cells were in line with those observed in other
studies [6-8].
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
446
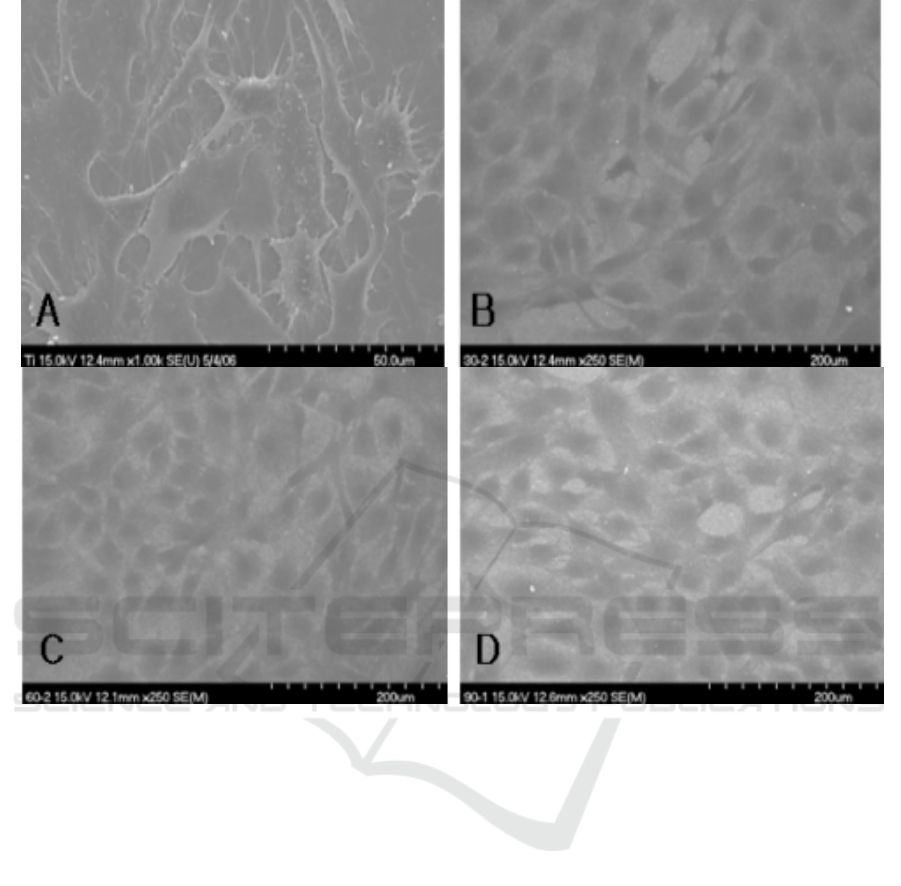
Figure 4. Morphology of primary rat calvarial cells on Ti surfaces.
A. Control (original magnification ☓500); B. Group I (original magnification ☓250).
C. Group II (original magnification ☓250); D. Group III (original magnification ☓250).
3.4. Cell viability test
The cells in all groups proliferated actively within culture period, showing good cell viability. There
was no significant difference in the response of fetal rat calvarial cells to anodized titanium surfaces
with different treatment conditions (Figure 5). This result was in contrast to other reports that
anodized TiO
2
surfaces induced a significant increase in growth and proliferations [5, 6]. It was not
clear why anodized TiO
2
surfaces slightly reduced cell proliferation than control in this study. A
possible explanation of conflicting results was the type of cells for studies. In most of studies,
permanent cell lines such as MC3T3-E1 or MG63 cells, were used for cell proliferation assay,
whereasthe primary cells in this study were used for cell proliferation. Whether primary cell or
immortalized cell lines would be used is controversial. In this study, primary osteoblasts were
obtained from fetal rat calvaria. This is an excellent source of osteoblasts because cells from young
animals proliferate rapidly. Cells from the third, fourth and fifth digests were collected because these
later digests provide a more pure culture, containing most cells that expressed an osteoblast-like
phenotype. Primary cell strains derived from living tissues are necessary and have been
recommended by the ISO for specific testing to simulate the in vivo situation [9]. However, further
studies are required to clearly understand the reason for the difference in cell proliferation.
Osteogenic Responses of Murine Pre-osteoblastic Cells to Anodized TiO2 Surfaces
447
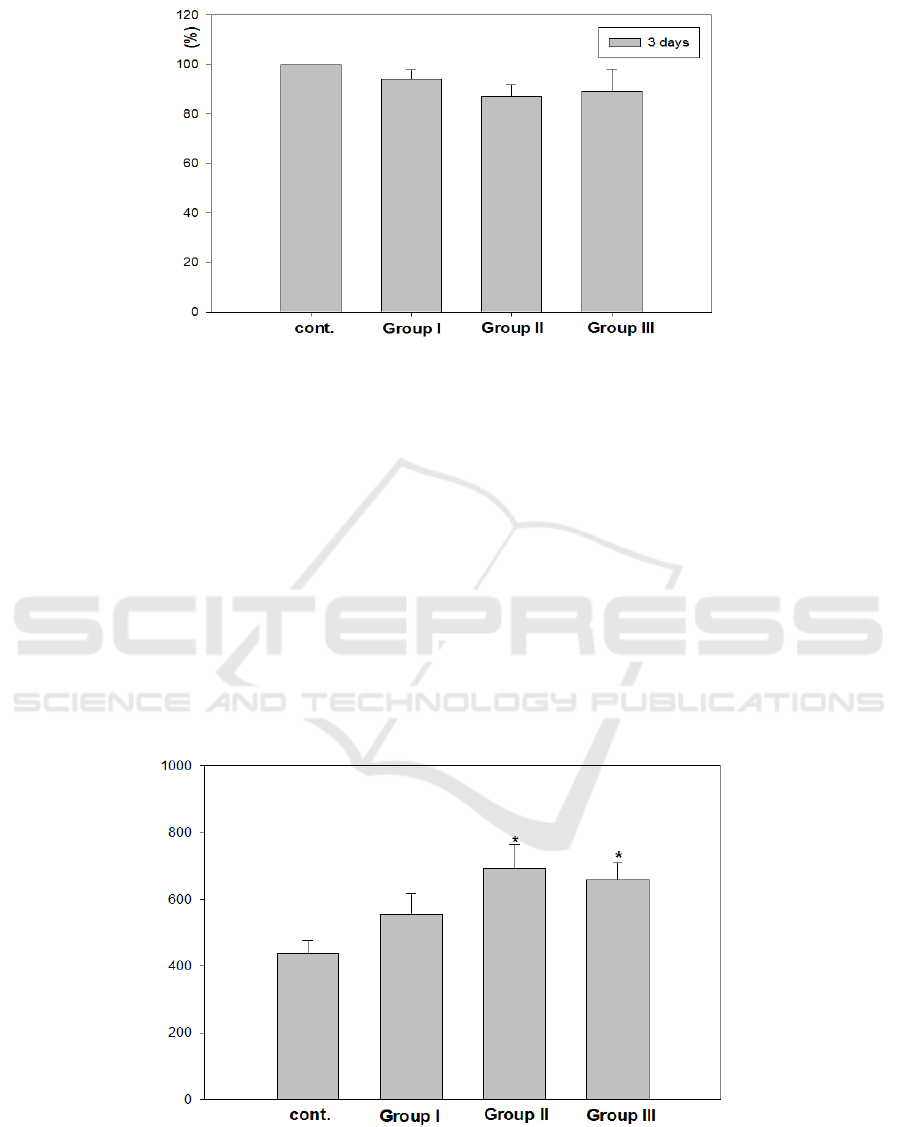
Figure 5. Cell viability test after 3 days on control, Group I, Group II, and Group III.
3.5. Alkaline phosphatase (ALP) activity
Since ALP can mediate bone mineralization by decomposing phosphate compounds and stimulating
the combination of phosphate and calcium in extracellular matrix, the ALP activity is used as a
biomarker for expressing osteoblast activity. Cells grown on Group II and III showed higher alkaline
phosphatase levels compared to those on control and Group I (p<0.01, Figure 6). This result indicates
that anodized TiO
2
surfaces seemed to affect alkaline phosphatase activity. Enhancement of alkaline
phosphatase activity on anodized TiO
2
surface was due to production of matrix vesicles, indicating a
facilitation of osteoblast differentiation. Matrix vesicles are extracellular organelles enriched in
alkaline phosphatase specific activity and are associated with initial calcification in vivo. In
summary, our study suggest that anodized TiO
2
surfaces treated at 60 and 90 seconds should promote
cellular activity of osteoblasts compared with machined Ti surface. However, cellular response of
osteoblasts was different according to different treatment times, thickness of anodized TiO
2
films.
Thus, further studies will be needed to elucidate the relation between thickness of TiO
2
film and
cellular response before clinical applications are considered.
Figure 6. Alkaline phosphatase activity of primary rat calvarial cells on control.
Group I, Group II, and Group III at day 7 (U/μg/protein).
*: Significantly different compared to control (p<0.01).
IWMCE 2018 - International Workshop on Materials, Chemistry and Engineering
448

4. Conclusions
In conclusion, anodized TiO2 surfaces treated at 60 and 90 seconds should promote cellular activity
of osteoblasts compared with machined Ti surface.
References
[1] Okazaki Y and Gotoh E 2005 Biomaterials 26 11-21
[2] Yao C and Webster T J 2006 J Nanosci Nanotechn. 6 2682-2692
[3] Minagar S, Wang J, Berndt CC, Ivanova EP and Wen C 2013 J Biomed Mater Res A. 101
2726-2739
[4] Oh E J, Nguyen T D, Lee S Y, Jeon Y M, Bae T S and Kim J G 2014 Korean J Orthod. 44
246-253
[5] Saldaña L, Vilaboa N, Vallés G, González-Cabrero J and Munuera L 2005 Biomed Mater Res
A. 73 97-107
[6] Cho K P, Kim J H, Chung Y S, Kim O S, Lee K K, Lee D J, Lee K M, Chung S S and Kim Y J
2010 J Nanosci Nanotechn. 10 3581-3585
[7] Oh S, Daraio C, Chen L H, Pisanic T R, Finones RR and Jin S 2006 J Biomed Mater Res. 78
97-103
[8] Wang Y, Wen C, Hodgson P and Li Y 2014 J Biomed Mater Res. 102 743-751
[9] Schmalz G 1994 J Dent. 22 Suppl2 6-11
Osteogenic Responses of Murine Pre-osteoblastic Cells to Anodized TiO2 Surfaces
449
