
Informative Oscillatory EEG Components and their Persistence in Time
and Frequency
Michael Tangermann and Andreas Meinel
Brain State Decoding Lab, Cluster of Excellence BrainLinks-BrainTools, Department of Computer Science,
Albert-Ludwigs-University of Freiburg, Albertstr. 23, Freiburg im Breisgau, Germany
Keywords:
Electroencephalogram, EEG, Oscillatory Component, Oscillatory Subspace, Hyperparameter Optimization,
Brain-Computer Interface, Common Spatial Patterns, CSP, Source Power Comodulation, SPoC, Visualization,
Characterization, Tracking.
Abstract:
Oscillatory brain activity measured by the electroencephalogram, local field potentials or magnetoencephalo-
gram can reflect cognitive processes. It can be used to run brain-computer interfaces or to analyze information
processing, user learning and rehabilitation progress, e.g., after stroke. To extract oscillatory components,
which are informative about a users task and which show an enhanced signal-to-noise compared to raw mul-
tivariate recordings, data-driven spatial filtering methods are widely applied. Some of these approaches can
learn spatial filters from labeled data. They typically require the data analyst to at least define a frequency
band of interest and time interval relative to the course of events in the experiment. These hyperparameters
are exploited by the filtering method in order to extract informative oscillatory features. Their choice typically
is domain-specific and may require adaptations to individuals. Post-hoc data analysis, however, should not be
restricted to the initial hyperparameter ranges. Thus we present an approach, which allows to characterize a
given oscillatory component with respect to the frequency bands and the temporal windows for which it con-
tains task-relevant information. The approach allows to track task-informative persistence of components over
multiple experimental sessions and may be helpful to monitor motor learning and rehabilitation over time.
1 INTRODUCTION
Neurotechnological applications like brain-computer
interface (BCI) systems for patients or non-medical
use (Wolpaw and Wolpaw, 2012; H
¨
ohne et al., 2014;
van Erp et al., 2012) tap into multivariate brain sig-
nals. Their goal is to either drive an online applica-
tion, or to monitor mental processes, which are in-
formative about the tasks executed by the user. But
also outside the field of BCI, oscillatory signal com-
ponents of electrophysiological recordings like the
magnetoencephalogram, the electroencephalogram
(EEG), invasive recordings of the electrocorticogram
or local field potentials have long been studied, as
they can contain information about the user task or
task performance (Klimesch, 1999). The exploita-
tion of these oscillatory signal components, however,
is not straight forward due to the low signal-to-noise
ratio especially with non-invasive recordings. Here,
sophisticated data driven machine learning meth-
ods (M
¨
uller et al., 2008) proved helpful to extract sub-
spaces containing informative oscillations with en-
hanced signal-to-noise ratio. Among these methods,
common spatial patterns (CSP) (Ramoser et al., 2000;
Koles, 1991; Fukunaga, 1990) and variants thereof
are widely used (Tangermann et al., 2012; Lotte and
Guan, 2011). The algorithm allows to extract oscil-
latory components, which display contrastive behav-
ior, e.g. event-related de-synchronization (ERD) and
-synchronization (ERS) effects. These ERD/ERS ef-
fects are time-locked e.g. to the cueing time point
of discrete motor tasks (Pfutscheller et al., 1997),
and are extracted for a pre-selected frequency band.
More recently, source power comodulation (SPoC)
was proposed by D
¨
ahne and colleagues (D
¨
ahne et al.,
2014) as a regressing subspace filtering approach.
SPoC allows to extract oscillatory components from
bandpass-filtered EEG, which comodulate in their
band power amplitude with a known variable. This
variable in practice can be derived e.g. from a task-
wise behavioral metric or represent the intensity
of stimuli. At training time, when spatial filters
(they determine the oscillatory subspace components)
are derived, an optimization problem needs to be
solved. Depending on the algorithm, this can be time-
consuming as it typically involves an iterative gra-
Tangermann M. and Meinel A.
Informative Oscillatory EEG Components and their Persistence in Time and Frequency.
In NEUROTECHNIX 2017 - Extended Abstracts (NEUROTECHNIX 2017), pages 17-21
Copyright
c
2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
-2.0 -1.0 1.0 2.0
0
10
20
30
40
time t
test
relative to trial start (s)
frequency f
test
(Hz)
f
train
z-AUC
-0.63
-0.60
-0.58
-0.55
-0.53
-0.51
-0.47
-0.45
t
train
A B
Filter
Pattern
f
train
=28.8 Hz
subject S5
t
train
= -0.05 s
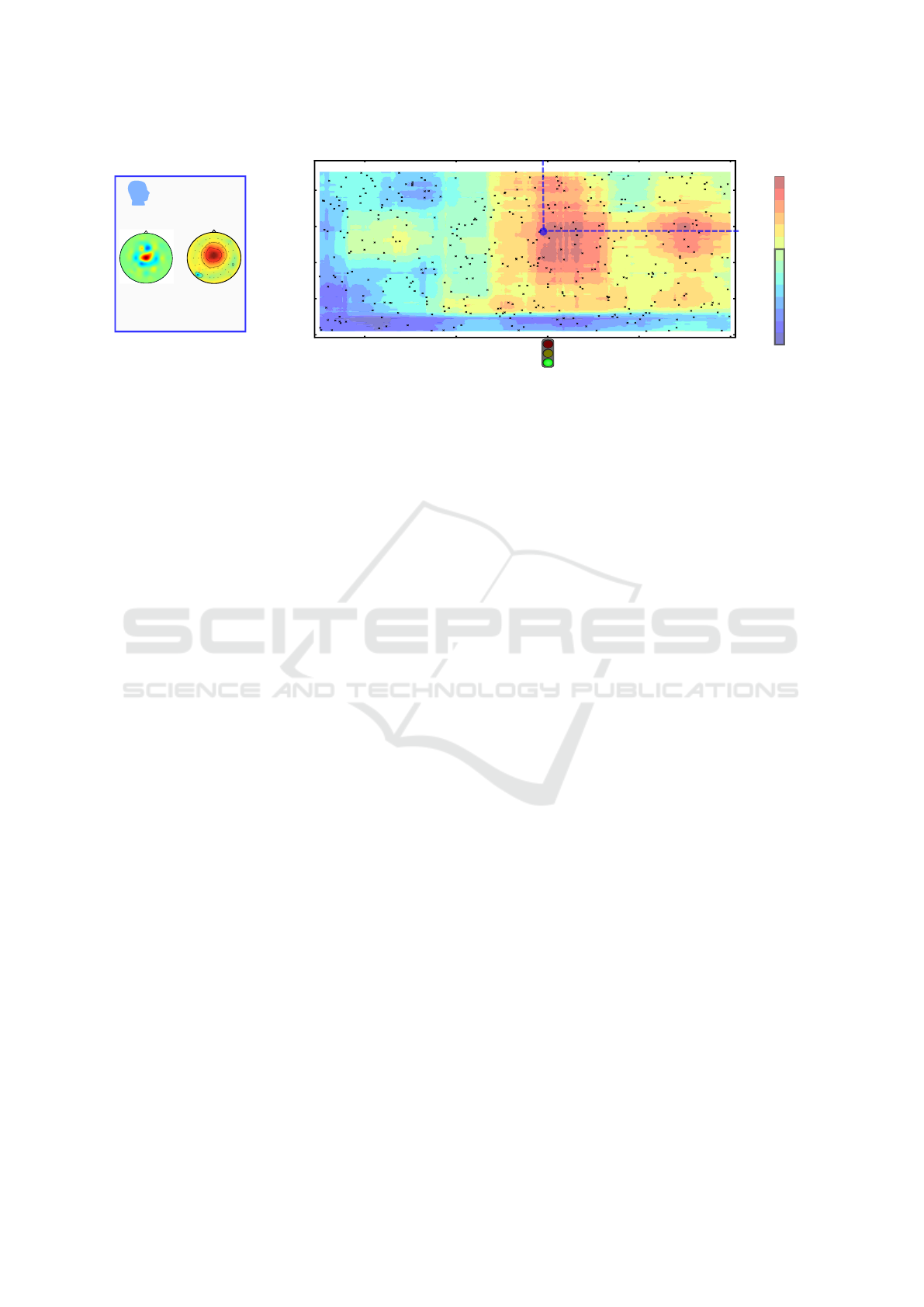
Figure 1: (a) Characterization of an exemplary oscillatory component (spatial filter and spatial pattern) derived by parameters
(t
train
= -50 ms, f
train
= 28.8 Hz) using the source power comodulation (SPoC) method. (b) The z-AUC performance of this
SPoC component for varying pairs of hyperparameters (t
test
, f
test
) sampled at coordinates marked by single black dots is
interpolated and depicted in a color scheme. The chance level is provided by the blue-rimmed z-AUC values.
dient descent or the calculation of covariance matri-
ces and their inversion. At testing time, however, the
application of a known spatial filter usually is very
fast, as it can typically be realized by computing a
weighted linear combination of input channels.
No matter if supervised approaches like CSP,
SPoC or other subspace methods shall be used for
the analysis of multivariate brain signals, it usu-
ally is unclear a priori, in which exact task-related
time segment and in which frequency band the in-
formative oscillatory activity persists (Meinel et al.,
2016b). While knowledge about these method- and
task-specific hyperparameters would be desirable to
have already at training time, it provides valuable in-
sights also in the post-hoc analysis of experimental
data collected during repeated executions or sessions
of the task. For these reasons we present a method,
that allows to characterize an oscillatory component
in terms of its persistence over time and frequency
space. We are convinced, that this characterization
contributes valuable information which goes beyond
a description of ERD/ERS behavior and of the spatial
pattern.
2 METHODS
The decoding or even prediction of motor perfor-
mance from brain signal recordings is a recent re-
search topic (Meyer et al., 2014). In (Meinel et al.,
2016a), we studied the sequential visual isometric
pinch task (SVIPT, (Meinel et al., 2015)) as an ex-
ample of a repetitive hand force task. While we re-
fer the reader to (Meinel et al., 2015) for details on
this hand force training task, it is helpful to know,
that each repetitive trial required the user to control
the horizontal movement trajectory of a cursor on the
screen by applying varying levels of pinch force to
sensor. Per trial, behavioral performance metrics such
as the deviation from the optimal trajectory, reaction
time after the trial start etc. were measured. For de-
tails on SVIPT metrics and correlations among differ-
ent metrics please refer to (Tangermann et al., 2015).
The EEG activity of participants was recorded prior
and during the execution of SVIPT trials using d = 63
gel-based Ag/AgCl electrodes and BrainAmp ampli-
fiers. Thriving to find an explanation for the observed
strong inter-trial variability of the motor performance,
we were able to identify robust pre-trial oscillatory
components, i.e. components whose band power was
informative to predict the single-trial motor perfor-
mance. For a discussion on robustness scores please
refer to (Casta
˜
no-Candamil et al., 2015). Based on
the multichannel EEG recordings, spatial filters and
resulting oscillatory components were computed with
SPoC using trial-wise continuous performance labels
z. More precisely, the spatial filters were trained using
one epoch of EEG data per trial, which was extracted
as a 750 ms wide time segment. The segment’s posi-
tion within the trial is described by the hyperparam-
eter t
train
, which marks the end of the segment. In
addition, the data were filtered prior to the training of
the spatial filter method to a passband of 1.5 Hz width
around a central frequency f
train
of this band. For
SPoC training, epochs of the EEG had been extracted
from trial-wise time segments located just before the
go-cue (t
train
= −50 ms).
For this short paper, we selected a single, representa-
tive spatial filter derived by SPoC, which was trained
on data of the beta frequency band f
train
= 28.8 Hz
and used the trial-wise reaction time as label z. In
Fig. 1A, we show an example of a derived spatial fil-

ter w
train
∈ R
d
. The Figure shows the filter together
with the corresponding spatial pattern. For informa-
tion on the relation between filters and patterns in
spatial subspace decomposition methods we refer the
reader to (Haufe et al., 2014).
To obtain an estimate of the label z
est
for a novel
data epoch e, the trained spatial filter w
train
is applied
to the spatial covariance estimate Σ(e, t, f ) of the data
epoch:
z
est
(e) = w
T
train
Σ(e, t, f ) w
train
(1)
Please note, that the covariance estimate requires to
make an explicit choice of the hyperparameters (t, f ).
Equation 1 now allows to test the persistence
of a given oscillatory component on the same data
set. Therefore we evaluated the estimated labels z
est
according to Equation 1 with N = 500 novel, ran-
domly chosen time-frequency hyperparameter pairs
(t
test
, f
test
) within the frequency range of 1 to 46 Hz
and for time segment endpoints within -2.5 s to +2.0 s
relative to the go-cue of each SVIPT trial.
For characterizing the sensitivity of the compo-
nent with respect to varying hyperparameters the z-
AUC performance is reported. Related to the area un-
der the receiver-operator characteristics curve (AUC),
the z-AUC describes how well the estimated labels
z
est
gathered by the band power of the SPoC compo-
nent are in accordance with the measured trial-wise
motor performance labels z. We decided to use z-
AUC rather than the correlation coefficient r as an
evaluation score for the component’s persistance, as
it has shown to be less sensitive to varying training
set size. For further details see (Meinel et al., 2016a).
The interpolation and visualization of z-AUC re-
sulting from various hyperparameter pairs was per-
formed using using functional ANOVA toolbox (Hut-
ter et al., 2014).
3 RESULTS
An exemplary oscillatory component derived by
SPoC is characterized in Fig. 1A by the spatial fil-
ter and pattern of the component. Its band power was
found to predict the trial-wise SVIPT reaction time.
The pattern could be interpreted such, that the com-
ponent reflects the status of the motor system.
As shown in Fig. 1B, its persistence has been
tested for many hyperparameter pairs, which go be-
yond the frequency of 28.8 Hz and the temporal seg-
ment of [-800 -50] ms relative to the go-cue, on which
the component had been trained. It can be observed,
that the component is able to extract information
about the task performance (reaction time in this ex-
ample) also in time intervals after the go-cue. This in-
formation subsides at around 800 ms after the go cue,
which is not unexpected, as most of the motor reac-
tions already have happened at this latency. Interest-
ingly, a second informative time interval around 1.5 s
after the go cue is observed, which may be caused by
the repetitive structure within each single SVIPT trial.
The information extracted by the component is
visible in a large beta band (approximately in the
range of 15 to 35 Hz and also in the the gamma band
above 35 Hz. In this gamma band, however, the in-
formative time intervals are shorter than in the beta
band. Interestingly, these frequency ranges and time
interval, i.e. the existance range of the component, by
large extends the original parameters (t
train
, f
train
) that
have been used to extract the component with SPoC.
4 DISCUSSION
In previous work with SPoC on data derived with the
SVIPT hand motor training, we had identified a num-
ber of oscillatory components, which allowed to pre-
dict trial-wise SVIPT reaction time (and other per-
formance metrics). We reported these components
in (Meinel et al., 2016a), but have not yet described
a method to characterize their stability in the time-
frequency domain.
Based upon an oscillatory EEG component that
has the ability to predict or decode behavioral perfor-
mance, we have introduced a method, which allows
to describe how the task-related information of this
component persists over time and in frequency space.
We have evaluated the method for an exemplary com-
ponent which had been found in our earlier study. The
method will open the door for an re-analysis of large
collections of informative components and may in the
future contribute to their functional interpretation.
A similar sensitivity analysis termed event-related
spectral pertubation analysis has recently been pro-
posed by Mousavi and colleagues for a motor im-
agery BCI paradigm. Comparing class-informative
information in the oscillatory domain along the time-
frequency space (Mousavi et al., 2017), their ap-
proach involves multiple training repetitions of the
CSP spatial filtering method, while our proposed
method requires evaluations of a trained component
only, but does not require full re-training for every
hyperparameter pair.
The in-depth characterization of components with
our method clearly goes beyond a description based
on solely the ERD/ERS behavior or the corresponding
spatial patterns. While applied exemplarily to a SPoC

component, the proposed method is not restricted to
SPoC and can be utilized to characterize any type of
spatial filter / component.
We propose to use a component’s persistence in
the time- and frequency domain in order to track
changes over sessions and we argue that this is use-
ful in various scenarios. Examples are cognitive and
memory tasks (Klimesch, 1999), when changes of
oscillatory activity is induced by motor learning in
sports, or over the course of BCI-supported motor re-
habilitation after stroke — a field which recently re-
ceived a lot of attention (Soekadar et al., 2015; Rem-
sik et al., 2016). In experimental scenarios with a re-
stricted, similar functional context, this form of anal-
ysis may even help to identify corresponding oscil-
latory components across users and can thus support
novel forms of group level analyses.
ACKNOWLEDGEMENTS
The authors are thankful for support by the Clus-
ter of Excellence BrainLinks-BrainTools, funded by
the German Research Foundation (DFG, grant num-
ber EXC 1086) and by state of Baden-W
¨
urttemberg
through bwHPC and the German Research Founda-
tion (DFG) through grant no INST 39/963-1 FUGG.
Finally, we want to thank Katharina Eggensperger and
Frank Hutter for providing software on the hyperpa-
rameter analysis.
REFERENCES
Casta
˜
no-Candamil, J. S., Meinel, A., D
¨
ahne, S., and Tanger-
mann, M. (2015). Probing meaningfulness of oscilla-
tory EEG components with bootstrapping, label noise
and reduced training sets. In Engineering in Medicine
and Biology Society (EMBC), 2015 37th Annual Inter-
national Conference of the IEEE, pages 5159–5162.
IEEE.
D
¨
ahne, S., Meinecke, F. C., Haufe, S., H
¨
ohne, J., Tanger-
mann, M., M
¨
uller, K.-R., and Nikulin, V. V. (2014).
SPoC: a novel framework for relating the amplitude of
neuronal oscillations to behaviorally relevant parame-
ters. NeuroImage, 86(0):111–122.
Fukunaga, K. (1990). Introduction to statistical pattern
recognition (2nd ed.). Academic Press Professional,
Inc., San Diego, CA, USA.
Haufe, S., Meinecke, F., G
¨
orgen, K., D
¨
ahne, S., Haynes, J.-
D., Blankertz, B., and Biemann, F. (2014). On the
interpretation of weight vectors of linear models in
multivariate neuroimaging. NeuroImage, 87(0):96 –
110.
H
¨
ohne, J., Holz, E., Staiger-S
¨
alzer, P., M
¨
uller, K.-R.,
K
¨
ubler, A., and Tangermann, M. (2014). Motor im-
agery for severely motor-impaired patients: Evidence
for brain-computer interfacing as superior control so-
lution. PLoS ONE, 9(8):e104854.
Hutter, F., Hoos, H., and Leyton-Brown, K. (2014). An
efficient approach for assessing hyperparameter im-
portance. In Proceedings of International Conference
on Machine Learning 2014 (ICML 2014), pages 754–
762.
Klimesch, W. (1999). EEG alpha and theta oscillations re-
flect cognitive and memory performance: a review
and analysis. Brain Research Reviews, 29(2):169–
195.
Koles, Z. J. (1991). The quantitative extraction and topo-
graphic mapping of the abnormal components in the
clinical EEG. Electroencephalography and clinical
Neurophysiology, 79(6):440–447.
Lotte, F. and Guan, C. (2011). Regularizing common spa-
tial patterns to improve BCI designs: unified theory
and new algorithms. IEEE Transactions on Biomedi-
cal Engineering, 58(2):355–362.
Meinel, A., Casta
˜
no-Candamil, S., Reis, J., and Tanger-
mann, M. (2016a). Pre-trial EEG-based single-trial
motor performance prediction to enhance neuroer-
gonomics for a hand force task. Frontiers in Human
Neuroscience, 10:170.
Meinel, A., Castao-Candamil, J. S., D
¨
ahne, S., Reis, J., and
Tangermann, M. (2015). EEG band power predicts
single-trial reaction time in a hand motor task. In Proc.
Int. IEEE Conf. on Neural Eng. (NER), pages 182–
185, Montpellier, France. IEEE.
Meinel, A., Eggensperger, K., Tangermann, M., and Hut-
ter, F. (2016b). Hyperparameter optimization for ma-
chine learning problems in BCI. In M
¨
uller-Putz, G.,
Huggins, J., and Steyrl, D., editors, Proceedings of the
6th International Brain-Computer Interface Meeting:
BCI Past, Present, and Future, page 184, Asilomar
Conference Center, Pacific Grove, California, USA.
Verlag der Technischen Universit
¨
at Graz.
Meyer, T., Peters, J., Zander, T. O., Sch
¨
olkopf, B.,
and Grosse-Wentrup, M. (2014). Predicting motor
learning performance from electroencephalographic
data. Journal of Neuroengineering and Rehabilitation,
11(1):24.
Mousavi, M., Koerner, A. S., Zhang, Q., Noh, E., and
de Sa, V. R. (2017). Improving motor imagery BCI
with user response to feedback. Brain-Computer In-
terfaces, 4(1-2):74–86.
M
¨
uller, K.-R., Tangermann, M., Dornhege, G., Krauledat,
M., Curio, G., and Blankertz, B. (2008). Machine
learning for real-time single-trial EEG-analysis: From
brain-computer interfacing to mental state monitoring.
Journal of Neuroscience Methods, 167(1):82–90.
Pfutscheller, G., Neuper, C., Flotzinger, D., and Pregen-
zer, M. (1997). EEG-based discrimination between
imagination of right and left hand movement. Elec-
troencephalography and Clinical Neurophysiology,
103:642–651.
Ramoser, H., M
¨
uller-Gerking, J., and Pfurtscheller, G.
(2000). Optimal spatial filtering of single trial EEG
during imagined hand movement. Rehabilitation En-
gineering, IEEE Transactions on, 8(4):441–446.

Remsik, A., Young, B., Vermilyea, R., Kiekhoefer, L.,
Abrams, J., Evander Elmore, S., Schultz, P., Nair, V.,
Edwards, D., Williams, J., et al. (2016). A review
of the progression and future implications of brain-
computer interface therapies for restoration of distal
upper extremity motor function after stroke. Expert
Review of Medical Devices, 13(5):445–454.
Soekadar, S. R., Silvoni, S., Cohen, L. G., and Birbaumer,
N. (2015). Brain–machine interfaces in stroke neu-
rorehabilitation. In Clinical Systems Neuroscience,
pages 3–14. Springer.
Tangermann, M., M
¨
uller, K.-R., Aertsen, A., Birbaumer,
N., Braun, C., Brunner, C., Leeb, R., Mehring, C.,
Miller, K., M
¨
uller-Putz, G., Nolte, G., Pfurtscheller,
G., Preissl, H., Schalk, G., Schl
¨
ogl, A., Vidaurre, C.,
Waldert, S., and Blankertz, B. (2012). Review of the
BCI competition IV. Frontiers in Neuroscience, 6(55).
Tangermann, M., Reis, J., and Meinel, A. (2015). Common-
alities of motor performance metrics are revealed by
predictive oscillatory EEG components. In Proceed-
ings of the 3rd International Congress on Neurotech-
nology, Electronics and Informatics (NEUROTECH-
NIX), pages 32–38, Lissabon.
van Erp, J., Lotte, F., and Tangermann, M. (2012). Brain-
computer interfaces for non-medical applications:
How to move forward. Computer, pages 26–34.
Wolpaw, J. R. and Wolpaw, E. W., editors (2012). Brain-
computer interfaces : principles and practice. Oxford
University press. ISBN-13: 978-0195388855.
