Identifying Intra-Cortical Recording Instabilities
Maran Ma, Theodoros P. Zanos, Matthew R. Krause, Christopher C. Pack and Timothy E. Kennedy
Department of Neurology and Neurosurgery, Montreal Neurological Institute, McGill University, Montréal, Canada
1 OBJECTIVES
Cortical multielectrode-arrays (MEA) offer some of
the highest resolution technology for detecting
clinical user intent for controlling prosthesis, such as
robotics and functional electrical stimulation. The
long term performance of these neuron-machine-
interfaces (NMI) depends on both neural directional
tuning stability and signal recording stability (Wang
et al., 2014). Having more functional channels to
decode from a larger neural ensemble however, can
effectively compensate for directional tuning
fluctuations (Nuyujukian et al., 2014).
To improve MEAs to retain more stable channels,
it is critical to prioritize engineering requirements, as
the biological vs. non-biological causes of instability/
failure may require opposite design strategies. For
example, reducing gliosis may necessitate enhanced
biomimicry and softer substrates, while reducing
hardware failure may benefit from adsorption-
repelling and robust substrates.
This project aims to discern hardware vs. tissue
degradations that underlie recording instability, and
quantify MEA stability without relying on spike
sorting – as sorting is not essential to NMI, labour
intensive and/or has uncertainies (Einevoll et al.,
2012). To accomplish this we analysed chronic Utah
array recordings from inferotemporal and prefrontal
cortices of adult macaque monkeys, focusing on
individual channel trends.
2 METHODS
Existing recordings obtained from eight-months of
visual system studies on an adult macaque monkey
(monkey F) was used. MEAs were Iridium oxide
Utah arrays implanted in the left inferotemporal
(implant 1) and right prefrontal (implant 2) cortices.
Before each experiment an 8-minute baseline
recording was made with the same series of visual
stimuli (10 repetitions of 100 images per session) and
fixation point as previous sessions. These baseline
recordings are used in our stability analysis.
Data collected at 30Khz was digitally filtered at
250Hz-7.5KHz and thresholded at -4.5RMS for spike
detection. Channel spike rate distributions were
tabulated per session, fitted to gamma distribution,
and verified with chi-square goodness-of-fit test.
3 RESULTS
3.1 Stability Quantification
The most active channels were selected (based on
mean spike rate over all sessions), and analysed
individually. To examine channel data in detail, spike
counts within each 8-minute recording was tabulated
per second into histogram form (Figure 1). Since the
stimuli sequence included a wide variety of images,
each distribution represents a general range of
behaviour of the neurons at that electrode. If these
ranges change significantly between sessions (under
the same stimuli) the channel’s neural population has
likely changed, and is defined as unstable.
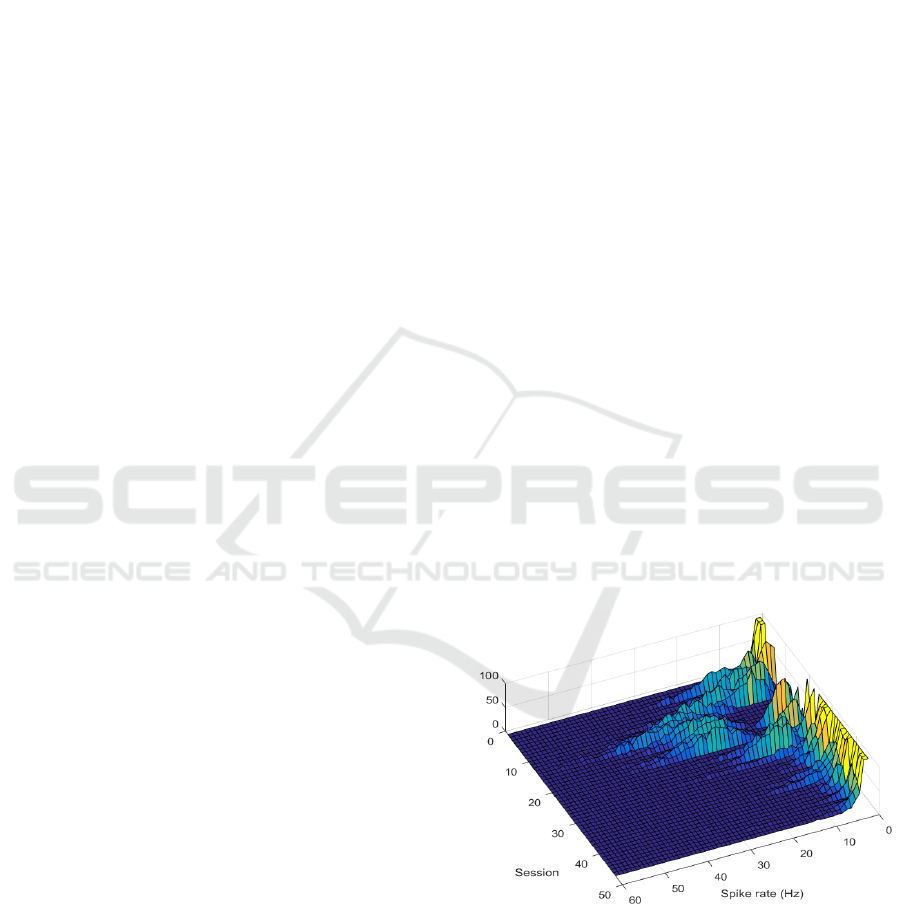
Figure 1: Spike rate histograms per session from channel
23, which showed notable discrepancy between sessions.
The spike rate histograms were found to fit
gamma distributions. Since each session contained 10
repetitions of identical stimuli, distribution
parameters extracted from single repetitions could
serve as a standard for nominal variability within a
stable period. This enables, for example, comparing
14
Ma, M., Zanos, T., Krause, M., Pack, C. and Kennedy, T.
Identifying Intra-Cortical Recording Instabilities.
In Extended Abstracts (NEUROTECHNIX 2016), pages 14-15
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
the parameters’ percent change between sessions vs.
within sessions as a criterion of channel stability. We
intend to further this analysis and quantify stability
over time for both implants.
3.2 Biological vs. Non-biological Causes
Intra-cortical recordings consist of high-amplitude
action potentials and a dense core of device and
biological noise. The latter is the larger component
(Lempka et al., 2011), and contains the overlapping
activity of a sea of distant neurons. Hence if the loss
of spike detection is due to hardware degradation, a
change should also be reflected in the below-
threshold portion of the recording. Inspection of
representative sessions (Figure 2) show that when
spikes are absent, the core portion is unaffected, and
signal loss is reversible. Thus hardware failure is not
the cause of this form/instance of instability.
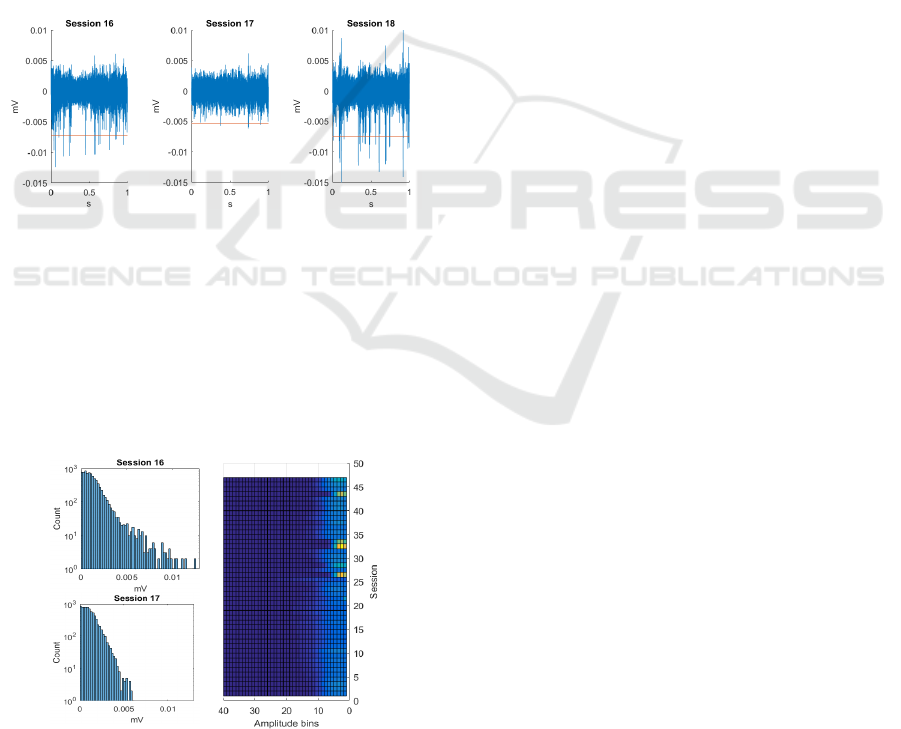
Figure 2: Time domain signal from channel 23, sessions 16
to 18; red line is the spike detection threshold (-4.5RMS).
Histograms of waveform amplitudes distinguish the
bulk that is biological noise (Figure 3). Measuring the
low-amplitude side of this distribution quantifies
noise consistency, hence hardware stability across
sessions. A simple colour map visualization of all
sessions also implicates instances of hardware failure
(unusual distributions at the core signal component).
Figure 3: A) Un-thresholded signal amplitude histogram
showing noise consistency regardless of spike stability. B)
Top view of the stack of histograms for channel 23.
4 DISCUSSION
It is helpful for new NMI design and evaluation to
have simple methods to assess recording stability and
distinguish tissue or hardware induced signal loss.
Typically, stability is quantified from combined
activity of all channels. Such statistics are compelling
but hide the profile of single channels, which suggest
underlying mechanisms of failure. Also common is to
apply thresholding to eliminate noise and isolate units
prior to analysis, but this imposes a zone around the
electrode where only neural activity within is kept as
data, and more distant activity is discarded as noise
(or heavily filtered for LFP). This "biological noise"
holds robust information on hardware performance
unaffected by the state of local neurons.
We examined long term intra-cortical recordings
from sensory & processing areas of macaque cortex -
where neural activity can be regulated by stimuli - and
analysed individual channel spike rates and biological
noise: 1) Quantifying and fitting the distribution of
spike rates per session gave an illustrative statistic of
channel stability. This method does not rely on spike
sorting, which is non-trivial to perform and the
margins of error are harmful to stability assessment.
2) Tabulating all peaks in the recording showed that
the "core" of the amplitude distribution (comprised of
biological noise and system noise) can remain
constant when the spike count is low, indicating
cellular causes such as poor health of a neuron. This
analysis also identified sessions with abnormal signal
core shape, suggesting true hardware failure.
REFERENCES
Einevoll, G. T., Franke, F., Hagen, E., Pouzat, C. & Harris,
K. D. 2012. Towards reliable spike-train recordings
from thousands of neurons with multielectrodes. Curr
Opin Neurobiol, 22, 11-7.
Lempka, S. F., Johnson, M. D., Moffitt, M. A., Otto, K. J.,
Kipke, D. R. & Mcintyre, C. C. 2011. Theoretical
analysis of intracortical microelectrode recordings. J
Neural Eng, 8, 045006.
Nuyujukian, P., Kao, J. C., Fan, J. M., Stavisky, S. D., Ryu,
S. I. & Shenoy, K. V. 2014. Performance sustaining
intracortical neural prostheses. J Neural Eng, 11,
066003.
Wang, D., Zhang, Q., Li, Y., Wang, Y., Zhu, J., Zhang, S.
& Zheng, X. 2014. Long-term decoding stability of
local field potentials from silicon arrays in primate
motor cortex during a 2D center out task. J Neural Eng,
11, 036009.
Identifying Intra-Cortical Recording Instabilities
15
