
Proteotronics: Application to Human 17-40 and Bacteriorhodopsin
Receptors
Eleonora Alfinito
1
, Lino Reggiani
2
, Rosella Cataldo
2
, Giorgio De Nunzio
2
,
Maria Rachele Guascito
3
and Livia Giotta
3
1
Dipartimento di Ingegneria dell`Innovazione, Università del Salento, via Monteroni, Lecce, Italy
2
Dipartimento di Matematica e Fisica “Ennio de Giorgi”, Università del Salento, via Monteroni, Lecce, Italy
3
Dipartimento Di.S.Te.B.A., Università del Salento, via Monteroni, Lecce, Italy
Keywords: Protein Electrical Properties, Proteotronics.
Abstract: Human olfactory 17-40 and Bacteriorhodopsin are two protein receptors that received particular attention in
electronics, due to the possibility of implementing nano-biodevices able to detect odours and light and thus
useful for medical and green energy harvesting applications. Some recent experiments concerning the
electrical responses of these receptors are reviewed. Data are interpreted in the framework of a new science
exploiting the complexity in biology and biomedical engineering called proteotronics. In particular, the
single protein is modelled as an impedance network whose topological properties affect the electrical
response as measured by experiments.
1 FOREWORD
Recent advances in science and technology, such as
the development of techniques and devices for
health care and green and renewable energy, are the
successful products of the synergy among different
bailiwicks. As a matter of fact, cross-fertilization has
diffused a consolidated knowledge beyond the
boundaries of specific cliques, thus allowing the
birth of a more comprehensive methodological
approach which integrates and makes more powerful
chemical, computational, biological, engineering and
physical strategies (Alfinito, 2015a; De Nunzio,
2015; Guascito, 2011; Nagy, 2013).
Complexis 2016, being the first international
conference on complex information systems, offers
the relevant opportunity to introduce proteotronics, a
new emerging discipline aiming to propose and
develop innovative electronic devices, based on the
selective action of specific proteins. The word
originates from the combination of proteomics, the
science devoted to the large-scale study of proteins
structures and functions, and electronics, the science
devoted to the development of devices manipulating
electrical current to perform useful tasks.
2 INTRODUCTION
Proteins are the core of the cellular functions. They
are macro-assemblies of hundreds to thousands of
amino acids. Amino acids are taken by a set of about
20 elements and are structurally similar molecules,
which differ for the specific R-group. Proteins carry
out very different functions: they produce energy,
transform chemicals, build tissues, etc. Their
function is intimately connected to structure (Berg,
2002), which, in most cases, changes
(conformational change) when the protein performs
its activity. Therefore, a growing interest is devoted
to determine protein 3D structures in the different
phases of the protein activity. At present, the number
of classified structures of proteins in their native
state is quite large, while the structures of activated
proteins are quite a few. As a matter of fact, the
determination of the structure of proteins in their
active state is much more complex than in their
native state. This because structure and function are
strongly connected and the measurement of the
former may change the latter (Shrödinger, 1944).
Protein activation itself is a big challenge of
investigation, involving the mechanisms of internal
binding rearrangements (Kobilka, 2007) and the
modification of the protein free-energy landscape
(Alfinito, 2015b). These and other questions have
been recently investigated on a special family of
32
Alfinito, E., Reggiani, L., Cataldo, R., Nunzio, G., Guascito, M. and Giotta, L.
Proteotronics: Application to Human 17-40 and Bacteriorhodopsin Receptors.
In Proceedings of the 1st International Conference on Complex Information Systems (COMPLEXIS 2016), pages 32-38
ISBN: 978-989-758-181-6
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
proteins, the receptors, i.e. proteins able to capture
an external ligand (small proteins, molecules, light,
etc.), then converting this capture into a biochemical
signal. Recently, proteins have gained a primary role
in advancing electronics performances toward a
more friendly, green and renewable guise. The
cornerstone of this interest is the possibility to equip
electrical devices with the peculiar abilities of these
proteins in recognizing, selecting and capturing
specific ligand. These biochemical activities have a
natural portability to the electronic world since they
can be converted into electrical signals (Hou, 2007;
Benilova, 2008; Vidic, 2006; Jin, 2006; Ron, 2010;
Casuso, 2007). To take on this challenge it is
necessary to gain knowledge about the protein
features in vivo and in vitro, to achieve the
experience for producing electronic nanodevices, to
develop the ability for integrating organic and
inorganic components, to collect information
sufficient for producing a model of the physico-
chemical mechanisms underlying the device
response. The body of these studies has been
recently named proteotronics (Alfinito, 2015a), by
combining proteomics, the large-scale study of
proteins, and electronics. In particular, we already
showed some breaking results in protein electrical
measurements and the way these results are
interpreted into the framework of proteotronics. This
is carried out by representing the protein like a
complex network able to mimic the electrical
responses observed in experiments and to be
predictive for novel results.
In this paper we present the application of
proteotronics for two receptors, the human olfactory
receptor OR 17-40 and the bacteriorhodopsin. They
received wide experimental investigation for their
electrical properties as biological transducers when
used as active parts of electronic devices. We
selected those two cases as the best representative
among the experimental datasets on which the
theoretical model, drawn in the next section, has
been validated. The content is organized as follows:
Section 3 reports experimental and theoretical
results concerning the electrical responses of human
olfactory receptor OR 17-40, of high interest for the
realization of bioelectronic noses. Section 4 reports
experimental and theoretical results concerning the
light receptor bacteriorhodopsin, of great interest for
future applications in the field of green energy
production; finally, Section 5 sketches the main
conclusions.
3 HUMAN OR 17-40
3.1 Generalities and Experiments
Recent investigations have confirmed the possibility
of detecting the protein activity by using electrical
measurements (Vidic, 2006; Jin, 2006; Hou, 2007;
Benilova, 2008; Guascito, 2011; Nagy, 2014). All
the analyzed proteins (mainly olfactory and light
receptors) show a characteristic impedance response,
which strongly depends on the protein structure and
on the environmental conditions. In other terms,
measurements of the electrical responses are a
sensitive tool for detecting protein conformational
change (activation). The present case concerns the
activation of human olfactory receptor OR 17-40.
This protein shows a very good sensitivity to
helional and heptanal odorants (Levasseur, 2003;
Vidic, 2006; Benilova, 2008), although the results
deviate from a standard expectation and need for a
microscopic interpretation (Alfinito, 2011b).
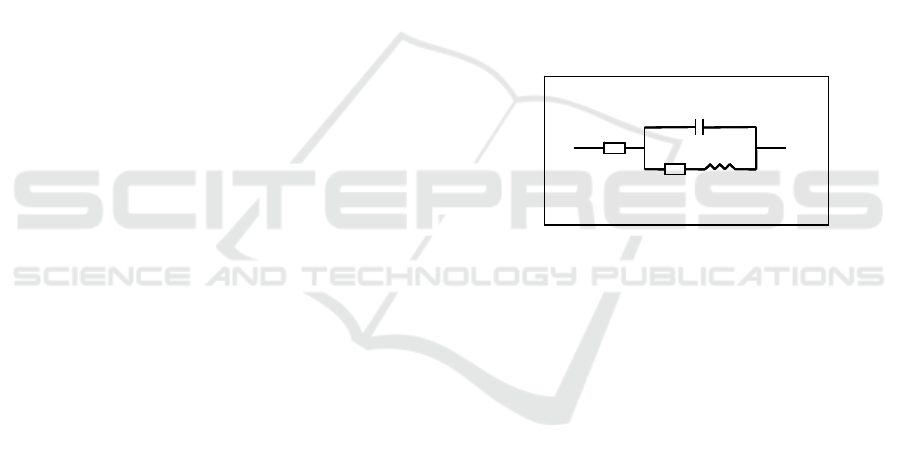
Figure 1: Randles cell. Rs is the solution resistance and
Rp, Zw and CPE, the polarization resistance, the Warburg
impedance, and the constant phase element of the sample,
respectively.
Here we report experiments performed by using
the electrochemical impedance spectroscopy (EIS)
(Benilova, 2008).
The experimental set-up involves protein
receptors in their membrane fraction, anchored on
properly functionalized specific substrates and used
to detect the in vitro dose- response to their specific
ligands. In particular, EIS measurements were
performed under different concentrations of the
protein specific odorants and for each concentration
an impedance spectrum was recorded. The standard
procedure to interpret the impedance spectrum is to
give a simplified representation of the apparatus by
using a Randles cell, like that shown in Figure 1. In
particular, Rp is the element more sensitive to the
protein activation. Therefore, measurements of its
value with and without the odorant were performed.
At increasing values of the specific odorant
concentration, the polarization resistance shows a
peculiar bell-shaped behavior, centered at 10
-10
M
(Benilova, 2008). Furthermore, by using
Rs
CPE
R p
Z
W
Proteotronics: Application to Human 17-40 and Bacteriorhodopsin Receptors
33

complementary techniques such as the differential
surface plasmon resonance (SPR) and the
differential bioluminescence response, also a second
peak was observed, at a higher concentration of the
odorant (around 10
-5
M) (Vidic, 2006). This kind of
response, that does not exhibit a saturation at the
highest concentrations, is unexpected.
3.2 Theory
The protein structure-function correlation is here
interpreted at the amino acid level, building a graph
whose nodes correspond to the amino acids. Each
node contains several data, like the amino acid
position, taken by the public data banks (Berman,
2002), and its electrical polarization in terms of a
specific dielectric constant (Alfinito, 2011a). These
data are used to assign the links between nodes that
are associated with an elemental impedance
responsible of charge transfer. The procedure to
construct the analogous network of the protein
requires two steps. In the first step, an interaction
radius, R
C
, is assigned. It determines the degree of
the graph nodes, because two nodes are connected
only if the physical distance between the
corresponding amino acids is less than R
C
(Figure
2).
Figure 2: Graphical representation of human OR 17-40 in
its native state. The network is obtained by using R
C
= 6 Å.
Furthermore, this kind of network preserves the
memory of the protein structure, i.e. it changes if the
protein 3D structure changes. In the second step, the
protein function is then introduced, by attributing to
the links the role of a specific physical interaction.
In the present case, it is an electromagnetic
interaction, that describes the response of the protein
to different electrical solicitations, thus the links
correspond to elementary impedances. In particular,
since we are interested in monitoring the impedance
variation due to the protein activation, we use the
impedance of a simple RC parallel circuit, like the
couple CPE-Rp in the Randles cell.
Finally, the elemental impedance between the i,j-
th nodes is:
i,j
i,j
-1
i,j
i.j 0
l
1
Z=
A
ρ +iεεω
(1)
where A
i,j
=
π
(R
C
2
- l
i,j
2
/4), is the cross-sectional area
between two spheres of radius R
C
centered on the i-
th and j-th node, respectively; l
i,j
is the distance
between these centers, ρ is the resistivity, taken to be
the same for every amino-acid;
i= -1 is the
imaginary unit, ε
0
is the vacuum permittivity, ω is
the circular frequency of the applied voltage. The
relative dielectric constant of the couple of i-th and
j-th amino-acids, ε
i,j
, is expressed in terms of the
intrinsic polarizability of each amino acid. The
network is connected to an external bias by using
ideal contact on the first and last amino acid and
solved by using standard techniques. In particular,
analogously to the well known Hodgkin-Huxley
model, the problem statement consists in a set of
linear equations whose solution is performed by a
computational procedure, based on the Kirchhoff’s
laws. The network global impedance spectrum is
represented by a Nyquist plot for each configuration
of the protein 3D structure. The role of the
interaction radius, R
C
,
is still an open problem.
Recent investigations strongly suggest that it is
related to the level of protein activation (Kobilka,
2007; Alfinito, 2015b) and in the following section
we make use of this conjecture to interpret the
experiments.
Besides the electrical characterization, also a
preliminary description in terms of graph topology
was attempted. The molecule networks resulting
from setting the interaction radius to reasonable
values were found to be graphs with a small-world
structure (Albert, 2002). Small-world networks
emerge as intermediate configurations between the
limiting cases of regular lattices and completely
random graphs. Artificial generation of a small-
world network can be obtained by the Watts-
Strogatz model, in which a fraction p of the links of
a regular lattice is replaced with random links: of
course p is 0 for the regular lattice, and becomes 1
for a completely random network. In small-world
networks the distribution of node degrees is Poisson-
like, and the Average Path Length is short, like in
random networks, but the Clustering Coefficient is
quite high, like in regular lattices.
COMPLEXIS 2016 - 1st International Conference on Complex Information Systems
34
3.3 Results
As a preliminary test, we analyze the relative
variation of static impedance (resistance) for the
single protein in the presence/absence of the ligand.
Globally small differences are detectable and, in
agreement with experiments only two regions of R
C
values centred around 20 and 50 Å are of interest,
because the native state resistance exhibits a higher
value than the active state (Benilova, 2008). In
agreement with (Kobilka, 2007) we have postulateda
functional dependence of the odorant concentration
on the value of R
C
(Alfinito, 2011b).
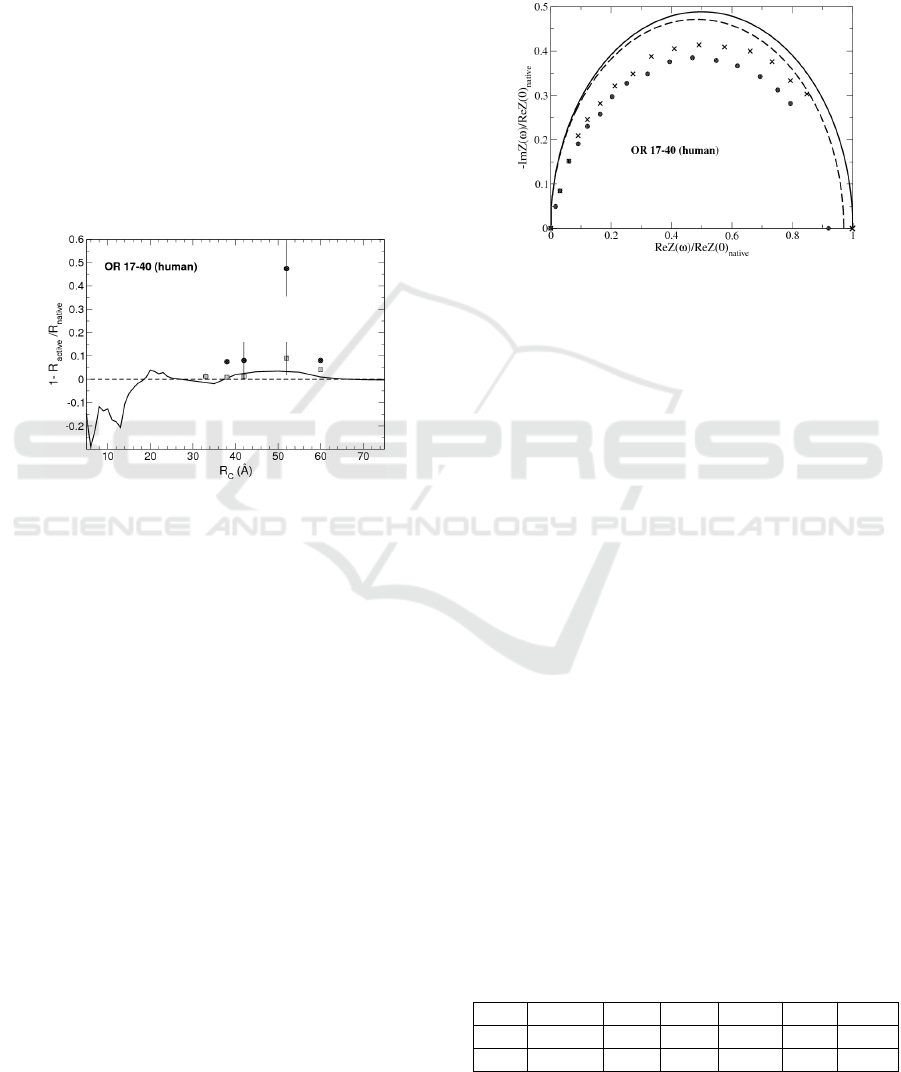
In Figure 3 we report the relative resistance
variations, calculated with our model. Furthermore,
symbols describe the same quantity as observed in
experiments performed with helional and heptanal.
Figure 3: Relative resistance variation for human olfactory
receptor OR 17-40. Continuous line is the calculated
result, full circles ( empty squares) are the dose response
to heptanal (helional) by experimental data (Benilova,
2008), here given in terms of R
C
(Alfinito, 2011a).
The bump at lower value of R
C
, should be, in a
similar way, attributed to the second bump observed
by SPR. As we can see, the agreement is qualitative
and more close to the heptanal than to the helional
data.
Further improvement of the model are necessary
to quantitatively fit experimental outcomes (Alfinito,
2015b).
These results are confirmed by the analysis of the
impedance spectrum. The Nyquist plots, as
calculated by using the native and active state of OR
17-40 are reported in Figure 4. Continuous line
describes the receptor impedance spectrum in the
native state calculated by using R
C
= 50Å and the
dashed line to the active state with the same value of
R
C
. As a comparison, data obtained with protein
exposed to a concentration of 10
-10
M of heptanal,
normalized to the static impedance of the protein not
exposed to the odorant are also reported (dots)
(Alfinito, 2011b). The agreement is qualitatively
correct, i.e. the spectrum is represented by a simple
semicircle and the shrinking of the semicircle, when
going to the activated state, is also reproduced. As a
general outcome, the comparison between theory
and experiment is quite good and able to explain the
quite novel results concerning the protein response
to the specific ligands.
Figure 4: Normalized Nyquist plot of human OR 17-40.
Lines (continuous for native state, dashed for active state)
refer to calculated data, with R
C
=50Å. Dots refer to
experimental data for the protein with heptanal at a
concentration of 10
-10
M, crosses to the protein without
heptanal (Benilova, 2008).
Finally, concerning the small-world features of
the network, the node degree distribution was
calculated, giving a bell-shaped curve. The
calculation was done at two values of the interaction
radius, R
C
= 6Å and R
C
= 12Å, and the behaviour of
the frequency curve in the transition from the
smaller to the larger R
C
value was as expected, i.e.
the bell width increases while its mean value gets
larger like in the Poisson distribution, which is
considered a good approximation for random
networks (Figure 5).
The average path length L and the clusterization
coefficient C were also calculated, together with the
same values normalized to C
R
and L
R
(which are the
clusterization coefficient and the average path length
calculated for a regular lattice with equal number of
nodes N and equal mean degree, MD). The result
was a large C (far greater than O(N
-1
) which would
be typical of random graphs, and similar to C
R
) and
a relatively low average path length, which is
compatible with the hypothesis of a small-world
network with significant clusterization. This is
particularly true for the lower R
C
case (see Table 1).
Table 1: Network parameters for human OR 17-40.
N R
C
(Å) MD C C/C
R
L L/L
R
315 6 6.1 0.56 0.93 8.3 0.31
315 12 29.5 0.59 0.81 3.0 0.53
Proteotronics: Application to Human 17-40 and Bacteriorhodopsin Receptors
35
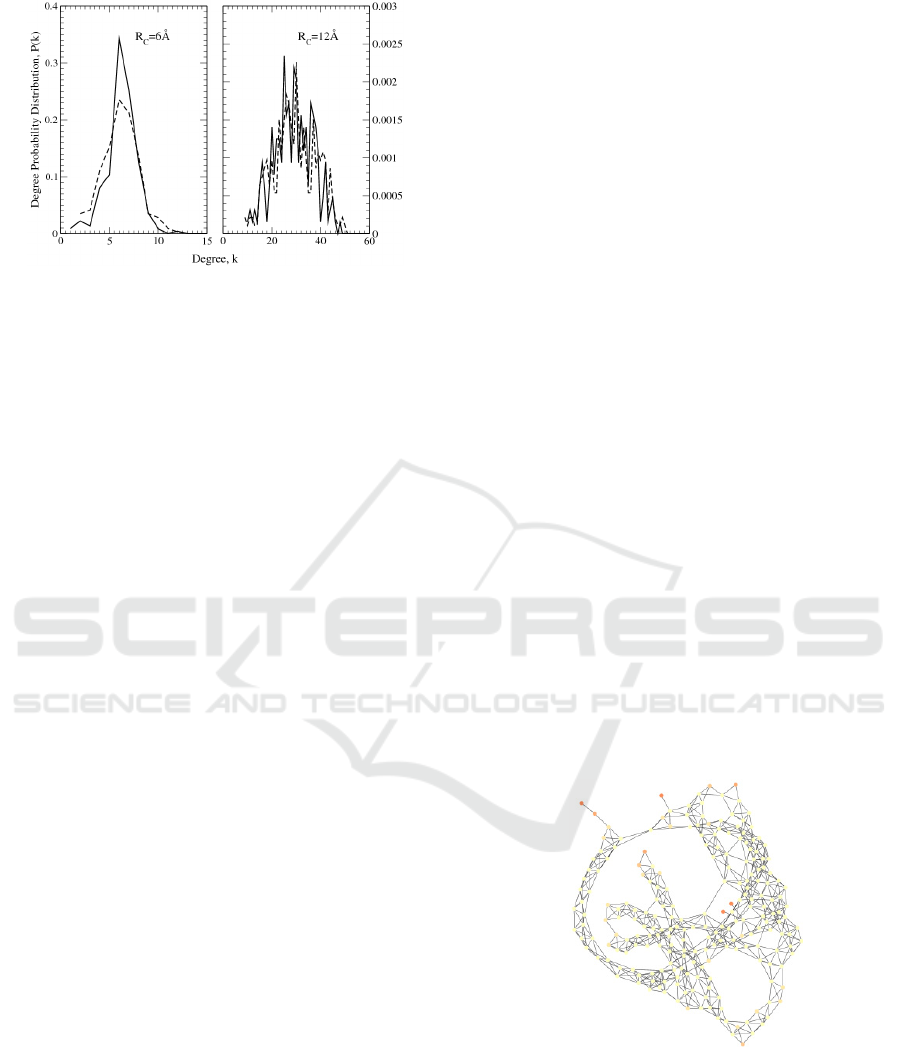
Figure 5: Degree distribution of human OR 17-40 (dashed
lines) and bacteriorhodopsin (continuous lines), obtained
by using R
C
= 6 Å and R
C
= 12 Å.
4 BACTERIORHODOPSIN
4.1 Generalities and Experiments
Another receptor of interest for applications is
bacteriorhodopsin (bR), a protein found in a
primeval organism, the Halobacterium salinarum,
specifically in a part of its cell membrane called the
purple membrane (PM). This membrane is a very
thin lipidic film of 5 nm, about the protein height,
and shows a quite stable structure.
Bacteriorhodopsin is able to convert the sun light
into an electrical potential across the host cell
membrane. In doing so, the conjugated dye, the
retinal, changes its structure, also inducing the
complete protein conformational change.
Recently, several experiment have analyzed bR,
in vitro, both in dark and light. The aim was to test
the possibility of recovering the activity of this
protein outside its natural environment and finally to
convert the activation due to light into an electrical
signal useful for technological applications.
In doing so, patches of PM were anchored on a
conductive substrate and connected to an external
circuit. The connection was made mainly with two
different techniques, i,e, by using an extended
transparent conductive contact (Jin 2006, Ron 2010)
or the tip of a c-AFM (Casuso, 2007;
Mukhopadhyay, 2014).
These investigations focused on the
measurement of the current-voltage (I-V)
characteristics in static conditions and revealed the
great stability of bacteriorhodopsin toward thermal,
mechanical and electrical stress (Casuso, 2007). This
protein shows a medium-gap conductivity in dark
which can be significantly enhanced by light. The
observed I-V characteristics are super-linear, and
this feature becomes more evident by increasing the
applied bias. Therefore, the charge transport across
the protein is mainly attributed to a tunneling
mechanism. Tunneling can be described like the
crossing of rectangular barriers at low bias (direct
tunneling), or the crossing of triangular barriers
(injection tunneling), at high bias. In the latter
regime, a growth of the protein current of about 5
orders of magnitude has been observed (Casuso,
2007). All these impressive features require a deep
investigation about the microscopic origin of the
responsible mechanisms. Furthermore, they have
arisen great expectations in technology, so inspiring,
for example, the realization of a Grätzel cell based
on it (Renugopalakrishnan, 2014).
It has been observed that the light protein
activation may produce an enhancement of the
current as large as the 100% (Jin, 2006). In the
perspective of a future use of this protein in solar
cell, this outcome is of extraordinary relevance.
4.2 Theory
The network protein analogue has been also used to
describe the observed I-V characteristics in bR and
other proteins (Alfinito, 2015a). In Figure 6, the plot
of the protein graph, drawn by using R
C
= 6 Å, is
reported. As a matter of fact, light induces a
conformational change that this model is able to
account for. Accordingly, a sequential tunneling
mechanism is introduced by using a stochastic
approach within a Monte Carlo solving technique.
Figure 6: Graphical representation of bacteriorhodopsin in
its native state. Network is obtained by using R
C
= 6 Å.
The non-linear shape of the I-V characteristic,
reported in (Jin, 2006; Casuso, 2007), is accounted
for by considering a tunneling probability including
direct and injection regimes, respectively, as in
(Alfinito, 2011a; Alfinito, 2014):
COMPLEXIS 2016 - 1st International Conference on Complex Information Systems
36

Φ≥
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
Φ
Φ
β−=
Φ<
⎟
⎟
⎠
⎞
⎜
⎜
⎝
⎛
−Φβ−=
ij
ij
ij
IN
ij
ij
ij
DT
eV ,
2eV
expP
eV ,
2
eV
expP
(2)
where V
ij
is the potential drop between the couple of
i-j amino acids,
=/2m
ij
2lβ
⎟
⎠
⎞
⎜
⎝
⎛
=
, m the electron
effective mass. In this model, to tunnel an energy
barrier is equivalent to reduce the resistivity from its
maximum value down to ρ
Τ
(V):
ρ
Τ
(V) =
ρ
min
(3a)
in direct tunneling regime (rectangular barrier), and:
Φ≥
⎟
⎠
⎞
⎜
⎝
⎛
Φ
−ρ+
Φ
ρ=ρ
Φ<ρ=ρ
eV ,
eV
1
eV
)V(
eV ,)V(
minMAX
MAX
(3b)
in the injection tunneling regime (triangular barrier).
4.3 Results
The I-V characteristics of bR in dark and light have
been calculated by using the aforementioned
theoretical approach. In particular, the model
parameters have been tuned on the data given by
(Casuso, 2007), which cover the largest bias range.
In such a way, the experiments have been
reproduced with good accuracy (Alfinito, 2011a).
Data in light were given by (Jin, 2006) in the range
0-1 V. A fine agreement with these data has been
found by taking into account the multiple effects that
light produces on a sample of light receptors. In
particular, light irradiation transfers energy to the
single protein by means of two basic processes: (i)
the protein activation, specifically the change of its
3D structure consequent the ligand capture; and (ii)
the protein excitation, specifically the increasing of
its free-energy without conformational change
(Alfinito, 2015).
In the framework of our model, we introduce
process (i) by changing the 3D protein structure
input, while process (ii) is described by changing the
value of R
C
. Finally a sample of proteins irradiated
with light of appropriate wavelength experiences
both the processes, with some proteins activated and
some proteins excited. The percentage of
activated/excited proteins has been fitted by using a
Hill-like equation, and it is associated with a specific
value of R
C
(Alfinito, 2015). Therefore, following
this scheme, the current response of bR samples has
been reproduced by using a binary mixture of native
and active states:
(i) the sample in light corresponds to R
C
= 6.3 Å
with 96% of activated proteins, (ii) the sample in
dark corresponds to R
C
= 5.8 Å and 100% of
proteins in the native state. By using these
guidelines, we reproduce the photocurrent measured
in experiments. Figure 7 reports the photocurrent of
bR calculated within our model, in the same bias
range explored by (Casuso, 2007).
Figure 7: Photocurrent for the bacteriorhodopsin,
calculated as described in the text. In the inset, the same
data in the bias range 0-1 V and the experimental
outcomes (circles) (Jin, 2006).
Furthermore, the theoretical approach is able to
foresee photocurrent over a larger (a priori, arbitrary
large) bias range. At present only the bias range
described in the inset has been explored by
experiments (Jin, 2006).
Also for this protein we checked the network
small-world character. The node degree distribution
was again bell-shaped (not shown for the sake of
brevity) and very similar to what was found for OR
17-40 (see Fig.5). The (absolute and relative)
average path length and clusterization coefficient are
shown in Table 2.
Table 2: Network parameters for bacteriorhodopsin.
N Rc (Å) MD C C/C
R
L L/L
R
222 6 6.2 0.58 0.96 7.0 0.37
222 12 28.9 0.59 0.82 2.6 0.60
Again, the lower R
C
case is more easily framed
in a small-world context, having large C/C
R
and low
L/L
R
.
5 CONCLUSIONS
Proteotronics is an emergent branch of electronics,
Proteotronics: Application to Human 17-40 and Bacteriorhodopsin Receptors
37

able to describe the electrical properties of proteins.
The model is physically plausible and sufficiently
flexible to be tailored for describing different
experimental conditions. Here we have investigated
two specific case concerning human OR 17-40 and
bacteriorhodopsin protein receptors with promising
chances of being used in developing a new
generation of electronic devices. The results are also
of basic interest in advancing the present knowledge
on the microscopic mechanisms responsible of
protein functioning inside living cells.
REFERENCES
Albert, R. and Barabási, A.L. 2002. Statistical mechanics
of complex networks. Reviews of modern physics,
74(1), p.47.
Alfinito, E, Millithaler, J-F. and Reggiani, L. 2011.
Charge transport in purple membrane monolayers….
Physical Review E 83, no. 4 pp. 042902.
Alfinito, E., Millithaler, J.F., Reggiani, L., et al. 2011.
Human olfactory receptor 17-40 …. RSC Advances,
1(1), pp.123-127.
Alfinito, E and Reggiani, L. 2014 Opsin vs opsin: New
materials for biotechnological applications. Journal of
Applied Physics 116 (6), p. 064901.
Alfinito, E., Pousset, J.-F., and Reggiani, L. 2015.
Proteotronics: Development of Protein-Based
Electronics, Pan Stanford, Singapore.
Alfinito, E. and Reggiani, L. 2015. Mechanisms
responsible for the photocurrent in bacteriorhodopsin.
Physical Review E, 91(3), p.032702.
Benilova, I.V., Vidic, J.M., Pajot-Augy, et al. 2008.
Electrochemical study of human olfactory receptor OR
17–40…. Materials Science and Engineering: C,
28(5), pp.633-639.
Berg, J.M., Tymoczko, J.L, Stryer, L. 2002 Biochemistry,
W H Freeman, New York, V edition.
Berman, H.M., Westbrook, J., Feng, Z., et al. 2000. The
protein data bank. Nucleic acids research, 28(1),
pp.235-242.
Casuso, I., Fumagalli, L., Samitier, J., et al. 2007.
Nanoscale electrical conductivity of the purple
membrane monolayer. Physical Review E vol 76(4),
pp. 04191.
De Nunzio, G., Cataldo, R. and Carlà, A. 2015. Robust
Intensity Standardization …. Journal of digital
imaging, pp.1-11.
Guascito, M.R., Chirizzi, D., Malitesta, C. et al. 2011.
Mediator-free amperometric glucose biosensor ….
Analyst, 136(1), pp.164-173.
Hou, Y., Jaffrezic-Renault, N., Martelet, et al. 2007.A
novel detection strategy for odorant molecules…
Biosensors and Bioelectronics, 22(7), pp.1550-1555.
Jin, Y., Friedman, N., Sheves, M., He, T. and Cahen, D.
2006. Bacteriorhodopsin (bR) as an electronic
conduction medium: Current transport through bR-
containing monolayers. Proceedings of the National
Academy of Sciences, 103(23), pp.8601-8606.
Kobilka, B.K. and Deupi, X. 2007. Conformational
complexity of G-protein-coupled receptors. Trends in
pharmacological sciences, 28(8), pp.397-406.
Levasseur, G., Persuy, M.A., Grebert, D., et al. 2003.
Ligand-specific dose–response…. European Journal
of Biochemistry
, 270(13), pp.2905-2912.
Nagy, L., Magyar, M., Szabo,T., et al. 2013. Photosyntetic
Machineries in Nano-Systems. Current Proteins and
Peptide Science, I5 pp.363-373
Renugopalakrishnan, V., Barbiellini, B., King, C., et al.
2014. Engineering a Robust Photovoltaic Device with
Quantum Dots and Bacteriorhodopsin. J. Phys. Chem.
C, 118, pp.16710-16717
Ron, I., Sepunaru, L., Itzhakov, S., et al 2010. Proteins as
electronic materials…Journal of the American
Chemical Society, 132(12), pp.4131-4140.
Shrödinger, E. 1944 What is life? Cambridge University,
Cambridge.
Vidic, J.M., Grosclaude, J., Persuy, M.A., et al. 2006.
Quantitative assessment of olfactory receptors
activity… Lab on a Chip, 6(8), pp.1026-1032.
COMPLEXIS 2016 - 1st International Conference on Complex Information Systems
38
