
Healthcare Software Process Model
Doctoral Consortium
Marie Travers
Lero, University of Limerick, Limerick, Ireland
1 RESEARCH PROBLEM
Software can provide opportunities for innovation
and competitive differentiation. There are challenges
to this, for example software in products increases
complexity and, in turn, can compromise quality. By
improving process efficiencies, industries are able to
focus on areas such as innovation and reduce time to
market.
Health Information Systems (HIS) are used in
healthcare to make decisions on:
Diagnosis and treatment
Financial and administrative matters
Best practices
Manipulation of clinical data which needs to be
secure, accurate and timely
Developing Health Information Systems (HIS) is a
complex task for a number of reasons. For example
healthcare mistakes can have serious consequences
that can affect patients’ lives as well as having a
high financial cost all within a highly regulated
industry
Currently there is no comprehensive
methodology for developing HIS. Relevant HIS
regulations state what needs to be done to comply
but not how. Technology is evolving quickly.
2 OUTLINE OF OBJECTIVES
This PhD research has carried out case studies and
action research of various areas within health and
innovation to inform the research development. A
research fellowship in innovation was undertaken
for a duration of 10 months. A further 3 months was
spent in a medical device company observing how
change is managed. Interviews were also carried out
in hospitals with key staff members to tease out the
key concepts/issues/concerns/etc. of implementing a
new IT healthcare system.
The following research objectives are addressed
in this PhD:
To improve the way software is developed in a
highly competitive regulated domain such as
Healthcare informatics
To identify areas that aid successful design,
development and implementation of health
information systems using a model to support it
To develop a model that addresses the needs of
complex healthcare projects
To develop an integrated set of process models
that combine recommended practices with the
needs of the information systems domain with
inputs from literature, medical device industry,
hospitals and a successful innovation
programme.
3 STATE OF THE ART
Software development within the health domain is
different from other domains for a number of
reasons. Healthcare is a fragmented industry with,
for example, independent hospitals, medical device
companies etc. Within healthcare, different
stakeholders have different objectives such as non-
profit, profit etc. There are specific industry-focused
regulations. There are also healthcare regulations.
For such reasons, change becomes a complex task
within healthcare. Indeed, change management
requires a specific approach to transition an
organisation to a desired future state (Benjamin and
Levinson 1993). Within a hospital context, the
various steps required to achieve a desired future
state is of particular importance to ensure that
patient safety is a priority and quality is not
jeopardized (Cazzaniga and Fischer 2015). The
objective of change management is typically to
provide a structured approach to implement change
in a controlled manner while adhering to specific
requirements on functionality, budget and time
through various deliverables or milestones. The
Oxford handbook of innovation (2006) points out
that innovation in healthcare and software industries
is more complex due to regulators sometimes
Travers, M.
Healthcare Software Process Model - Doctoral Consortium.
In Doctoral Consortium (DCBIOSTEC 2016), pages 35-39
35

restricting innovation. Gottlieb and Makower (2013)
point out that innovation in technology offers
perhaps the best chance to tackle rising healthcare
costs while maintaining high-quality care. In the
healthcare industry medical devices are
manufactured to aid patients. To safeguard patient
safety and minimize risk such devices are regulated.
In America the regulatory body is the Food and
Drug Administration (FDA) whereas in Europe the
regulatory body is the European Commission (EC)
using the Medical Devices Directive (MDD)
(Travers and Richardson 2015). The FDA issues
guidance on development stating what needs to be
done but how to do it is up to the software producer.
Regulators can approve standards also. Recently the
MDD amended its definition of a medical device to
include software; therefore software could be
classed as a standalone medical device (Travers and
Richardson 2015). This clarified that standalone
software can be a device in its own right, software
can be embedded within a medical device or be used
in the manufacturing of a device (Travers and
Richardson 2015). Travers and Richardson (2015)
point out that the medical device industry faces
challenges, including competitors, government
regulations, and productivity and quality issues.
Standards and guidelines have been developed to aid
in achieving the safest possible product and
individual companies can decide which
methodology to use (Travers and Richardson 2015).
There does not seem to be a method for quantifying
just how much process is enough (Travers and
Richardson 2015). To ensure high quality products
companies attempting to improve their products,
also have to change their development processes
(Travers and Richardson 2015). Companies
implementing process change can benefit from using
a change management model but usually published
models relate to organization change as opposed to
process changes (Travers and Richardson 2015).
Travers and Richardson (2015) state that
introducing change must be a formalised planned
process. There are many change models in existence
but the three more common ones are:
Lewin’s Change Management Model
McKinsey 7-S Model
Kotter’s 8 Step Change Model (2005)
Lewin’s Change Management Model was developed
in the 1950s. It is easy to use but it is timely to
implement. McKinsey 7-S Model was developed in
the 1970s. It provides a more holistic approach and
each part is interrelated so changes affect all parts.
Kotter’s model was developed in the 1990s it also
provides a more holistic approach but the focus is on
preparing for change instead of the actual change
Kotter’s model was chosen as the most
appropriate model to research innovation and change
management in this research. The healthcare
industry can learn a lot from existing business
models that have successfully been used in other
industries. Kotter’s 8 step change model (2005) lists
the following:
1. Establish a Sense of Urgency
2. Form a Powerful Guiding Coalition
3. Create a Vision
4. Communicate the Vision
5. Empower Others to Act on the Vision
6. Plan for & Create Short-Term Wins
7. Consolidate Improvements and Produce Still
More Change
8. Institutionalise new approaches
4 METHODOLOGY
To understand innovation in health a 10 month
research fellowship was undertaken. This fellowship
uses immersion and observation in hospitals to
facilitate an understanding of procedures and
innovations in health.
A single case study was commenced within a
Medical Device company. The researcher spent
three months onsite. In addition to being a
participant-observer on the project, the researcher
held one-to-one interviews with software team
members.
To study hospitals within healthcare the
approach taken was to undertake a single case study
considering the unique opportunity to capture an
empirically-rich account of specific phenomena (Yin
2013) within a healthcare context. Thus from a
epistemological and analytical standpoint, a single
case study was deemed suitable to test and build a
hypotheses on IT change management within a
publically funded hospital.
One-to-one interviews were held with eleven key
staff members who were all involved in IT change to
various degrees. Since the interviewees were
healthcare experts within public hospitals, some
were difficult to access. To overcome this, a
snowballing sampling strategy was employed to
identify other experts in this field within the sample
population. This proved to be useful since each
expert was able to recommend the next relevant
expert. Through a structured interview technique,
this provided a more balanced insight to uncover the
DCBIOSTEC 2016 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
36

change process. The structured interviews supported
the research methodology by ensuring consistency,
i.e. each interview was presented with exactly the
same questions in the same order. The questions had
to be short as the health experts had limited time
available to partake in the case study.
The interviewees’ answers were reliably
aggregated and comparisons were made between the
different interviewees. A number of emerging
themes were identified using coding to categorise
the text – this allowed the building of a story around
specific events, facts, and interpretations.
The eleven interviewees were all experienced in
software change and processes. They included
software developers, clinicians and IT managers.
The interviewees’ work experience spanned from 4
to 30 years. Participant’s interview data was
analyzed to understand the change process within
the case study. The data was reviewed within the
structure of Kotter’s change model steps 1 to 8,
which allowed the researcher to understand how
change had been made within the hospital setting.
This facilitated gaining a rich insight of the working
environment.
5 EXPECTED OUTCOME
Currently there is no comprehensive methodology
for developing HIS
Relevant HIS regulations state what needs to be
done to comply but not how
Technology is evolving quickly
The proposed methodology has the potential to:
Improve decision-making, monitoring and cost
management
Improve communication and learning
Create better quality of life for patients
Innovation usually begins with an idea. An idea is
just the first step on a sometimes-long path to
successful innovation. Technical change usually
requires organizational changes also. Organisation
and process support or buy-in is required for
successful implementation as this type of change is
difficult due to potential resistance, competing ideas,
or failure to be sustained. Therefore innovators not
only need their original idea but also a vision of how
things would change if the innovation succeeds.
Organizational and process change is needed for
implementation of ideas in achieving success.
Significant innovations can be resisted, fall victim to
competing ideas, or fail to be sustained.
For the medical device company case study
Kotter’s change model was appropriate. Travers and
Richardson (2015) point out that process
improvement should be managed through the use of
a model so that the change is implemented
completely in an organisation. Travers and
Richardson (2015) state that Kotter’s change model
was a good basis, but note that there were aspects of
the model that were overlooked and some elements
were unnecessary. Travers and Richardson (2015)
point out that a more tailored and specific model is
required.
Analysing the findings from the hospital study
key themes were identified. The results indicate that
some aspects of Kotter’s change model is useful to
successfully manage change but would need to be
modified for a healthcare context. This case study
facilitated analysis from a hospital perspective and
the findings informed and enhanced a proposed
model which is called the Healthcare Innovation and
Quality Change (HIQC) Model (See Figure 1). The
HIQC model is split into three relevant sections
which acknowledges that change occurs through key
iterative processes namely identification, ideation
and strategy. These three phases are similar to the
phases in the innovation research fellowship. Each
phase comprises of a number of requirements and
practices which emerged from the research.
Figure 1: Proposed model version 1.
6 STAGE OF THE RESEARCH
A literature review was undertaken to understand the
health, software and innovation requirements of the
healthcare industry. This identified gaps, which are
reflected in the research questions. An innovation
research fellowship was completed which involved
access to both public and private hospitals in Ireland
to ascertain where innovations could help improve
Healthcare Software Process Model - Doctoral Consortium
37
existing practices or treatments. A placement in a
medical device company was also completed to
understand how process improvement is undertaken
in such a regulated healthcare industry. A model has
been researched and developed (see figure 1). The
next step is the further refinement and validation of
this model, which will be useful as currently there is
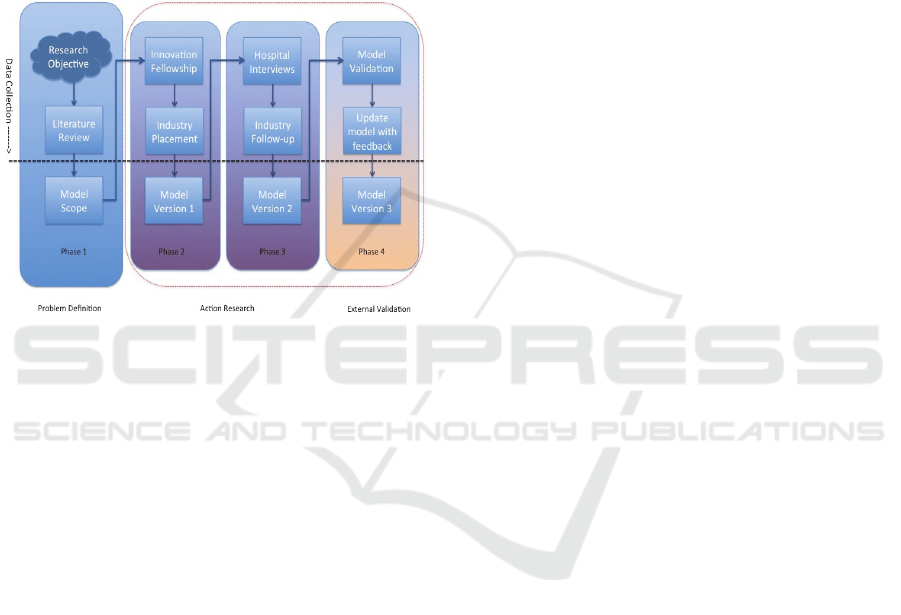
no model currently available. Figure 2 is a diagram
that illustrates my Phd progression to date by
identifying the various phases involved in this
research.
Figure 2: Research plan.
Each phase above the dotted line in the diagram
above starts with extensive data collection.
Phase 1:
The problem definition involved extensive data
gathering by analysing existing research and
software engineering practices to aid in scoping an
initial model.
Phase 2:
Action research involved carrying out case studies
and action research to aid in the research and
development and enhancement of a version 1 of the
model. The case studies were an innovation research
fellowship and an industry placement in a medical
device company. This allowed the researcher to gain
an understanding of healthcare innovation.
Phase 3:
Action research involved carrying out more case
studies to aid in the development of a version 2 of
the model again enhancing it. The case studies were
hospital interviews and a follow-up industry
placement in a medical device company. Currently
the researcher is completing this phase. This allowed
the researcher to gain an understanding of healthcare
process improvement.
Phase 4:
External Validation involves legitimising the model
proposed in this PhD research. This involves
evaluating said model by validating it with experts
such as entrepreneurs or software engineers. After
validation the model will have to be then updated
after gathering feedback and the results
disseminated. The model identified has the potential
to aid the development of innovation in health
software.
It is envisaged that this validation and update stage
should take 6 months. This research builds a new
model to address shortcomings identified in my
research. As part of future research it is planned to
hold focus groups to use expert opinion to validate
the new healthcare model. This new model will be
used in a follow-up case study to examine the
implementation of a new IT healthcare system.
Moving forward the new model will act as a guide
for IT personnel considering the implementation of a
new hospital system, i.e. they use the framework as
a step-by-step guide.
ACKNOWLEDGEMENTS
This research is supervised by Prof. Ita Richardson,
Lero, University of Limerick, Ireland.
REFERENCES
Moore, R., Lopes, J., 1999. Paper templates. In
TEMPLATE’06, 1st International Conference on
Template Production. SCITEPRESS.
Smith, J., 1998. The book, The publishing company.
London, 2
nd
edition.
AAMI (2012) TIR45:2012 Guidance on the use of AGILE
practices in the development of medical device
software 2012, Association for the Advancement of
Medical Instrumentation.
ANSI/AAMI/IEC, 62304:2006 Medical Device Software-
Software life cycle processes, 2006, Association for
the Advancement of Medical Instrumentation. p. 67.
Benjamin, R. I., and Levinson, E. (1993). A framework for
managing IT-enabled change. Sloan Management
Review, 34(4), 23-33.
Burton, J., (2008) A Software Risk Management
Capability Model for Medical Device Software,
Unpublished thesis (PhD), University of Limerick.
Cawley, O., Wang, X., Richardson, I., (2013) Regulated
Software Development-An Onerous Transformation,
in Foundations of Health Information Engineering and
Systems: Springer, 72-86.
Cawley, O., Richardson, I., Wang, X., (2011) Medical
Device Software Development - A Perspective from a
DCBIOSTEC 2016 - Doctoral Consortium on Biomedical Engineering Systems and Technologies
38

Lean Manufacturing Plant, O’Connor, R. V., Rout, T.,
McCaffery, F., and Dorling, A., ‘Software Process
Improvement and Capability Determination’, Berlin,
Springer, 84 – 96.
Cazzaniga, S., and Fischer, S. (2015). How ICH Uses
Organizational Innovations to Meet Challenges in
Healthcare Management: A Hospital Case Study. In
Challenges and Opportunities in Health Care
Management (pp. 355-361). Springer International
Publishing.
Donabedian A. (1980). Explorations in Quality
Assessment and Monitoring, Volume I. The Definition
of Quality and Approaches to its Assessment. Ann
Arbour, MI , Health Administration Press, pp. 1–164.
EU, Council Directive 93/42/EEC of the European
Parliament and of the Council, Concerning Medical
Devices, E. Council, Editor 1993, Official Journal of
the European Union.
EU, Directive 2007/47/EC of the European Parliament and
of the Council, 2007, Official Journal of the European
Union.
FDA, Code of Federal Regulations 21 CFR Part 820,
(2009) U.F.a.D. Administration, Editor April 2009.
Forte, G., (1997) ‘Managing Change for Rapid
Development’, IEEE Software 14(6), 114–123.
Scott, Josh, A Role for Entrepreneurs: An Observation on
Lowering Healthcare Costs via Technology
Innovation, American Journal of Preventive Medicine,
Volume 44, Issue 1, Supplement 1, January 2013,
Pages S43-S47
Hayes, S. & Richardson, I., (2008), Scrum Implementation
using Kotter’’s Change Model, 9th International
Conference on Agile Processes and eXtreme
Programming in Software Engineering, Limerick,
Ireland, Lecture Notes in Business Information
Processing 2008, vol 9, Part 6, 10th-14th June, pp.
161-171.
Innovation in HealthcareFrom Research to Market to
Health-systems to Patient Main conclusions from
2010, 2011, 2012 conferences
Innovation in Ireland 2008.
Kissick, (W). (1994). Medicine's Dilemmas: Infinite
Needs versus Finite Resources, Yale University Press.
Kotter, J., (2005) Leading Change: Why Transformation
Efforts Fail, Harvard Business School Press, Boston.
Malerba Franco (2006) The Oxford Handbook of
Innovation Sectoral Systems: How and Why
Innovation Differs across Sectors Edited by Jan
Fagerberg and David C. Mowery
McCaffery, F., Casey, V., Sivakumar, M.S., Coleman, G.,
Donnelly, P., Burton, J., (2012) Medical Device
Software Traceability, Software and Systems
Traceability, Ed. Zisman A., Cleland-Huang J. and
Gotel, O., Springer Verlag Publishers, pp 321 – 340.
MEDDEV 2.1/6 (2012) Guidelines on the qualification
and classification of stand alone software used in
healthcare within the regulatory framework of medical
devices, European Commission.
Miles, M., Huberman, A. (1994) Qualitative Data
Analysis, 2nd edn. SAGE Publications, USA.
Spence, J.W. (2005) There has to be a better
way![software development] in AGILE Conference,
July 24 - July 29, 2005. Denver, CO, United states:
Inst. of Elec. and Elec. Eng. Computer Society, 272-
278.
Sammon, D., & Adam, F. (2007). Information Systems as
change agents–the case of a failed implementation in
the Irish Health Service. Cahier de la Recherche de
l’ISC Paris (CRISC), 15, 223-246.
Travers, M. and Richardson, I. (2015) 'Medical Device
Software Process Improvement – A Perspective from a
Medical Device company', in 8th International
Conference on Health Informatics, Healthinf 2015,
Lisbon, Portugal.
Yin, R. K. (2013). Case study research: Design and
methods. Sage publications
Healthcare Software Process Model - Doctoral Consortium
39
