
Preliminary Evaluation of a Silent Speech Interface based on
Intra-Oral Magnetic Sensing
Lam A. Cheah
1
, Jie Bai
1
, Jose A. Gonzalez
2
, James M. Gilbert
1
, Stephen R. Ell
3
,
Phil D. Green
2
and Roger K. Moore
2
1
School of Engineering, The University of Hull, Kingston upon Hull, U.K.
2
Department of Computer Science, The University of Sheffield, Sheffield, U.K.
3
Hull and East Yorkshire Hospitals Trust, Castle Hill Hospital, Cottingham, U.K.
Keywords: Assistive Technology, Silent Speech Interface, Permanent Magnet Articulography, Intraoral Magnetic
Sensing.
Abstract: This paper addresses the hardware challenges faced in developing a practical silent speech interface (SSI)
for post-laryngectomy speech rehabilitation. Although a number of SSIs have been developed, many are
still deemed as impractical due to a high degree of intrusiveness and discomfort, hence limiting their
transition to outside of the laboratory environment. The aim of this paper is to build upon our previous
work, in developing a user-centric prototype and enhancing its desirable features. A new Permanent Magnet
Articulography (PMA) system is presented which fits within the palatal cavity of the user’s mouth, giving
unobtrusive appearance and high portability. The prototype is comprised of a miniaturised circuit
constructed using commercial off-the-shelf (COTS) components and is implemented in the form of a dental
retainer, which is mounted under roof of the user’s mouth and firmly clasps onto the upper teeth.
Preliminary evaluation via speech recognition experiments demonstrates that the intraoral prototype
achieves word recognition accuracy of 75.7%, slightly lower than its predecessor. Nonetheless, the intraoral
design is expected to improve the stability and robustness of the PMA system with a much improved
appearance since it can be completely hidden inside the user’s mouth.
1 INTRODUCTION
Speech is a key capability and the most natural form
of communication of human beings. However, there
are a variety of situations in which people wish to
communicate orally but where normal speech can be
either impossible or undesirable. For instance,
people with speech impairments who have
undergone laryngectomy: the surgical removal of
larynx as part of treatment for cancer or other
diseases affected the vocal cords. These post-
laryngectomy patients, who have lost their voice,
often find themselves struggling with their daily
communication and may experience a severe impact
on their quality of life (Braz et al., 2005; Fagan et
al., 2008). However, there are currently only a
limited number of post-laryngectomy voice
restoration methods available for these individuals:
oesophageal speech, the electrolarynx and speech
valves. Unfortunately, these methods are often
limited by their usability and abnormal voice quality
(Fagan et al., 2008; Gilbert et al., 2010). Whereas,
typing-based augmented and alternative
communication (AAC) devices are limited by slow
manual text input (Wang et al., 2012). Although
some improvements were achieved in term of
voicing quality of the electrolarynx and oesophageal
speech (Doi et al., 2010; Toda et al., 2012),
emerging assistive technologies (ATs) such as silent
speech interfaces (SSIs) have shown promising
potential in recent years as an alternate solution.
In principle, SSIs are devices that enable speech
communication to take place in the absence of
audible acoustic signals (Denby et al., 2010). Hence,
aside from use as a communication aid for speech
impaired individuals, SSIs can also be deployed in
acoustically challenging environment or where
privacy/confidentially is desirable. To date, a
number of SSIs have been proposed in an attempt to
extract non-acoustic information generated during
speech production and reproduce audible speech
using different sensing modalities. A comprehensive
108
Cheah, L., Bai, J., Gonzalez, J., Gilbert, J., Ell, S., Green, P. and Moore, R.
Preliminary Evaluation of a Silent Speech Interface based on Intra-Oral Magnetic Sensing.
DOI: 10.5220/0005824501080116
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 108-116
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

summary on different SSIs technologies were
presented in (Denby et al., 2010). Permanent magnet
articulography (PMA) is a type SSI that is based on
sensing the changes in the magnetic field generated
by a set of permanent magnet markers attached onto
the vocal apparatus (i.e. lips and tongue) during
speech articulation by using an array of magnetic
sensors located around the mouth (Fagan et al.,
2008; Gilbert et al., 2010), which shares some
similarities with the electromagnetic articulography
(EMA) (Toutios and Margaritis, 2005; Toda et al.,
2008; Wang et al., 2012). In contrast to EMA, PMA
does not explicitly provide the position of the
markers, but rather a summation of the magnetic
fields from magnets that are associated with a
particular articulatory gesture. Previous work
(Gilbert et al., 2010; Hofe et al., 2013a, 2013b;
Cheah et al., 2015) demonstrated the possibility of
performing automatic speech recognition (ASR) on
PMA data.
Despite the attractive attributes of SSIs, two
major challenges of building an effective SSI exist
in the form of hardware and processing software.
Preliminary discussions on the influential factors
(e.g. invasiveness, market readiness, potential
costing and etc.) affecting the SSIs’ implementation
were presented in (Denby et al., 2010). Earlier
PMA-based prototypes (Gilbert et al., 2010; Hofe et
al., 2013b) showed acceptable speech recognition
performance, but were not particularly satisfactory
in terms of their appearances, comfort and
ergonomic factors for the users. To address these
hardware challenges, a PMA prototype in the form
of a wearable headset (design based on a customised
pair of spectacles or a headband) comprising of
minituarised sensing modules and wireless
capability was developed (Cheah et al., 2015). The
second generation prototype was re-designed based
on a user-centred approach through utilising
feedback from user questionnaires and through
discussion with stakeholders including clinicians,
potentials users and their families. The appearance
and comfort of the prototype was much improved
and it demonstrated comparable performances to its
predecessors.
As illustrated in figure 1, the second generation
PMA system consists of a set of six cylindrical
Neodynium Iron Boron (NdFeB) permanent
magnets, four on the lips (ø1 mm × 5 mm), one at
the tongue tip (ø2 mm × 4 mm) and one on the
tongue blade (ø5 mm × 1 mm). These magnets are
currently attached using Histoacryl surgical tissue
adhesive (Braun, Melsungen, Germany) during
experimental trials, but will be surgically implanted
for long term usage. The remainder of the PMA
system is composed of a set of four tri-axial
Anisotropic Magnetoresistive (AMR) magnetic
sensors mounted on the wearable headset, a set of
microcontrollers, rechargeable battery and a
processing unit (e.g. computer/ tablet PC). Although
the prototype has many desirable hardware features,
it is not without limitations.
Figure 1: (a) A wearable PMA prototype designed in a
form of spectacles. (b) & (c) Placement of six magnets on
lips (pellets 1-4), tongue tip (pellet 5) and tongue blade
(pellet 6).
The present work builds upon the work of
(Cheah et al., 2015), to further improve and alleviate
the shortcomings from a hardware perspective. The
proposed prototype has several distinctive features,
such as being miniature in size, highly portable,
discreet and unobtrusive since it is hidden from sight
within the user’s mouth. The remainder of this paper
is structured as follows. Section 2 outlines the design
challenges of the intraoral version of the PMA
device. Section 3 describes the architecture of the
intraoral PMA prototype, followed by the
performance evaluation in Section 4. The final
section concludes this paper and provides an outlook
for future work.
2 DESIGN CHALLENGES
Despite the improvements made in the second
generation PMA prototype, it also has drawbacks.
Firstly, the performance of the external headset
cannot be maintained in certain real-life conditions
(i.e. exaggerated movement or sports activity) due to
issues with instability. If there is a considerable
movement of the headset on the user’s head, the
PMA system may need re-calibration/re-training to
avoid degradation in performance.
Secondly, wearing the headset over long periods
may not be comfortable, despite the fact that the
Preliminary Evaluation of a Silent Speech Interface based on Intra-Oral Magnetic Sensing
109
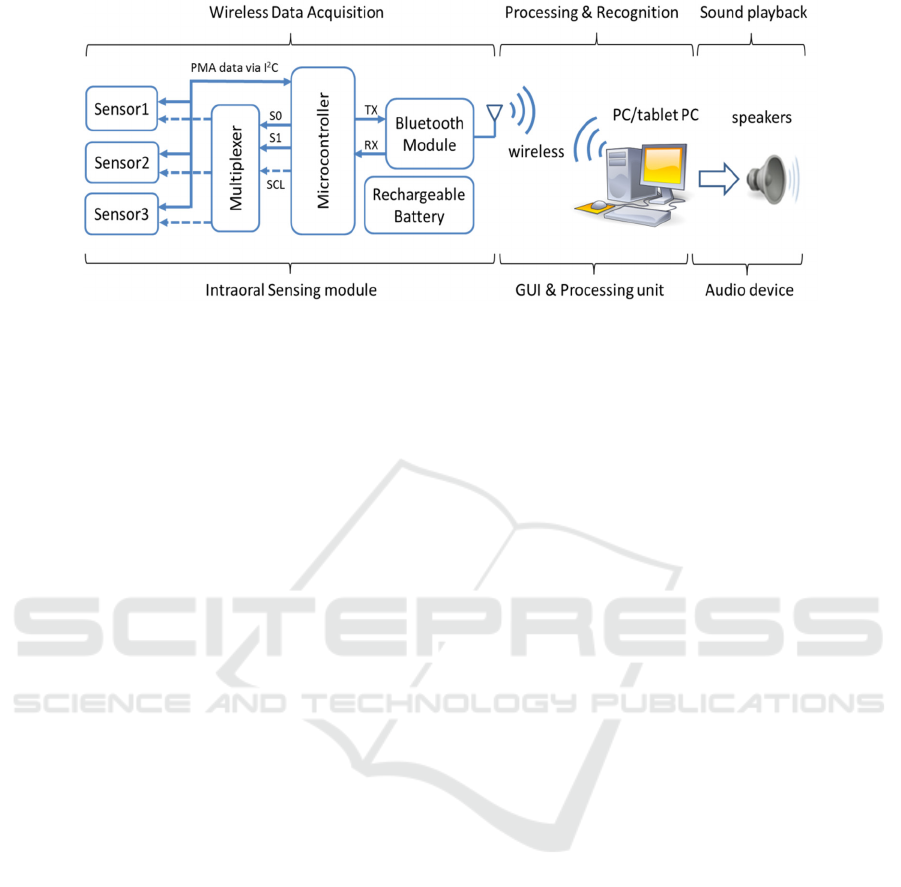
Figure 2: Simplified operation block diagram.
device was designed to be lightweight and
ergonomically friendly. Thirdly, the external version
of the PMA device may still be cosmetically
unacceptable to some users. Previous studies
indicated that the appearance is one of the most
important factors that affect the acceptability of any
AT by their potential end users (Hirsch et al., 2000;
Martin et al., 2006; Bright and Conventry, 2013).
To overcome these limitations, an intraoral
version of the PMA prototype, which fits under the
palate inside the user’s mouth in a form of a dental
retainer, was proposed. Being tightly clamped onto
the upper teeth means that the device would be more
stable than the previous wearable headset. Due to the
fact that the device is completely hidden from sight
during normal use, it is cosmetically inconspicous.
In addition, since the sensors are much closer to the
articulators than the external headset, the size of the
implants can be significantly reduced. Similar
intraoral-based designs have been previously
implemented for other non-speech related ATs with
various degree of success (Tang and Beebe, 2006;
Lontis et al., 2010; Park et al., 2012).
3 SYSTEM DESCRIPTION
3.1 Space Budget
The intraoral circuitry necessary to implement a
PMA system is made up of: three tri-axial magnetic
sensors, a wireless communication module, a
microprocessor to synchronise data capture and
communications and a suitable power source
capable of providing an appropriate operating
lifetime. This must be accommodated within the oral
cavity, without interfering with the natural tongue
articulation during speech. A recent study (Bai et al.,
2015) suggested that the palatal cavity is suitable to
house the intraoral circuitry because of its relatively
flat surfaces and proximity to the articulators. The
estimated space available in the palatal cavity on our
test subject is 59.7mm
3
.
3.2 Description of the Intraoral
Circuitry
In order to fit all necessary circuitry inside the
mouth, the size of the electronics and rechargeble
battery of the external version of PMA prototype
had to be shrunk down. The major components of
the PMA prototype are shown in figure 2. These are
implemented using a low-power ATmega328P
microcontroller, three tri-axial HMC5883L magnetic
sensors (AMR), a rechargeable Li-Ion coin battery
(capacity of 40mAh, 3.7V and 20 mm diameter ×
3.2 mm
thickness), and a wireless transceiver
(Bluetooth 2.0 module). The remainder of the
system shown in figure 3 consists of a processing
unit (e.g. computer/tablet PC) and a set six
permanent magnets (NdFeB) attached onto lips and
tongue in the same locations as illustrated in figure
1. The elements of the intraoral sensing system
(which have a total volume of 36.7 mm
3
) are
arranged as shown in figure 3(a). These may be
encapsulated and placed in the oral cavity as shown
in figure 3(d).
The positions of the magnets remained
unchanged from the earlier prototype but because of
the proximity of the sensors, significantly smaller
magnets (see Figure 3c) can be used: four on lips (ø1
mm × 4 mm), one on the tongue tip (ø1 mm × 1
mm) and one on the tongue blade (ø1 mm × 1 mm).
Note that the magnetic field strength decreases with
the cube of the distance away from the magnets.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
110
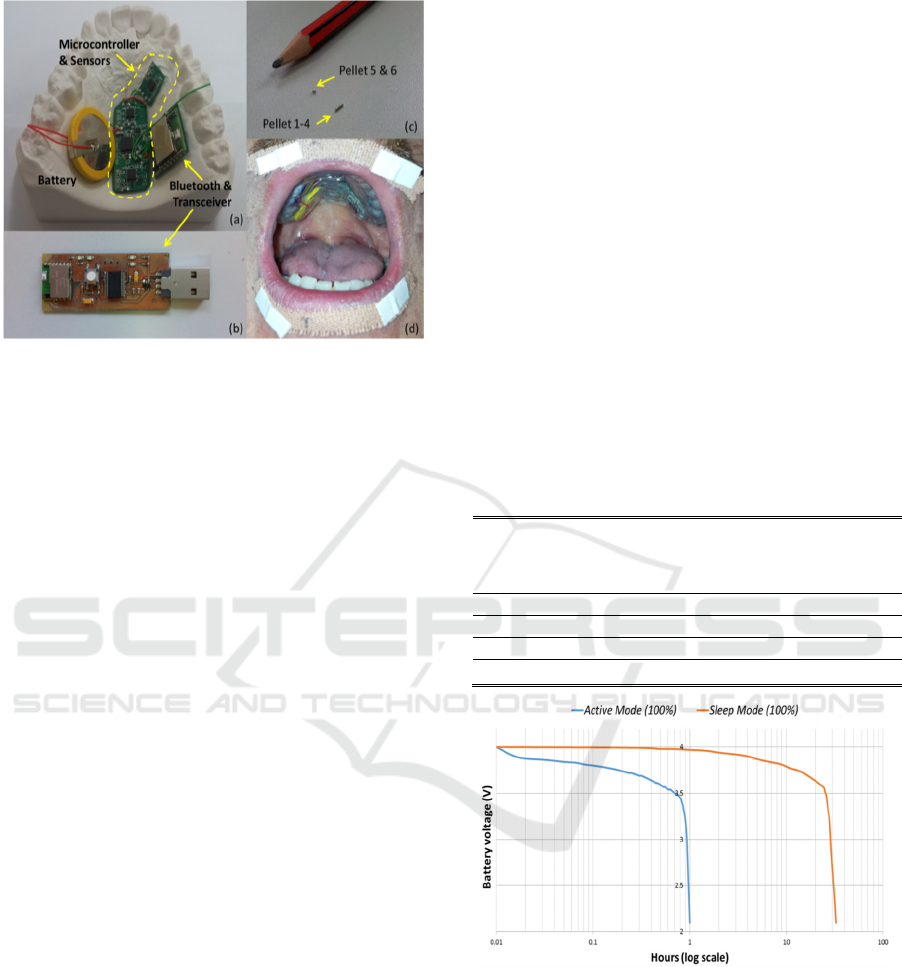
Figure 3: (a) & (b) Circuitry of the intraoral version of the
PMA system. (c) Placement of magnets on lips (pellets 1-
4), tongue tip (pellet 5) and tongue blade (pellet 6). (d)
View of the device when worn by user.
3.3 Circuit Operation
Figure 2 shows an operational block diagram of the
intraoral version of the PMA system. A command is
sent wirelessly from the processing unit to the
intraoral sensing module via Bluetooth to trigger
data acquisition. All three tri-axial magnetic sensors
then measure the magnetic field and digitize it with
12-bit resolution. The microcontroller acquires these
measurements (9 PMA channels sampled at 80 Hz)
through managing a multiplexer using three control
signals (S0, S1 and SCL). The multiplexer acts as a
switching device to route the serial clock (SCL) to
the desired magnetic sensor through the I
2
C
interface. The acquired samples are then transmitted
back to a processing unit wirelessly via the Blutooth
transceiver and custom designed Bluetooth dongle
(Figure 3(b)) for further processing. Unlike the
external version of the PMA prototype, the intraoral
device is restricted to only operate wirelessly from
inside the mouth. Wired connectivity is impossible,
as the sensing modules are to be sealed and
packaged inside a dental retainer. A description of
the operational and timing diagrams of the sub-
modules are presented in (Bai et al., 2015).
In terms of software, an ad-hoc MATLAB-based
graphical user interface (GUI) developed in (Cheah
et al., 2015) was adapted, where all speech
processing and recognition algorithms were
embedded. During silent speech recognition, if the
acquired PMA signal is correctly matched to an
articulated gesture from the training database, the
corresponsed utterance will be identified. A text-to-
speech synthesiser (TTS) is then used to generate an
acoustic signals (e.g. pre-recorded individual’s own
voice) for the recognised utterance through built-in
speakers.
3.4 Power Budget
Since the circuitry is to be sealed into a dental
retainer, the intraoral device can only acquire power
from a battery. Given the limited space available, a
small, low capacity battery must be used (in the
current design, the battery takes 27% of the total
volume of the circuitry). In addition, any measures
to extend the battery life will be of interest. Power
hungry components such as the microcontroller, the
magnetic sensors and the Bluetooth may be set to
standby mode or sleep mode to reduce the current
consumption when they are inactive. A shown in
table 1, sleep mode gives a saving of 93% over
standby mode or a saving of 97% over active mode.
Table 1: Current consumption in difference operational
modes.
Current
Consumption
Active
mode
(mA)
Standby
mode
(mA)
Sleep
mode
(mA)
Sensors 5.1 0.006 0.006
Microcontroller 5.4 4.4 0.7
Bluetooth 19.0 7.22 0.07
Total 29.5 11.626 0.776
Figure 4: Battery discharging over time under active mode
and sleep mode.
If the system is to operate continuously (in active
mode), the battery will last approximately one hour
before being depleted below the minimum operating
voltage (cut-off voltage) required by the Bluetooth
module of 2.1V. The battery can then be recharged
through a charging point located at the bottom side
of the dental retainer. In contrast, if the system was
inactive at all times (in sleep mode), the battery
would last about 32 hours. Figure 4 shows a
Preliminary Evaluation of a Silent Speech Interface based on Intra-Oral Magnetic Sensing
111
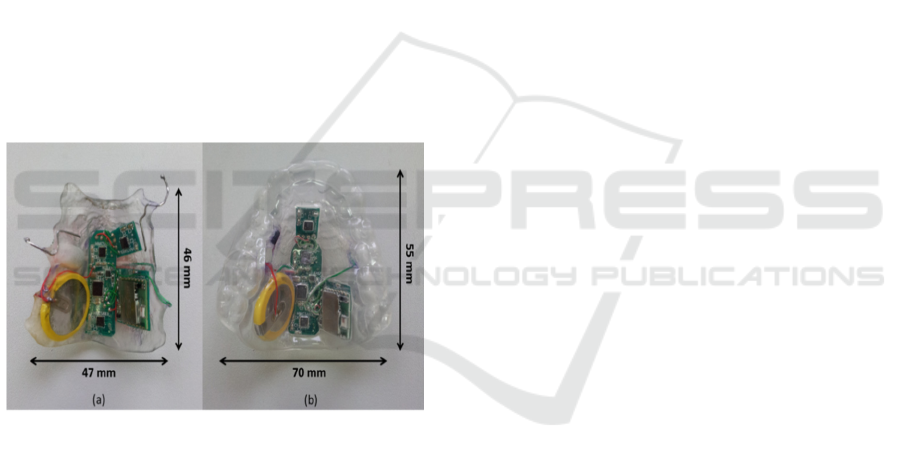
summary of the discharging cycle of the battery in
different modes. Neither of these operating regimes
is fully representative of the expected use since they
correspond to continuous speech and no speech
respectively. Based on the measurements in table 1
and figure 4, a more realistic regime would be to
allow 30 minutes of speech with a further 16 hours
in sleep mode. Hence, the estimated usage time is
considered to be sufficient for a typical day before
charging is required.
3.5 Construction of the Dental Retainer
The circuit described in the previous section must be
encapsulated to protect it from damage and short
circuits due to saliva and to ensure it is held in place
within the palate. The retainer must be customised
according to the individual’s oral anatomy. This may
be achieved by forming it on a dental impression of
the user’s oral cavity (seen in the background of
figure 3a). The intraoral PMA prototype was
implemented in the form of dental retainers utilising
both hard and soft materials, as illustrated in figure
5.
Figure 5: PMA circuitry embedded inside a (a) Hawley
dental retainer and (b) soft bite raiser like dental retainer.
The hard retainer is similar to a Hawley retainer
and is made of dental acrylic resin, which is
commonly used in fabrication of orthodontic
appliances. On the other hand, the soft retainer is
similar to a soft bite raising appliance and is made of
polypropylene or polyvinylchloride (PVC) material.
To allow stable fitting in the palate, the Hawley
retainer utilises a set of ball clasps to tightly secure it
onto the upper teeth. In contrast, the soft bite raiser
is fitted over the entire arch of the upper teeth. Note
that only soft retainer was used in the preliminary
experiments, but similar performance is expected
from the hard retainer.
4 PERFORMANCE EVALUATION
4.1 Experimental Design and Setup
The PMA-based SSIs (both intraoral and external
version) are speaker dependant systems because
their designs need to be individually tailored based
on the speaker’s head or oral anatomy for optimal
performance. The data used for evaluating the new
intraoral prototype were collected from a male
native English speaker who is proficient in the usage
of the external PMA device. Magnets were
temporary attached on the subject using Histoacryl
surgical tissue adhesive (Braun, Melsungen,
Germany).
Recordings of PMA and audio data for training
and evaluation were performed using a bespoke
Matlab-based GUI. The software provides a visual
prompt of randomised utterances to the subject at
interval of 5 seconds during the training session. The
subject’s head was not restrained during the
recording sessions, but the subject was requested to
avoid any large head movements. This was
necessary to ensure that interference induced by
movement relative to earth’s magnetic field was at
its minimum, so that it did not corrupt or distort the
desired signal. This is because the current prototype
is not yet equipped with a non-articulatory
cancellation/removal mechanism.
For optimal sound quality, the recordings were
conducted in an acoustically isolated room. The
audio data were recorded using a shock-mounted
AKG C1000S condenser microphone via a dedicated
stereo USB-sound card (Lexicon Lambda) to a PC,
with a 16 kHz sampling rate. Meanwhile, the PMA
data were captured at a sampling frequency of 80 Hz
via the intraoral PMA device and transmitted to the
same PC wirelessly via Bluetooth, as illustrated in
figure 2. Since both data streams (PMA & audio) are
acquired from separate modality, synchronisation
between the two data streams is necessary. Hence,
an automatic timing re-alignment mechanism was
implemented utilising start-stop markers generated
in additional to both data streams.
4.2 Data Recording
Our long term goal is to explore the feasibility of
using the intraoral device for continuous speech
reconstruction. For preliminary testing, the TIDigits
database (Leonard, 1984) was selected because the
limited size of the vocabulary enables whole-word
model training from relatively sparse data and
because of the simplicity of the language involved.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
112

The corpus consists of sequences of connected
English digits with up to seven digits per utterance.
The vocabulary is made up of eleven individual
digits, i.e. from ‘one’ to ‘nine’, plus ‘zero’ and ‘oh’
(both representing digit 0).
The experimental data were collected from two
independent sessions, with each session consisted of
four datasets containing 77 sentences each. A total
of 308 utterances containing 1012 individual digits
were recorded during each session. To prevent
subject fatigue, short breaks in between each
recording session were allowed.
4.3 HMM Training and Recognition
Prior to the training and recognition processes, the
acquired PMA data were segmented and checked
using the audio data. Inappropriate endpoints were
manually corrected if necessary. In addition, any
mis-labelled utterances were corrected using the
acquired audio data.
The PMA data was then subjected to offset
removal via median subtraction over 2s windows
with 50% overlap and followed by data
normalization. Next, the delta parameters were
computed for all PMA channels and added to its
original time series data, resulting in a feature vector
of size 18. The delta-delta parameters were not
included as part of the feature vector as they did not
produced significant improvement in performance
(Hofe et al., 2013a, 2013b). The recognition
performance based on the audio data was also
evaluated for comparison purposes. In this case, 13
Mel-frequency cepstral coefficients (MFCCs) were
extracted from the audio signals using 25ms analysis
windows with 10ms overlap. Next, the delta and
delta-delta parameters were computed and appended
to the static parameters, resulting in a feature vector
of dimension 39.
The extracted PMA and audio features were used
for training two independent speech recognisers
using the HTK toolkit (Young et al., 2009). In both
cases, the acoustic model in the recogniser uses
whole-word Hidden Markov Models (HMMs)
(Rabiner, 1989) for each of the eleven digits. Each
HMM has 21 states and 5 Gaussians per state. The
selected parameters were not optimised, but were
known for their performances based on previous
work (Hofe et al., 2013a, 2013b). The HMM
training and recognition was carried out in four
validation cycles. In each cycle, three out of four
sets within a session were used for training and the
remaining one for testing. The recognition results
were averaged over four cycles and across two
independent sessions.
4.4 Recognition Performance
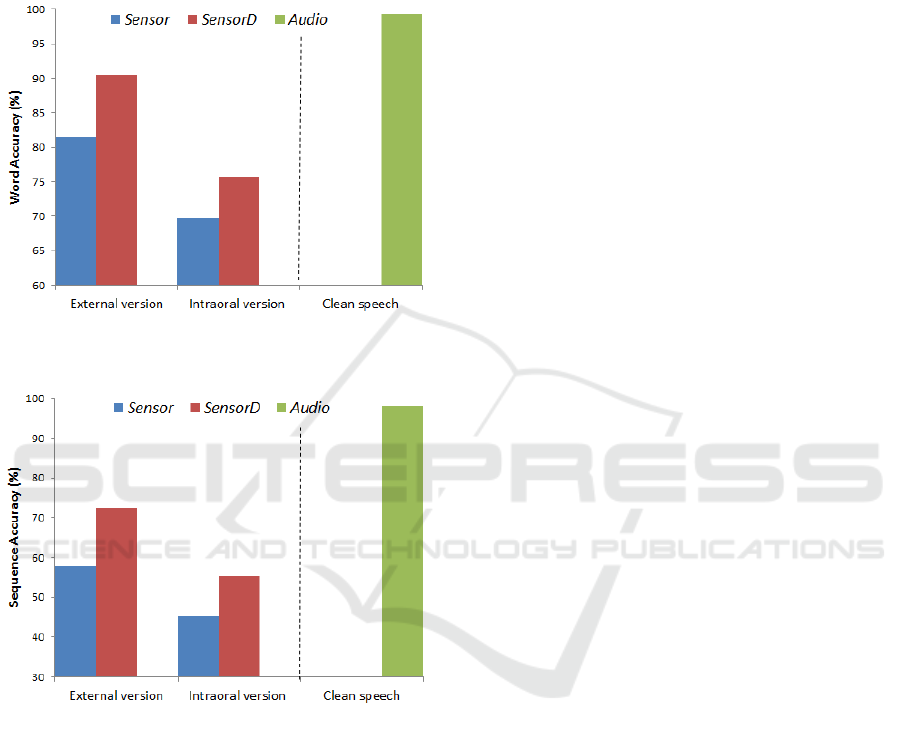
Both word and sequence accuracy results for the
intraoral and external versions of the PMA device
are presented in figure 6 and figure 7. In addition,
the performances of the PMA devices were
compared with audio-based recognition. The blue-
coloured bars indicate the performance achieved
using only static PMA data (vector size of 9),
whereas the red-coloured bars are the results
achieved using both static and dynamic features
(vector size of 18). In addition, the green-coloured
bars are the speech-recognition performance
achieved using audio data (vector size of 39). We
will refer to these three conditions as Sensor,
SensorD and Audio features, respectively.
The results reflect the mean of the data collected
across the two sessions, but were initially analysed
independently session-by-session. In order to avoid
the inconsistency of magnets placement during
individual training sessions, data were not merged
across different sessions. This however could be
solved, as magnets are to be surgically implanted for
long term usage. Alternatively session-independent
approaches, such as those presented for other SSIs
methods in (Maier-Hein et al., 2005) and (Wand and
Schultz, 2011) could be investigated.
As shown in both figure 6 and figure 7, it is quite
obvious that SensorD produced better recognition
performance on both occasions than using Sensor
alone Similar trends were also reported in (Hofe et
al., 2013b; Cheah et al., 2015). As expected, for this
simple task, recognition using Audio performed very
well (i.e. 99%). Preliminary evaluations indicated
some degradation (i.e. 15% for word accuracy and
17% for sequence accuracy) in recognition
performances in the intraoral device as compared to
the previous external version, as illustrated in figure
6 and figure 7. There are a number of possible
explanations for this degradation: 1) the presence of
the intraoral prototype affects articulation and, in
particular limits the tongue movements. This may
lead to inconsistent articulation, 2) the subject
was new to the intraoral version, but had prior
experiences on the external PMA version, 3) non-
articulatory features arise from unintentional
movements (e.g. swallowing, licking the lips, head
movements) which could have corrupted the data or
been confused with utterances, and 4) the magnets
are able to come much closer to the sensors in the
intraoral device than in the external device, resulting
Preliminary Evaluation of a Silent Speech Interface based on Intra-Oral Magnetic Sensing
113
in a more significant non-linear effect (since the
field strength decreases with cube of the distance).
This means that small unintentional articulator
movements can generate very large signals in some
instances. Further work is required to understand the
significance of each of these possible causes.
Figure 6: Comparison of word accuracy in the connected
digits.
Figure 7: Comparison of sequence accuracy in the
connected digits.
4.5 Hardware Evaluations
As discussed in section 2, one major obstacle to the
acceptability of an AT (e.g. SSI) is its appearance if
it is considered unattractive. Similar views were also
concluded through discussions with potential users
who have undergone a laryngectomy and an opinion
survey of 50 laryngectomees and their families/
friends, the appearance of the PMA-based device
was considered to be of a very high priority (Cheah
et al., 2015). To enhance its appeal to users,
influential factor such as appearance needed to be
accounted for during device development. The
challenge here is to satisfy the design objective and
continue improving on the PMA device’s
appearance but without compromising its speech
reconstruction performance. The latest intraoral
prototype employs the same functional principles as
the previous design reported in (Gonzalez et al.,
2014; Cheah et al., 2015), but implemented in a
different form. A summary of the hardware features
of the new intraoral PMA system compared to its
predecessor is presented in table 2.
Despite the improved appearance of the second
generation PMA system in the form of a wearable
headset, it might not yet to be appealing to all. To
address this shortcoming, the latest intraoral
circuitry was implemented in the form of a dental
retainer. To achieve this, the circuit was re-designed
to use fewer and smaller components. In addition,
the power consumption of the circuit was carefully
managed to allow it to operate from a small battery
suitable for inclusion within the dental retainer.
Hence, this led to a much smaller and lighter (i.e.
one tenth of previous weight) prototype as compared
to its predecessor. In addition, the intraoral prototype
is highly portable, it operates and can be controlled
wirelessly via Bluetooth using a computer/tablet PC.
Also, a higher signal-to-noise ratio (SNR) was
obtained with smaller magnetic tracers, due to their
proximity to the magnetic sensors. The tongue
magnets used with the intraoral sensor system were
16 to 25 times smaller volume than those used for
the external headset, potentially making them less
invasive when implanted.
A significant drawback with the intraoral device
is the limited battery size and capacity (i.e. 40mAh).
In contrast, the external version is less restricted in
term of size and weight of the battery. Hence, this
significantly reduces the operational time of the
intraoral device per charging. A number of steps
have been introduced to reduce its power
consumptions: a lower operational voltage is
selected and power-efficient components, lower data
sampling and transmission rates were chosen. In
addition, software was developed to switch from an
active mode to sleep mode when not in use. Using
these measures, it is estimated that the battery life
cycle could be extended from one hour to about 16.5
hours including 30 minutes of speech.
5 CONCLUSIONS
In this paper we have described a new intraoral
PMA prototype using commercial off-the-shelf
(COTS) components and embedded inside a dental
retainer constructed using the subject’s dental
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
114

Table 2: Summary of the PMA devices’ specifications and comparison [*Note that although the external sensing system has
12 channels, only 9 are used for speech recognition and 3 are used for cancellation of background magnetic fields].
Specifications Intraoral Sensing External Sensing
Appearance Dental retainer Wearable headset
Operating voltage 2.1 V 5 V
Magnets
Tongue Blade ø1 mm × 1 mm ø5 mm × 1 mm
Tongue Tip ø1 mm × 1 mm ø2 mm × 4 mm
Lips ø1 mm × 4 mm ø1 mm × 5 mm
Magnetic
Sensing
Dimension 12 × 12 × 3 mm
3
12 × 12 × 3 mm
3
Sensitivity 230 LSb/gauss 440 LSb/gauss
Samplig rate 80 Hz 100 Hz
Channels 9 12*
Data
Transmission
Type Bluetooth 2.0 Bluetooth 2.0 / USB
Frequency 2.4 GHz 2.4 GHz
Data rate 57.6 kbps 500 kbps
Power
Supply Rechargeable battery Rechargeable battery / USB
Battery Li-Ion 40 mAh Li-Ion 1080 mAh
Current consumption 30.5 mA 93.5 (wireless) / 67.1 (wired) mA
Lifetime 1 hour 10 hours
Prototype
Dimension 70 × 55 × 25 mm
3
160 × 160 × 150 mm
3
Weight 15 g 160 g
Material Acrylic resin / polypropylene VeroBlue / VeroWhitePlus resin
impression. Preliminary evaluation of the intraoral
prototype indicated a recognition performance,
slightly lower than the previous external PMA
device. However, there are a number of avenues for
further investigation to improve its performance.
Nonetheless, there are several advantages over
its predecessor. It is considered to be more stable
and robust against unintentional movement as it is
implemented in a form of a dental retainer, which
securely sits in the palatal cavity and is clasping
firmly on the upper teeth. Secondly, significantly
smaller magnets may be used for the intraoral
version (because of their proximity to the magnetic
sensors) while also giving a higher SNR. In addition,
the dental retainer can be completely hidden inside
the user’s mouth and out of sight. Hence, this would
eliminate the concern of being a sign of disability.
However, a downside of the intraoral design
would be the possibility of limiting the natural
movement of the tongue, because the device
occupies part of the user’s oral cavity. Further work
is required to assess whether users become
accustomed to the presence of the device and are
able to achieve more consistent articulation.
Encouraged by the results so far, extensive work
is needed to: 1) further reduce the size of future
intraoral prototypes, 2) improve the circuitry power
efficiency, 3) incorporate inductive charging for the
battery, and 4) introduce a background cancellation
mechanism for movement-induced interference.
Though there are still limitations, the present work
demonstrates a major step towards creating a viable
SSI that would appeal to speech impaired users. For
further information on the PMA-based SSI and its
speech restoration technique, please visit
www.hull.ac.uk/speech/disarm/demos.
ACKNOWLEDGEMENTS
The authors would like to thank Helen Dehkordy
from Hull and East Yorkshire Hospitals NHS Trust
for prototyping the dental retainers. The study is an
independent research funded by the National
Institute for Health Research (NIHR)’s Invention for
Innovation Programme. The views stated in this
report are those of the authors and should not be
interpreted as representing the official thoughts of
the sponsor.
REFERENCES
Bai, J., Cheah, L. A., Ell, S. R., and Gilbert, J. M. (2015).
Design of an intraoral device based on permanent
magnetic articulography. In Proceedings of Macau
Conference on Engineering, Technology and Applied
Science, pages 1-12, Macau, China.
Preliminary Evaluation of a Silent Speech Interface based on Intra-Oral Magnetic Sensing
115

Braz, D. S. A., Ribas, M. M., Dedivitis, R. A., Nishimoto,
I. N., and Barros, A. P. B. (2005). Quality of life and
depression in patients undergoing total and partial
laryngectomy. Clinics, 60(2):135-142.
Bright, A. K., and Conventry, L. (2013). Assistive
technology for older adults: psychological and socio-
emotional design requirements. In Proceedings of 6
th
International Conference on PErvaesive Technologies
Related to Assistive Environments, pages 1-4, Rhodes,
Greece.
Cheah, L. A., Bai, J., Gonzalez, J. A., Ell, S. R., Gilbert, J.
M., Moore, R. K., and Green, P. D. (2015). A user-
centric design of permanent magnetic articulography
based assistive speech technology. In Proceedings of
8
th
BIOSIGNALS, pages 109-116, Lisbon, Portugal.
Denby, B., Schultz, T., Honda, K., Hueber, T., Gilbert, J.
M., and Brumberg, J. S. (2010). Silent speech
interfaces. Speech Communication, 52(4):270-287.
Doi, H., Nakamura, K., Toda, T., Saruwatari, H., and
Shikano, K. (2010). Esophageal speech enhancement
based on statistical voice conversion with Gaussian
mixture model. IEICE Transactions on Information
and Systems, 93(9):2472-2482.
Fagan, M. J., Ell, S. R., Gilbert, J. M., Sarrazin, E., and
Chapman, P. M. (2008). Development of a (silent)
speech recognition system for patients following
laryngectomy. Medical Engineering & Physics,
30(4):419-425.
Gilbert, J. M., Rybchenko, S. I., Hofe, R., Ell, S. R.,
Fagan, M. J., Moore, R. K. and Green, P. D. (2010).
Isolated word recognition of silent speech using
magnetic implants and sensors. Medical Engineering
& Physics, 32(10):1189-1197.
Gonzalez, J. A., Cheah, L. A., Bai, J., Ell, S. R., Gilbert, J.
M., Moore, R. K., and Green, P. D. (2014). Analysis
of phonetic similarity in a silent speech interface based
on permanent magnetic articulography. In Proceedings
of 15
th
INTERSPEECH, pages 1018-1022, Singapore.
Hirsch, T., Forlizzi, J., Goetz, J., Stoback, J., and Kurtx, C.
(2000). The ELDer project: Social and emotional
factors in the design of eldercare technologies. In
Proceedings on the 2000 conference of Universal
Usability, pages 72-79, Arlington, USA.
Hofe, R., Bai, J., Cheah, L. A., Ell, S. R., Gilbert, J. M.,
Moore, R. K., and Green, P. D. (2013a). Performance
of the MVOCA silent speech interface across multiple
speakers. In Proceedings of 14
th
INTERSPEECH,
pages 1140-1143, Lyon, France.
Hofe, R., Ell, S. R., Fagan, M. J., Gilbert, J. M., Green, P.
D., Moore, R. K., and Rybchenko, S. I. (2013b).
Small-vocabulary speech recognition using silent
speech interface based on magnetic sensing. Speech
Communication, 55(1):22-32.
Leonard, R. G. (1984). A database for speaker-
independent digit recognition. In Proceedings of 9
th
ICASSP, pages 328-331, San Diego, USA.
Lontis, E. R., Lund, M. E., Christensen, H. V., Gaihede,
M., Caltenco, H. A., and Andreasen-Strujik, L. N.
(2010). Clinical evaluation of wireless inductive
tongue computer interface for control of computers
and assistive devices. In Proceedings of International
Conference on Engineering in Medicine and Biology
Society, pages 3365-3368, Beunos Aires, Argentina.
Maier-Hein, L., Metze, F., Schultz, T., and Waibel, A.
(2005). Session independent non-audible speech
recognition using surface electromyography. In
Automatic Speech Recognition and Understanding
Workshop, pages 331-336, Cancun, Mexico.
Martin, J. L., Murphy, E., Crowe, J. A., and Norris, B. J.
(2006). Capturing user requirements in medical
devices development: the role of ergonomics.
Physiological Measurement, 27(8):49-62.
Park, H., Kiani, M., Lee, H. M., Kim, J., Block, J.,
Gosselin, B., and Ghovanloo, M. (2012). A wireless
magnetoresistive sensing system for an intraoral
tongue-computer interface. IEEE Transactions on
Biomedical Circuits and Systems, 6(6):571:585.
Rabiner, L. R. (1989). A tutorial on Hidden Markov
Models and selected applications in speech
recognition. Proceedings of the IEEE, 77:257-286.
Tang, H., and Beebe, D. J. (2006). An oral interface for
blind navigation. IEEE Transactions on Neural
Systems and Rehabilitation Engineering, 14(1):116-
123.
Toda, T., Black, A. W., and Tokuda, K. (2008). Statistical
mapping between articulatory movements and acoustic
spectrum using a Gaussian mixture model. Speech
Communication, 50(3): 215-227.
Toda, T., Nakagiri, M., and Shikano, K. (2012). Statistical
voice conversion techniques for body-conducted
unvoiced speech enhancement. IEEE Transactions on
Audio, Speech and Language Processing, 20(9):2505-
2517.
Toutios, A., and Margaritis, K. G. (2005). A support
vector approach to the acoustic-to-articulatory
mapping. In Proceedings of 6
th
INTERSPEECH, pages
3221-3224, Lisbon, Portugal.
Wand, M., and Schultz, T. (2011). Session-independent
EMG-based speech recognition. In Proceedings of 4
th
BIOSIGNALS, pages 295-300, Rome, Italy.
Wang, J., Samal, A., Green, J. R., and Rudzicz, F. (2012).
Sentence recognition from articulatory movements for
silent speech interfaces. In Proceedings of 37
th
ICASSP, pages 4985-4988, Kyoto, Japan.
Young, S., Everman, G., Gales, M., Hain, T., Kershaw, D.,
Liu, X., Moore, G., Odell, J., Ollason, D., Povery, D.,
Valtchev, V., and Woodland, P. (2009). The HTK
Book (for HTK Version 3.4.1). Cambridge: Cambridge
University Press.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
116
