
Development of Multi-parameter Analyser based on Electrochemical
Urea Biosensors and Electrolyte Electrodes for Monitoring of
Hemodialysis Patients
Julija Razumiene
1
, Vidute Gureviciene
1
, Marius Dagys
1
, Ieva Sakinyte
1
, Algimantas Jonuska
1
,
Laurynas Rimsevicius
2
, Svitlana Marchenko
3
and Alexey Soldatkin
3
1
Institute of Biochemistry, Vilnius University, Mokslininku 12, 08662, Vilnius, Lithuania
2
Nephrology Centre,
Vilnius University, Santariskiu 2, 08661 Vilnius, Lithuania
3
Laboratory of Biomolecular Electronics, Institute of Molecular Biology and Genetics, National Academy of Sciences of
Ukraine, Zabolotnogo Street 150,03143, Kyiv, Ukraine
Keywords: Biosensor, Amperometric Urea Detection, Potentiometric Urea Detection, Urease, Sodium and Potassium
Electrodes, Blood Dialysis.
Abstract: The idea of developing multi-parameter urea analyser comprising urea, Na
+
and K
+
selective electrodes has
been considered. For this purpose the urea biosensors based on urease and recombinant urease working in
amperometric and potentiometric way were developed. The working parameters of both urea biosensors were
studied and optimized. Possibilities of real samples analysis using the developed biosensors were shown by
measuring urea concentrations in blood dialysate taken from patients with renal failure. Both the
potentiometric and the amperometric biosensors demonstrated high degree of signal reproducibility (the
relative standard deviation of responses did not exceed 5 %). Change of sodium and potassium concentrations
during blood hemodialysis is dangerous life-threatening condition and their monitoring is an important feature
of point-of-care analyser. For this purpose high integrity commercial Na
+
and K
+
selective electrodes were
analysed and our own signal amplification and processing system proposed.
1 INTRODUCTION
Urea is the final product of protein metabolism and it
is synthesized in the liver as a result of amino acid
deamination (Kuralay et al., 2005). Excessive urea in
organism is excreted by renal system during blood
filtration, and elevated levels of urea concentration in
blood or serum usually indicate dangerous kidney
disease. Regular level of urea in serum varies from 15
to 40 mg/dl (2.5 – 6.7 mM), while in patients
suffering from renal failure urea concentrations in
serum can reach 180 – 480 mg/dl (30 – 80 mM), and
patients with such elevated concentrations have to
undergo blood dialysis treatment (Dhawan et al.,
2009). It is a dangerous condition – 5 year survival of
men older than 64 years who are starting dialysis is
worse than that of men with colon cancer and prostate
cancer (Parfrey and Foley, 1999).
For such reasons the methods for assessment of
urea concentration in blood, serum and spent
dialysate solutions are being developed. Urea
measurements are important in medical diagnostics
for clinical evaluation of renal function and
monitoring the effectiveness of dialysis treatment.
One of the first methods for urea determination was
based on spectrophotometric measurements after
sample treatment with specific compounds leading to
coloured solution with distinctive spectra (Patton and
Crouch, 1977; With et al., 1961). Such methods are
fairly accurate and are being used in medical practice,
but they are not suitable for real-time sample analysis.
Alternative technologies for urea measurements
being developed are based on biosensor electrodes.
They have different design approaches such as
potentiometric (Kuralay et al., 2005, Liu et al., 1993;
Ahuja et al., 2011; Boubriak et al., 1995),
conductometric (Soldatkin et al., 2014; Chen et al.,
1994; Sangodkar et al., 1996) and amperometric
(Sangodkar et al., 1996; Tiwari et al., 2009), they
usually employ urease (EC 3.5.1.5) as a catalyst for
urea breakdown, which is immobilized by a number
of methods on electrode surface (Dhawan et al.,
2009). The urease catalyses the hydrolysis of urea to
Razumiene, J., Gureviciene, V., Dagys, M., Sakinyte, I., Jonuska, A., Rimsevicius, L., Marchenko, S. and Soldatkin, A.
Development of Multi-parameter Analyser based on Electrochemical Urea Biosensors and Electrolyte Electrodes for Monitoring of Hemodialysis Patients.
DOI: 10.5220/0005814100930101
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 93-101
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
93
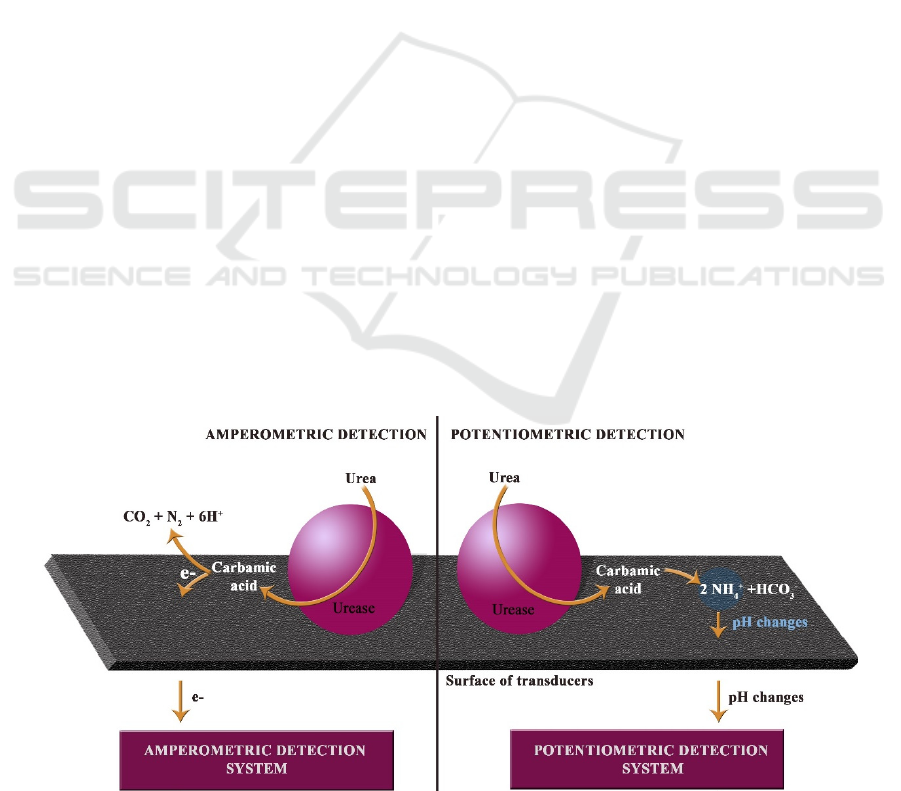
yield ammonia and carbamic acid (equation 1) which
spontaneously decomposes into carbonic acid and a
second ammonia molecule (equation 2) (Carter et al.,
2009), as shown below:
H
2
N-C(O)-NH
2
+H
2
O+H
+
→NH
4
+
+H
2
N-COOH
(1)
H
2
N-COOH+H
2
O→NH
4
+
+HCO
3
-
(2)
The principle of enzyme based action of
amperometric and potentiometric detection systems
are presented in figure 1.
Monitoring of electrolyte composition in blood
during dialysis treatment is also very important.
Serum electrolytes (sodium, potassium, calcium,
phosphate) are usually elevated in chronic dialysis
patients, they have a high survival risk ratio (Iseki et
al., 1996). While sodium and potassium can be auto-
regulated during long dwell continuous ambulatory
peritoneal dialysis (CAPD) (Nolph et al., 1980),
patients undergoing hemodialysis exhibit high
incidence of cardiac arrhythmias during dialysis days
particularly due to sudden imbalance of blood
electrolytes (Ramirez et al., 1984). While the
strategies of retaining sodium and potassium levels in
blood and extracellular fluid are being constantly
developed (Much and Wilcox, 1982; Maduell et al.,
2013; Mc Causland et al., 2012), real-time monitoring
of sodium and potassium concentrations during
hemodialysis could be crucial to determine rapid
change of such electrolytes in order to take according
revival actions.
The purpose of the study is to find best sensor
candidates for point-of-care urea, sodium and
potassium analyser, useful for monitoring of patients
undergoing hemodialysis treatment. The electrodes
and bioelectrodes (biosensors) used in such
equipment should be accurate, not expensive, stable,
simple to use in field measurement, etc. In case of
possible urea biosensor, our scientific group until
recently has been developing several types of urea
biosensors (Boubriak et al., 1995; Soldatkin et al.,
2014; Mc Causland et al., 2012; Laurinavicius et al.,
2013). In this case a specific attention is paid to
previously developed and published potentiometric
(Kulys et al., 1986) and amperometric (Mc Causland
et al., 2012) urea biosensors that were further
developed, as described in this work. In case of
sodium and potassium measurements, we will adapt
to our needs one of many commercially available
electrolyte measurement system, consisting of
potentiometric flow-through electrode cell. It is used
in several electrolyte analysers, which electronics and
signal analysis algorithms are hardcoded into
microcontrollers, subject to copyright material, so in
this study we will devise our own signal amplification
and processing system.
2 EXPERIMENTAL
2.1 Chemicals and Reagents
In this work the enzyme used for assembly of urea
amperometric biosensors was urease from Canavalia
ensiformis (E.C. 3.5.1.5.), activity of 343.0 U/mg
from Calbiochem (Germany). The enzyme substrate
was used as the phosphate buffer solution, pH 7.2,
containing 1 M of urea. Thermally reduced graphene
oxide (TRGO) was used as electrode materials.
TRGO have been synthesized by us as proposed in
the protocol (Razumiene et al., 2015).
Figure 1: The principle of enzyme based action of amperometric and potentiometric urea detection systems.
Urease
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
94

For potentiometric biosensor creation
recombinant urease (R. urease, E.C. 3.5.1.5) from
USBiological (USA) expressed in E. coli, was used,
its activity was 150 U/mg. Bovine serum albumin
(BSA, fraction V) and urea were obtained from
Sigma-Aldrich Chemie; poly(vinyl alcohol)
containing styrylpyridinium (PVA-SbQ) from Toyo
Gosei Kogyo Co. Ltd (Japan). The working
phosphate buffer (KH
2
PO
4
-NaOH), pH 7.4, was
prepared by using reagents from Helicon (Moscow,
Russia). Sensor chips with differential pair of pH-
sensitive field effect transistors produced at the JSC
“Kwazar” facilities (Kiev).
The samples of blood dialysate and serum for the
potentiometric measurements of urea content were
obtained from Kiev municipal scientific and practical
centre of nephrology and hemodialysis (Ukraine). All
others chemical reagents were obtained from Sigma-
Aldrich and were of analytical grade unless otherwise
mentioned.
2.2 Construction and Electrochemical
Measurements of Biosensors
2.2.1 Amperometric Urea Biosensors
Amperometric measurements were performed using
an electrochemical system PARSTAT 2273
(Princeton Applied Research) with a conventional
three-electrode system composed of an auxiliary
platinum plate electrode, a reference Ag/AgCl
electrode and working TRGO (Ø 3 mm) electrodes as
transducer for amperometric biosensor (Razumiene et
al., 2015). Aiming to design amperometric biosensor
TRGO were extruded by forming a tablet. The tablet
was sealed in a Teflon tube then tablet surface was
covering by the semipermeable terylene membrane
containing immobilized urease from Canavalia
ensiformi. The response of the prepared
amperometric biosensors to the addition of substrate
was investigated under potentiostatic conditions at
+200 mV (vs. Ag/AgCl) in a stirred phosphate buffer
solution, pH 7.2, 20
o
C.
2.2.2 Potentiometric Urea Biosensor
Potentiometric biosensor was based on pH-sensitive
field effect transistors as current transducers
(Sheliakina et al., 2014; Pavluchenko et al., 2011).
Each transducer contained a differential pair of pH-
sensitive field effect transistors placed on a single
crystal with the total area of 8 mm × 8 mm. Signals
were recorded from both transistors and then signal
from reference transistor (covered with BSA
membrane) was subtracted from the signal of
transistor covered with enzyme membrane.
Transistors demonstrated pH-sensitivity of
approximately 40 mV/pH and transconductance of
400–500 mkA/V. More information about transistor
structure and their photo can be found in (Sheliakina,
2014) and description of a portative measuring device
– in (Pavluchenko et al., 2011).
A bioselective membrane on the transducer
surface was formed by immobilisation of R. urease in
PVA/SbQ photopolymeric membrane. 66 % of
PVA/SbQ and 10 % of R. urease were mixed at 1:1
ratio and the mixture (0,1 μl) was deposited on the
surface of the ISFET. Then sensor chip was exposed
under the UV lamp KF-4M (Ukrainian production) of
3.4V/m
2
for 20 min.
Measurements were carried out in the 5 mM
potassium phosphate buffer solution (KH
2
PO
4
-
NaOH), pH 7.4, with intensive stirring at room
temperature. The biosensor and Ag/AgCl reference
electrode were placed into an open 1.5 ml measuring
cell. The urea concentrations in the working cell were
obtained by the addition of aliquots of concentrated
stock solution or real samples.
2.2.3 Na
+
and K
+
Selective Electrodes – Cell
Construction and Signal Processing
Measurements of electrolytes – sodium and
potassium ions – concentration in blood or standard
solutions were performed by using commercially
available potentiometric Sensa K and Sensa Na
electrodes in dedicated five electrode cell containing
integrated Sensa reference electrode, bubble detector
and three blank electrodes, forming a single flow-
through channel for measurement of solutions of
interest. The whole set was purchased from Sensa
Core Medical Instrumentation Pvt Ltd, India. The
idea of the study was to not use any dedicated
commercial electronic amplification and signal
analysis equipment, thus such system was designed
from ground up.
The voltamperometric measurements of
electrodes revealed that the resistance of sodium,
potassium and reference electrodes was in range of 4
– 10 MΩ, thus two stage, closed loop operational
amplifier (op-amp) based circuit was used to amplify
the signals – one op-amp was used to follow the
changes of electrode potential difference without
voltage gain, and the output signal was further
amplified at 11-fold gain by second op-amp. The gain
level was selected to allow optimal voltage
measurements with 18-bit analogue-to-digital
converter (ADC) (for example, MCP3424 from
Development of Multi-parameter Analyser based on Electrochemical Urea Biosensors and Electrolyte Electrodes for Monitoring of
Hemodialysis Patients
95
Microchip Technology Inc.), capable of measuring
potential differences up to 3.3 V in our electronic
setup. To avoid electromagnetic interference, op-amp
circuit was encased in dedicated small metal shield
box and placed on top of electrode contacts. The
constructed urea analyser employs ADCs with
dedicated computer hardware and software for signal
analysis and implementation of measurement
algorithms; however, in this study we used bench type
UT804 multimeter with USB data output (Uni-Trend
Group Limited, Hong Kong) for analysis of amplified
signals.
The measurements were performed by following
procedure. First, the electrode cell was assembled and
connected; inner fluid path and tubing were rinsed by
de-ionised water. Then the solution of interest was
aspired into fluid channel and amplified output signal
(voltage) was recorded by computer software
controlled multimeter. The fluid path was rinsed by
de-ionised water between measurements, after work
the electrodes were disassembled, their channels
dried and stored in room temperature. In case of
sodium ions concentration measurements, standard
referenc solutions with NaCl concentrations 100 –
200 mM were used to measure potential difference
dependence on electrolyte concentration. In case of
potassium ions concentration measurements, KCl
solutions from 2 to 7 mM were used, they also
contained 150 mM NaCl in order to maintain ionic
strength. In blood measurements, equal amounts of
solutions with different NaCl or KCl concentrations
or pure water were added to blood samples. In result
several batches of blood samples were obtained with
varying concentration of sodium and potassium by 10
or 1 mM, respectively.
3 RESULTS
3.1 Study of Amperometric Urea
Biosensor
Amperometric biosensor after addition of urea into
electrochemical cell shows substrate-dependent
anodic response. The biosensor response was fast:
90% of steady state current achieved in 20 s. The
response was measured as a difference between the
steady state and the background current. T
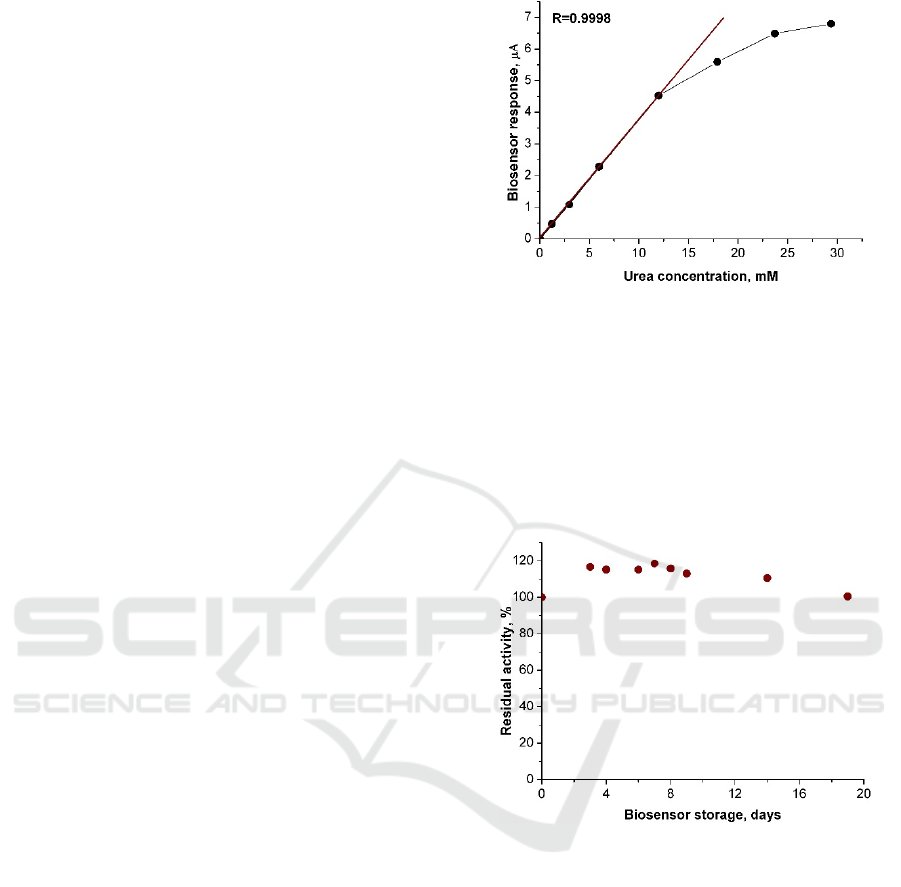
he urea
calibration curve with marked linear range is shown in
figure 2 and main characteristics of the biosensor are
shown in Table 1.
Figure 2: The urea calibration curve and the linear range
(solid line) obtained using the amperometric biosensor.
Applied electrode potential of 0.2 V, phosphate buffer
solution, pH 7.2.
Operational stability of the biosensors was tested by
consecutive measurements of the current response to urea
solution of 3 mM. At least twenty measurements of the
biosensor activity per day were done during three weeks.
Between the experiments the biosensor was stored at room
temperature. The averaged data are shown in figure 3.
Figure 3: Operational stability of the amperometric
biosensor.
As can be seen in figure 3 after a period of three
weeks the decreasing of sensitivity of the
amperometric biosensor was negligible.
The amperometric biosensor has been tested for
urea measurements in dialysate as well. Aiming to
validate responses of the biosensor measurements the
samples of dialysate in parallel were examined at the
hospital laboratory. The testing has been carried out
by investigating dialysate of four patients after one
hour of hemodialysis. Urea concentration data
obtained by both methods are presented in figure 4.
The results shown in figure 4 exhibited good
correlation between two methods.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
96
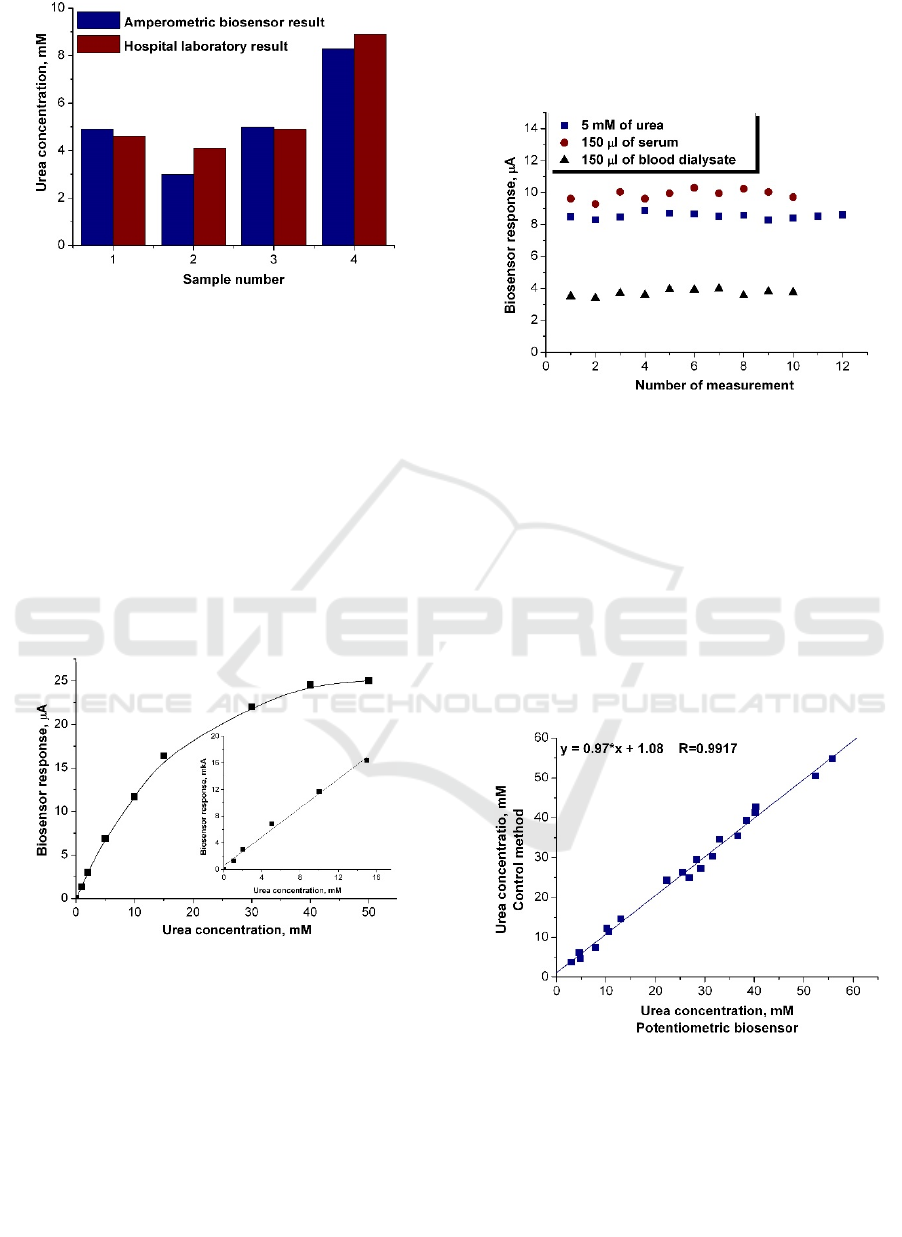
Figure 4: Comparison of results of urea determination in
dialysate after one hour of hemodialysis. The dialysates of
four patients were examined by the amperometric biosensor
and in the hospital laboratory.
3.2 Study of Potentiometric Urea
Biosensor
Firstly an investigation of analytical characteristics of
the potentiometric biosensor based on R. urease at
determination of urea concentration in model
solutions was done. The calibration curve for urea
determination by the potentiometric biosensor is
presented in figure 5 and main characteristics of the
biosensor are shown in Table 1.
Figure 5: The urea calibration curve – dependence of
current versus final urea concentration. Inset shows
measurements in linear response region. Measurements
were performed in 5 mM potassium-phosphate buffer, pH
7.4, at room temperature.
The signal reproducibility of the biosensor was
studied, in order to verify electrode suitability for
measurement of urea concentration both in model
solution and real samples. For this task the biosensor
responses to urea concentration of 5 mM in model
solution and 150 µl of the serum or blood dialysate in
working buffer were recorded during one workday at
30 minutes intervals. As shown in Figure 6,
potentiometric biosensor demonstrated high degree
of signal reproducibility in all cases (the relative
standard deviation of responses did not exceed 5 %).
Figure 6: Reproducibility of responses of potentiometric
biosensor to model and real samples. Measurements were
performed in 5 mM phosphate buffer, pH 7.4, at room
temperature.
The developed biosensor based on R. urease was
tested by the analysis of samples of blood dialysate
taken from patients with renal failure. For this task the
concentration of urea was analyzed in 10 samples of
blood dialysate and in 10 samples of serum. The
samples were added to the working cells (10-fold
dilution), the responses of biosensor were measured
and compared with the previously plotted calibration
curve.
Figure 7: Correlation between the data on urea
concentration in real samples obtained by biosensor and
control method.
To check the accuracy of urea determination by
the biosensor, the samples were analyzed in a
diagnostic laboratory by the control method - the
commercial Vitros 250 (Johnson & Johnson)
analyzer, based on "dry chemistry" technology – urea
Development of Multi-parameter Analyser based on Electrochemical Urea Biosensors and Electrolyte Electrodes for Monitoring of
Hemodialysis Patients
97
content being measured by using urease reaction with
colorimetric detection of produced ammonia. The
results of biosensor analysis and those obtained by
control method are shown in figure 7. The study
revealed high correlation between methods used (R =
0.9917).
3.3 Characterization of Amperometric
and Potentiometric Urea
Biosensors
The most important characteristics in terms to apply
the amperometric or potentiometric biosensors in
analytical device are summarized in Table 1.
Table 1: Analytical characteristics of amperometric and
potentiometric urea biosensors.
Biosensor
characteristic
Amperometric Potentiometric
Linear range, mM 0.1-12 0.5-15
Operational range,
mM
0.1-30 0.1-40
Detection limit, mM 0.1 0.1
Response
reproducibility
(relative standard
deviation of
responses), %
2-3 3-5
Response time, s 20 60-120
Sensitivity
after 20 days, %
100 110
Dilution of the
sample
10-20 10
References Present work
Marchenko et
al., 2015 and
present work
Comparison of characteristics of the
amperometric and the potentiometric biosensors
shows that both electrodes have adequate linearity
and operational range, detection limit and
reproducibility, which means they both could be
considered as good candidates for commercialization.
3.4 Studies of Na
+
and K
+
Selective
Electrodes
Commercial sodium and potassium electrodes were
used to analyse samples of standard solutions and
blood. This study was essential to mimic the
measurement procedure hardcoded into electrolyte
analysers which algorithms are sensitive copyright
material. However, the commercial electrodes we
purchased are being used in various applications,
each manufacturer having their own algorithms, and,
as consulted by representative of manufacturer, it is
legal to use them as long as one follows
recommended sample measurement procedure,
which is similar to ours as described before.
Consequently, the purpose of our study is to
analyse dependences of electrode potentials on
electrolyte concentrations in various solutions, also to
determine if our selected signal processing and
amplification method is adequate to obtain readable
data. Since the manufacturer provides calibration
information about each electrode and is responsible
for the quality of the product, we assumed that it is of
minor importance to perform extended time
dependent electrode response repeatability
measurements.
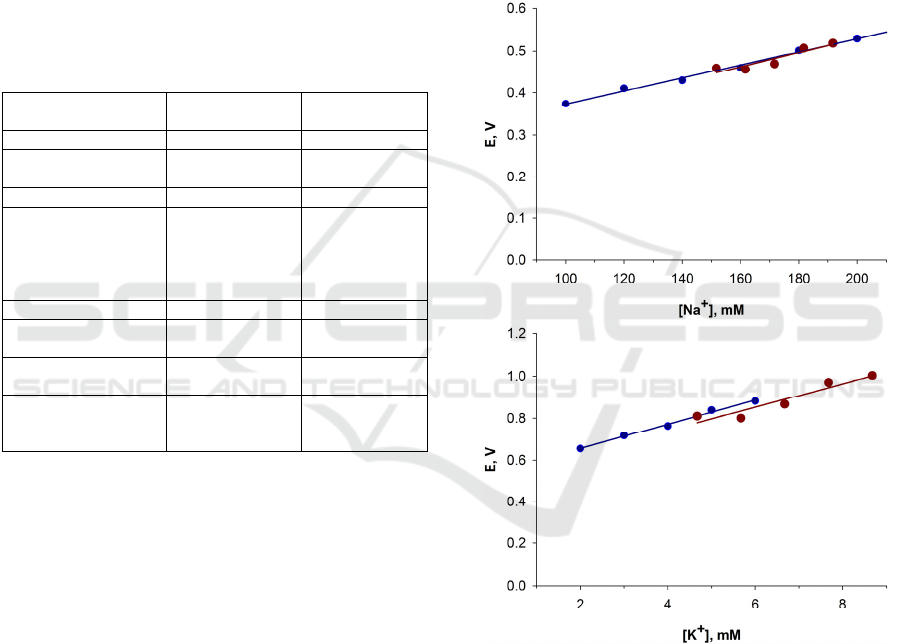
Figure 8: Sodium (top) and potassium (bottom) ion
selective electrode 11-fold amplified response dependence
on concentration. Standard solutions with known
concentrations are marked blue, blood with incremental
additions of sodium and potassium by 10 or 1 mM,
respectively, are marked red. Concentration of sodium or
potassium in unaltered blood samples are calculated from
measured potential and linear function from measurements
of standard solutions.
The measurements revealed that voltage signals
were stable (noise floor less than 2 mV of amplified
signal), immune to electromagnetic interference. In
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
98

fact, when one stage closed loop op-amp system with
direct 11-fold gain was used instead of two stage
system, signals were stable as well. After aspiration
of solution into electrode path, the signal in either
electrode setup asymptotically approaches stability in
2 – 5 minutes in exponential decay manner, at about
40 seconds the signal reaches 95 % of its final value.
For our study the responses were mathematically
fitted by exponential decay function, final offset
values were calculated and used in analysis.
Typical dependencies of amplified voltage
difference signals on electrolyte concentration from
measurements of blood and standard solutions are
presented in figure 8.
The study revealed that Na
+
and K
+
selective
electrodes responses exhibit linear concentration
dependence, as long as concentration range is within
the ones present in physiological media. The slope
values both in cases of standard and blood solutions
are similar. To estimate the best value, the device
algorithm should preferably contain exponential
decay minimization procedure; however,
measurement after waiting for about 40 seconds from
sample aspiration should be adequate.
3.5 Insights into Construction of
Multi-Parameter Urea Analyzer
Currently, urea measurement in blood and dialysate
samples are performed in laboratory using
commercial cumbersome equipment, the test itself is
termed and standardized as blood urea nitrogen
(BUN) measurement. Implementation of urea
biosensors (Dhawan et al., 2009) gives promising
leads to development of standalone point-of-care
compact urea measurement devices. Consequently,
our group together with colleagues has been
developing commercially viable biosensor designs
for about three decades (Boubriak et al., 1995;
Soldatkin et al., 2014; Maduell et al., 2013; Mc
Causland et al., 2012; Laurinavicius et al., 2013;
Kulys et al., 1986; Marchenko et al., 2015). This
study compares our best candidates to incorporate
into such device. Comparison of characteristics of the
amperometric and the potentiometric biosensors
shows that both electrodes have adequate linearity
and operational range, detection limit and
reproducibility, which means they both could be
considered as good candidates for commercialization.
However, several features of the amperometric sensor
such as short response time and higher accuracy due
to better linearity in relevant concentration range, as
well as technical benefits (lower sensitivity to
background noise, ease of production, almost
complete insensitivity to pH, etc.), not mentioned in
this study, make the amperometric urea sensor a
better option at this stage of designing of the multi-
parameter analyzer.
The study revealed that Na
+
and K
+
selective
electrode system is suitable for integration to device
as well. The signal amplification and analysis
techniques have been developed by technical cues
implied in sodium and potassium electrode cell
design. However, the amperometric biosensor and
ions selective electrode systems are of different
design nature and should be developed as different
parts of the multi-parameter analyzer. Specific care
must be taken when designing specimen and liquid
handling system, keeping in mind that electrical
amplification circuits would be separate – liquids
from both cells should not touch in order to avoid
signal interference from occurring common electric
plane.
4 CONCLUSIONS
The amperometric and potentiometric urea
biosensors based on urease and recombinant urease
were developed and possibilities of the real samples
analysis using the developed biosensors were shown.
The working parameters of the urea biosensors were
studied and optimized. Linear dynamic range of the
potentiometric urea determination was 0.5 – 15 mM,
detection limit - 0.1 mM. Urea concentrations were
determined in 20 samples of blood dialysate and
serum taken from patients with renal failure; the
potentiometric biosensor demonstrated a high
correlation of the results with the control method of
urea determination. Linear range of the amperometric
urea determination was 0.1 – 12 mM and detection
limit of 0.1 mM. The amperometric biosensor has
been tested for urea measurements in dialysate and
results correlated with data obtained in the hospital
laboratory. Both biosensors – the potentiometric and
the amperometric demonstrated high degree of signal
reproducibility in all cases (the relative standard
deviation of responses did not exceed 5 %). Thus,
both biosensors studied in this research can be
effectively used to diagnose the patients with renal
failure and to control the urea content in blood or
dialysis fluid during hemodialysis.
In this study the commercial sodium and
potassium ion selective electrodes were analysed
aiming to integrate them in to the designing analyser.
It was not used any dedicated commercial electronic
amplification and signal analysis equipment, thus,
such system was designed from ground up. The
Development of Multi-parameter Analyser based on Electrochemical Urea Biosensors and Electrolyte Electrodes for Monitoring of
Hemodialysis Patients
99

dependences of electrode potentials on electrolyte
concentrations in various solutions revealed that our
selected signal processing and amplification method
is adequate to obtain readable data.
Considering analytical and technical features of
the biosensor designs, it seems that benefits of the
amperometric sensor hold the edge over choosing the
latter in designing the commercial analyser. Together
with electrolyte electrodes, such multi-parameter
point-of-care blood and dialysis fluid analyser would
help in better outcomes and hemodialysis procedure
corrections for patients diagnosed with various stage
renal failures.
ACKNOWLEDGEMENTS
This work has been supported by Lithuanian Agency
for Science, Innovation and Technology Project E!
8835, National Academy of Sciences of Ukraine and
STCU project No. 6052.
REFERENCES
Ahuja, T., Kumar, D., Singh, N., Biradar, A. M., Rajesh,
2011. Potentiometric urea biosensor based on multi-
walled carbon nanotubes (MWCNTs)/silica composite
material. Materials Science and Engineering: C, vol.
31, pp. 90-94.
Boubriak, O. A., Soldatkin, A. P., Starodub, N. F.,
Sandrovsky, A. K., El'skaya, A. K., 1995.
Determination of urea in blood serum by a urease
biosensor based on an ion-sensitive field-effect
transistor. Sensors and Actuators B: Chemical, vol. 27,
pp. 429-431.
Carter, E. L., Flugga, N., Boer, J. L., Mulrooney, S. B.,
Hausinger, R. P., 2009. Interplay of metal ions and
urease. Metallomics, vol. 1, pp. 207-221.
Chen, K., Liu, D., Nie, L., Yao, S., 1994. Determination of
urea in urine using a conductivity cell with surface
acoustic wave resonator-based measurement circuit.
Talanta, vol. 41, pp. 2195-2200.
Dhawan, G., Sumana, G., Malhotra, B. D., 2009. Recent
developments in urea biosensors. Biochemical
Engineering Journal, vol. 44, pp. 42-52.
Iseki, K., Uehara, H., Nishime, K., Tokuyama, K.,
Yoshihara, K., Kinjo, K., Shiohira, Y., Fukiyama, K.,
1996. Impact of the initial levels of laboratory variables
on survival in chronic dialysis patients. American
Journal of Kidney Diseases, vol. 28, pp. 541-548.
Kulys, J. , Gurevičienė, V., Laurinavičius, V., Jonuška, A.
V., 1986. Urease sensors based on differential antimony
electrodes. Biosensors, vol. 2, pp. 35-44.
Kuralay, F., Özyörük, H., Yıldız, A., 2005. Potentiometric
enzyme electrode for urea determination using
immobilized urease in poly(vinylferrocenium) film.
Sensors and Actuators B: Chemical, vol.109, pp. 194-
199.
Laurinavicius, V., Razumiene, J., Gureviciene, V., 2013.
Bioelectrochemical Conversion of Urea on Carbon
Black Electrode and Application. Sensors Journal,
IEEE, vol. 13, pp. 2208-2213.
Liu, D., Meyerhoff, M. E., Goldberg, H. D., Brown, R. B.,
1993. Potentiometric ion- and bioselective electrodes
based on asymmetric polyurethane membranes.
Analytica Chimica Acta, vol. 274, pp. 37-46.
Maduell, F., Moreso, F., Pons, M., Ramos, R., Mora-Macià,
J., Carreras, J., Soler, J., Torres, F., Campistol, J. M.,
Martinez-Castelao, A., 2013. High-Efficiency
Postdilution Online Hemodiafiltration Reduces All-
Cause Mortality in Hemodialysis Patients. Journal of
the American Society of Nephrology, vol. 24, pp. 487-
497.
Marchenko, S. V., Kucherenko, I. S., Hereshko, A. N.,
Panasiuk, I. V., Soldatkin, O. O., El'skaya, A. V.,
Soldatkin, A. P., 2015. Application of potentiometric
biosensor based on recombinant urease for urea
determination in blood serum and hemodialyzate.
Sensors and Actuators B: Chemical, vol. 207, Part B,
981-986.
Mc Causland, F. R., Brunelli, S. M., Waikar, S. S., 2012.
Dialysate sodium, serum sodium and mortality in
maintenance hemodialysis. Nephrology Dialysis
Transplantation, vol. 27, pp. 1613-1618.
Much, W. E., Wilcox, C. S., 1982. Disorders of body fluids,
sodium and potassium in chronic renal failure. The
American Journal of Medicine, vol. 72, pp. 536-550.
Nolph, K. D., Sorkin, M. I., Moore, H., 1980.
Autoregulation of Sodium and Potassium Removal
During Continuous Ambulatory Peritoneal Dialysis.
ASAIO Journal, vol. 26, pp. 334-338.
Parfrey, P. S., Foley, R. N., 1999. The Clinical
Epidemiology of Cardiac Disease in Chronic Renal
Failure. Journal of the American Society of Nephrology,
vol. 10, pp. 1606-1615.
Patton, C. J., Crouch, S. R., 1977. Spectrophotometric and
kinetics investigation of the Berthelot reaction for the
determination of ammonia. Analytical Chemistry, vol.
49, pp. 464-469.
Pavluchenko, A. S., Kukla, A. L., Goltvianskyi, Y. V.,
Soldatkin, O. O., Arkhypova, V. M, Dzyadevych, S. V.,
Soldatkin, A. P., 2011. Investigation of Stability of the
pH-Sensitive Field-Effect Transistor Characteristics.
Sensor Letters, vol. 9, pp. 2392-2396.
Ramirez, G., Brueggemeyer, C. D., Newton, J. L., 1984.
Cardiac Arrhythmias on Hemodialysis in Chronic
Renal Failure Patients. Nephron, vol. 36, pp. 212-218.
Razumiene, J., Sakinyte, I., Barkauskas, J., Baronas, R.,
2015, Nano-structured carbon materials for improved
biosensing applications. Applied Surface Science, vol.
334, pp. 185-191.
Sangodkar, H., Sukeerthi, S., Srinivasa, R. S., Lal, R.,
Contractor, A. Q., 1996. A Biosensor Array Based on
Polyaniline. Analytical Chemistry, vol. 68, pp. 779-783.
Sheliakina, M., Arkhypova, V., Soldatkin, O., Saiapina, O.,
Akata, B., Dzyadevych, S., 2014. Urease-based ISFET
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
100

biosensor for arginine determination. Talanta, vol. 121,
pp. 18-23.
Soldatkin, O. O., Kucherenko, I. S., Marchenko, S. V.,
Ozansoy Kasap, B., Akata, B., Soldatkin, A. P.,
Dzyadevych, S. V., 2014. Application of
enzyme/zeolite sensor for urea analysis in serum.
Materials Science and Engineering: C, vol. 42, pp. 155-
160.
Tiwari, A., Aryal, S., Pilla, S., Gong, S., 2009. An
amperometric urea biosensor based on covalently
immobilized urease on an electrode made of
hyperbranched polyester functionalized gold
nanoparticles. Talanta, vol. 78, pp. 1401-1407.
Wang, X., Watanabe, H., Sekioka, N., Hamana, H.,
Uchiyama, S., 2007. Amperometric Urea Biosensor
Using Aminated Glassy Carbon Electrode Covered
with Urease Immobilized Carbon Sheet, Based on the
Electrode Oxidation of Carbamic Acid.
Electroanalysis, vol. 19, pp. 1300-1306.
With, T. K., Petersen, T. D., Petersen, B., 1961. A simple
spectrophotometric method for the determination of
urea in blood and urine. Journal of Clinical Pathology,
vol. 14, pp.202-204.
Development of Multi-parameter Analyser based on Electrochemical Urea Biosensors and Electrolyte Electrodes for Monitoring of
Hemodialysis Patients
101
