
Direct Detection of Bacteria on Fresh Produce
Shin Horikawa
1
, Yating Chai
1
, Howard C. Wikle
1
, James M. Barbaree
2
and Bryan A. Chin
1
1
Materials Research and Education Center, Auburn University, Auburn, Alabama 36849, U.S.A.
2
Department of Biological Sciences, Auburn University, Auburn, Alabama 36849, U.S.A.
Keywords:
Direct Detection, Bacteria, Fresh Produce, Magnetoelastic Biosensor, Phage, Surface-Scanning Detector.
Abstract:
This paper presents a revolutionary method of bacterial detection that directly detects and quantifies the pres-
ence of specific bacteria on the surfaces of fresh produce without sample preparation (water rinse, soak, stom-
aching) and/or enrichment. The speed of detection is from 2 to 10 minutes with a limit of detection in a
range of 10
2
to 10
4
cfu/mm
2
. The specificity of detection is 2 in 10
6
background bacteria. This technology
was awarded a $20,000 prize in the first United States Food and Drug Administration (FDA) Food Safety
Challenge. The method combines wireless magnetoelastic (ME) biosensors and a surface-scanning detector
for rapid determination of bacterial contamination. Tests were conducted on tomatoes and grapes spiked with
different concentrations of Salmonella Typhimurium. The resonant frequency changes of the biosensors were
found to be dependent on the surface concentration of Salmonella. Detection limits were found to be affected
by the surface roughness of the food. A 90-second video of a test for Salmonella on tomato can be viewed at
http://eng.auburn.edu/food-safety. The method presented in this paper is envisioned for use at ports of entry
for the swift screening of foods.
1 INTRODUCTION
The past decades have been marked by a global in-
crease in the outbreaks of food poisoning and asso-
ciated illnesses. These public health problems are
caused by the accidental supply and consumption of
contaminated food, largely due to improper safety
knowledge, perspectives, and practices of food pro-
ducers as well as insufficient consumer awareness.
Although substantial progress on food safety reg-
ulations has been made worldwide, up to 30% of
the population even in industrialized countries suffer
from foodborne illnesses each year (WHO, 2007). In
the United States, approximately 48 million cases of
foodborne illnesses are estimated to occur annually,
resulting in 128,000 hospitalizations, 3,000 deaths,
and $51.0 to $77.7 billion economic losses (Scallan
et al., 2011a; Scallan et al., 2011b; Scharff, 2012).
Salmonella is one of the most pervasive food prob-
lems today. In the past years in the United States,
$1.1 billion was lost, and over 3,000 individuals were
confirmed sick due to Salmonella food contamination
(2008 tomato, 2009 peanut butter, and 2010 egg out-
breaks). Since foodborne illnesses spread so easily,
rapid, on-site detection can play an important role
in preventing the spread of contamination. However,
current detection methods of Salmonella can take up
to several days, causing delays in contacting con-
sumers, removing food items from the marketplace,
and preventing producers from selling products while
the commodity remains stored in warehouses. In this
work, we present a revolutionary bacterial detection
method that allows for testing of contamination to be
completed in only a matter of minutes without sample
preparation (water rinse, soaking, stomaching, con-
centration, etc.) and/or enrichments in the testing pro-
cess. The method combines phage-coated magnetoe-
lastic (ME) biosensors and a surface-scanning detec-
tor, which can be used on site at ports of entry, food
processing facilities and in agriculture fields for food
inspection and outbreak investigation.
2 MATERIAL AND METHODS
2.1 Fabrication of ME Biosensor
Platforms
Strip-shaped ME biosensor platforms (length × width
× thickness: 1 mm × 0.2 mm × 30 µm) were fab-
ricated by dicing a commercially available Metglas
2826MB ribbon (Metglas, Inc.). The diced biosensor
platforms were, then, coated successively with thin
48
Horikawa, S., Chai, Y., Wikle, H., Barbaree, J. and Chin, B.
Direct Detection of Bacteria on Fresh Produce.
DOI: 10.5220/0005670100480053
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 48-53
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

layers of chromium (90 nm in thickness) and gold
(150 nm in thickness) by electron-beam induced de-
position. The chromium layer acts as an adhesive in-
terlayer. The gold layer provides corrosion resistance
as well as a ready surface for the immobilization of
a phage probe. Before and after the metal deposi-
tion, annealing was performed in vacuum at 220
◦
C
for 2 hrs to relieve residual internal stresses and cor-
rect any surface defects from the fabrication processes
(Chai et al., 2012; Chai et al., 2013c; Chai et al.,
2013b; Chai et al., 2013a; Li et al., 2010; Horikawa
et al., 2011; Horikawa et al., 2014b; Horikawa et al.,
2014a).
2.2 Preparation of E2 Phage and
Salmonella Typhimurium
E2 phage was derived from a landscape f8/8 phage
library (Sorokulova et al., 2005) and used as the
biomolecular recognition element for the ME biosen-
sor. E2 phage possesses 10- to 1,000-fold greater
binding affinity for S. Typhimurium over other bacte-
ria. The specificity of detection has been reported to
be 2 in 10
6
background bacteria (Lakshmanan, 2008).
Suspensions of E2 phage (1 × 10
12
virions/ml
in TBS) and S. Typhimurium cells (ATCC 13311,
5 × 10
8
cfu/ml in sterile distilled water) were pre-
pared and provided by Dr. James Barbaree’s group
at Auburn University, Auburn, Alabama, U.S.A. The
concentrated Salmonella suspension was diluted with
sterile distilled water as desired prior to use.
2.3 Phage Immobilization and Surface
Blocking
The fabricated biosensor platforms were individually
immersed in 330 µL of the E2 phage suspension in a
polypropylene PCR tube. The tubes were, then, ro-
tated with a Barnstead LabQuake tube rotator (Fisher
Scientific, Inc.) at 8 rpm for 1 hr. In this way, the
phage was allowed to uniformly attach to the biosen-
sor platforms via physical adsorption. Finally, a wa-
ter rinse was performed to remove loosely attached
phages and TBS buffer residues from the platform
surfaces.
In order to reduce non-specific binding of S. Ty-
phimurium on biosensor surfaces, surface blocking
was performed. The phage-coated biosensors, or
what we call ”measurement sensors,” were individ-
ually immersed in a 330-µl solution of SuperBlock
Blocking Buffer (Thermo Fisher Scientific, Inc.) in a
PCR tube. After 2 hrs of tube rotation at 8 rpm, the
biosensors were collected from the solution and thor-
oughly rinsed with sterile distilled water to be ready
for use. ”Control sensors,” which are not coated with
E2 phage but only surface-blocked with the blocking
buffer, were also prepared and used for determination
of the limits of detection.
∆f ≈ −
∆m
4L
2
WT
s
E
ρ
3
(1− ν)
, (1)
2.4 Principle of Detection
The ME biosensor used in this work is made of
Metglas 2826MB, a magnetostrictive alloy (Li et al.,
2012). Hence, the biosensors can be placed into me-
chanical resonance when subjected to an externally
applied time-varying magnetic field at the right fre-
quency. In this study, the fundamental resonant fre-
quency of longitudinal vibration, f, is of interest, and
thus, an external magnetic field was applied in the di-
rection parallel to the length of the biosensor to excite
such a mode of vibration. For a freestanding, strip-
shaped biosensor, f can be expressed by (Liang et al.,
2007)
f =
1
2L
s
E
ρ(1− ν)
, (2)
where L, E, ρ, and ν denote the length, modulus of
elasticity, density, and Poisson’s ratio of the biosen-
sor, respectively. When this biosensor comes into
contact with S. Typhimurium, E2 phage that is coated
on the biosensor binds specifically with the bacteria,
thereby increasing the total mass of the biosensor by
∆m. This change in mass causes a corresponding
decrease in the biosensor’s resonant frequency. The
mass-induced resonant frequency change, ∆ f, can be
approximated by (Grimes et al., 2011)
where W and T represent the width and thickness
of the biosensor, respectively. By recording ∆ f as a
function of time, real-time monitoring of the presence
of Salmonella on food surfaces can be performed.
Unlike a measurement sensor (with E2 phage), a con-
trol sensor (without E2 phage) does not bind specifi-
cally with S. Typhimurium, which gives a background
resonant frequency shift in the testing environment.
2.5 Construction of the
Surface-Scanning Detector
The surface-scanning detector is a microelectroni-
cally fabricated planar coil that serves as: (1) a driving
coil to magnetically excite the longitudinal vibration
of the biosensor and (2) a pick-up coil to read the res-
onant frequency of the biosensor. The detector was
fabricated using standard microelectronic fabrication
techniques. As shown in Fig. 1, the coil turns, leads,
Direct Detection of Bacteria on Fresh Produce
49
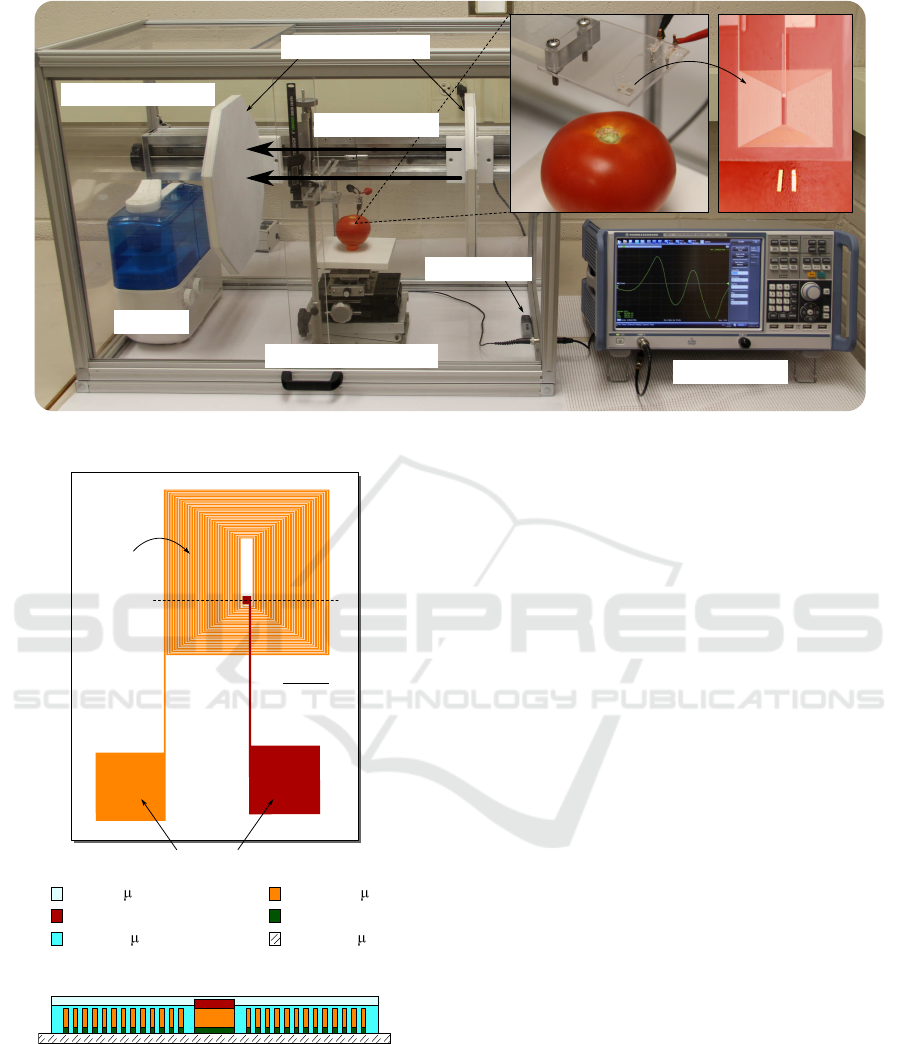
Network analyzer
Humidifier
Environmental chamber
Three-axis translation stage
Humidity sensor
Bias magetic field
Detector
Sensors
Pair of magnetic plates
Figure 2: Measurement setup for the direct detection of S. Typhimurium on food.
Glass (500 m)
Cu turns (8 m)
Ti (10 nm)
SU-8 (5 m)
SU-8 (10 m)
Cu lead and pad (200 nm)
Section AA'
(schematic, not to scale)
A'A
1 mm
Contact pads
Coil turns
Figure 1: Design of the surface-scanning detector.
and contact pads are made of copper, and they reside
on a glass substrate. To promote good adhesion of the
coil to the substrate, titanium was used as the inter-
layer. In addition, SU-8 3005 (MicroChem Corp.), an
epoxy-based photoresist, was used as the insulating
filler and topmost layer.
2.6 Spiking of Fresh Produce with S.
Typhimurium
Fresh tomatoes and grapes were purchased from a lo-
cal grocery store (Kroger) and used as-received. 20-µl
drops of S. Typhimurium with various concentrations
(5 × 10
5
to 5 × 10
8
cfu/ml) were spot-inoculated on
the surfaces of the foods. Salmonella were, then, al-
lowed to dry in air for 2 hrs. Finally, the area of
the Salmonella-spiked spots were measured, which
allows conversion of cfu/ml into cfu/mm
2
(i.e., sur-
face density of S. Typhimurium).
2.7 Measurement Setup and
Experimental Procedure
The measurement setup for the direct Salmonella de-
tection on food consists of a surface-scanning detec-
tor, a 3-axis translation stage, a network analyzer (Ro-
hde & Schwarz ZNC3), a humidifier, a pair of mag-
netic plates, and an environmental chamber as shown
in Fig. 2. ME biosensors were first placed on a
Salmonella-spiked spot on food. The food was, then,
placed between the magnetic plates in the environ-
mental chamber. At this time, the biosensors on the
food can spontaneously align parallel to the direction
of the external magnetic field, which is perpendicular
to the magnetic plates. The 3-axis translation stage
was next used to position the biosensors under the
surface-scanning detector. The detector is connected
to the ZNC3 network analyzer, operated in the S
11
reflection mode. An incident AC power is applied
across the detector to magnetically excite the longi-
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
50
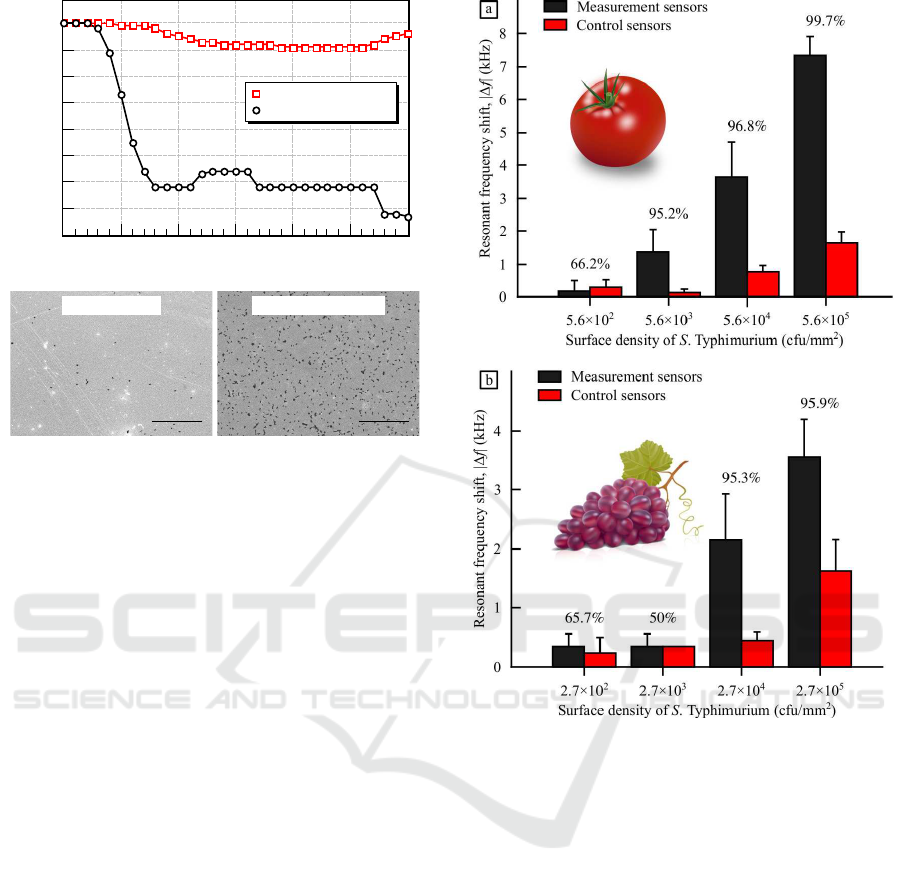
Time (seconds)
Resonant frequency shift, Δf (kHz)
0 50 100 150 200 250 300
0
-0.5
-1
-1.5
-2
-3
-2.5
-3.5
-3
-4
Measurement sensor
Control sensor
Measurement sensor
Control sensor
Figure 3: Resonant frequency shifts of biosensors on a
tomato that is spiked with 5.6 × 10
4
cfu/mm
2
S. Ty-
phimurium (open squares: control sensor and open circles:
measurement sensor). Scanning electron micrographs con-
firm the resonant frequency shift results. Salmonella cells
are shown as the black spots in the micrographs (scale bars:
50 µm).
tudinal vibration of the biosensor, and the resultant
reflected power is compared to the incident power
over a selected range of frequencies. In this man-
ner, frequency-dependant reflection coefficient, S
11
,
can be determined for the circuit. At the resonant fre-
quency of the biosensor, the largest change in the re-
flected power of the circuit occurs, which is visible
as a downward peak in the network analyzer output.
To enhance the magnitude of the resonance peak, a
proper bias magnetic field was applied to the biosen-
sor by adjusting the distance between the magnetic
plates. The measurement was conducted at 23
◦
C
and 85% relative humidity. Data were collected every
10 seconds (power: 0 dBm, bandwidth: 1 kHz, fre-
quency span: 100 kHz, number of date points: 2001,
averaging: 10 times, and smoothing aperture: 1 %).
3 RESULTS AND DISCUSSION
3.1 Direct Detection of S. Typhimurium
on Food
Resonant frequency shifts of ME biosensors placed
on the Salmonella-spiked foods were recorded every
10 seconds using the measurement setup described
in Subsection 2.7. Typical test results for a tomato
1
2
3
4
5
0
2 3 4
5
2 3
4
5
1
2
3
4
0
99.7%
96.8%
95.2%
66.2%
95.9%
95.3%
50%65.7%
Figure 4: Dose-response results for (a) tomatoes and (b)
grapes. The numbers shown above the bars are the con-
fidence levels of difference between the measurement and
control sensors at each Salmonella concentration.
spiked with 5.6 × 10
4
cfu/mm
2
Salmonella are shown
in Fig. 3. The resonant frequency shift of a con-
trol sensor (open squares) was found to be negligible
during the measurement. By contrast, a much larger
resonant frequency shift was observed for a measure-
ment sensor (open circles), due to the occurrence of
the phage-based specific binding of the bacteria on
the sensor. Depending on the surfaces of foods and
concentration of S. Typhimurium, the rates of reso-
nant frequencyshifts and detection speeds were found
to vary. In this particular example shown in Fig.
3, S. Typhimurium (5.6 × 10
4
cfu/mm
2
) was de-
tected within 2 minutes. Finally, scanning electron
microscopy was used to confirm the frequency mea-
surement results. A large cell count was found on
the measurement sensor as anticipated, indicating that
specific Salmonella binding had occurred.
In order to determine the limits of detection
Direct Detection of Bacteria on Fresh Produce
51
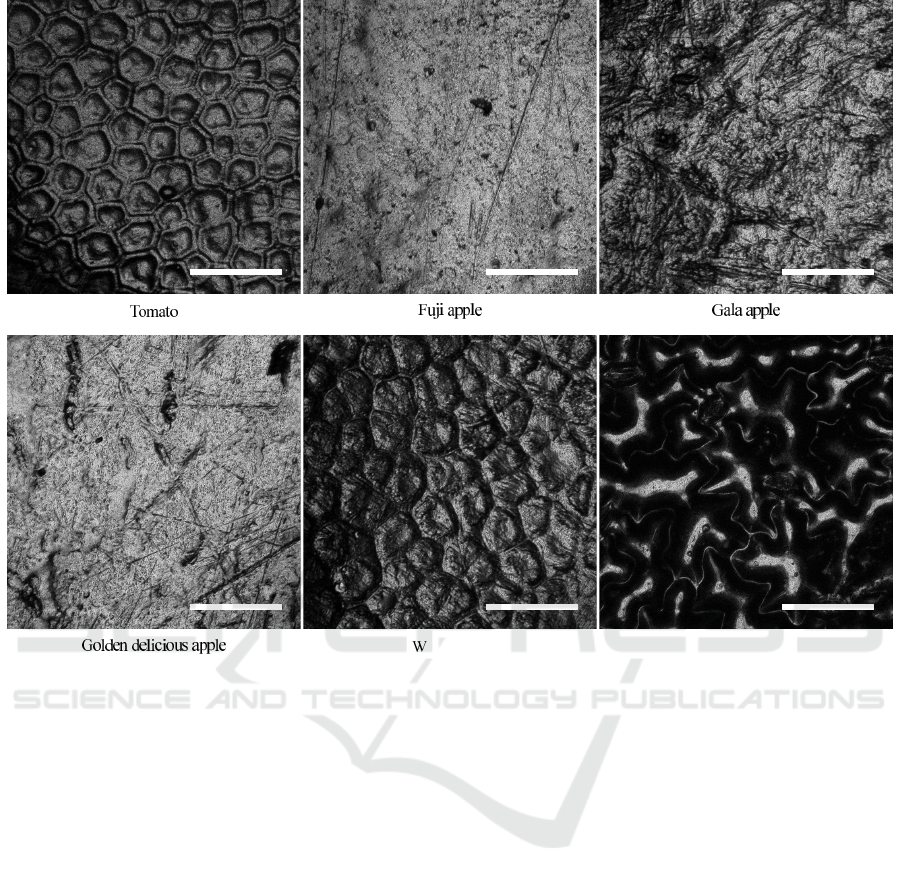
Spinach leaf
atermelon
Figure 5: Surfaces of various fresh produce (scale bars: 100 µm).
(LODs), the biosensors were tested at different
Salmonella concentrations on the food surfaces for 10
minutes. The dose-response results for tomatoes and
grapes are shown in Fig. 4. As anticipated, the reso-
nant frequency shifts of measurement sensors (black
bars) were found to be larger than those of control
sensors (red bars) at high Salmonella concentrations,
which is due to the specific binding of the bacteria
through E2 phage. By contrast, comparable sensor re-
sponses were observed at low Salmonella concentra-
tions, indicating that the limits of detection had been
reached. A one-tailed, unpaired Student’s t-test was
conducted to analyze the degree of dissimilarity be-
tween the measurement and control sensors. The con-
fidence levels of difference were calculated and pre-
sented above the bars at each Salmonella concentra-
tion in Fig. 4. With a confidence level of difference
higher than 95% (p < 0.05), the LODs were deter-
mined to be lower than: (a) 5.6 × 10
3
cfu/mm
2
for
tomatoes and (b) 2.7 × 10
4
cfu/mm
2
for grapes. The
method presented in this paper is a direct bacterial
detection method without sample preparation (con-
centration, purification, washing, etc.) and/or enrich-
ments in the testing process. The speed of detection
was found to be from 2 to 10 minutes, suited for rapid,
on-site pre-screening of food products.
4 FUTURE WORK
The current LODs can be improved by using ME
biosensors with smaller sizes. As can be seen in
Eq. 1, the mass-induced resonant frequency change,
∆f, is inversely proportional to L
2
WT, where L,
W, and T are the length, width, and thickness of
the biosensor, respectively. Smaller biosensors are,
hence, more mass-sensitive and capable of detecting
smaller amounts of pathogens that may be present on
food surfaces. The LOD is also largely dependent on
the surface topography of foods. As shown in Fig.
5, the surface pattern and roughness vary from one
food to another, which necessitates the use of appro-
priate sizes of biosensors for improved pathogen de-
tection. The authors have reported previously an ini-
tial model to calculate the LOD and probability of de-
tection as a function of the size and quantity of biosen-
sors (Horikawa, 2013). The model will be improved
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
52

and used to demonstrate a proof-in-concept in the fu-
ture.
5 CONCLUSIONS
A unique, revolutionary method of bacterial detection
on the surfaces of foods was presented. By combining
phage-coated ME biosensors and a surface-scanning
detector, S. Typhimurium was detected in a range of 2
to 10 minutes without sample preparation and/or en-
richment in the testing process. The method presented
in this paper can be used for rapid, on-site screening
of food products. The pathogen-positive foods deter-
mined by this method can then be sent to lab for con-
firmation and identification tests.
ACKNOWLEDGEMENT
This work was supported by the Auburn University
Detection and Food Safety Center and a grant from
the U.S. Department of Agriculture (USDA-2011-
51181-30642A).
REFERENCES
Chai, Y., Horikawa, S., Li, S., Wikle, H. C., and Chin,
B. A. (2013a). A surface-scanning coil detector for
real-time, in-situ detection of bacteria on fresh food
surfaces. Biosensors and Bioelectronics, 50(0):311 –
317.
Chai, Y., Horikawa, S., Wikle, H. C., Wang, Z., and Chin,
B. A. (2013b). Surface-scanning coil detectors for
magnetoelastic biosensors: A comparison of planar-
spiral and solenoid coils. Applied Physics Letters,
103(17):173510–173510–4.
Chai, Y., Li, S., Horikawa, S., Park, M.-K., Vodyanoy, V.,
and Chin, B. A. (2012). Rapid and sensitive detec-
tion of Salmonella Typhimurium on eggshells by us-
ing wireless biosensors. Journal of Food Protection,
75(4):631 – 636.
Chai, Y., Wikle, H. C., Wang, Z., Horikawa, S., Best, S.,
Cheng, Z., Dyer, D. F., and Chin, B. A. (2013c). De-
sign of a surface-scanning coil detector for direct bac-
teria detection on food surfaces using a magnetoelastic
biosensor. Journal of Applied Physics, 114(10):4504.
Grimes, C. A., Roy, S. C., Rani, S., and Cai, Q. (2011). The-
ory, instrumentation and applications of magnetoelas-
tic resonance sensors: A review. Sensors, 11(3):2809
– 2844.
Horikawa, S. (2013). Real-Time, in-situ Detection of
Pathogenic Bacteria on Food Surfaces Using a
Surface-Scanning Coil Detector and Phage-Based
Magnetoelastic Biosensors. PhD thesis, Auburn Uni-
versity.
Horikawa, S., Bedi, D., Li, S., Shen, W., Huang, S., Chen,
I.-H., Chai, Y., Auad, M. L., Bozack, M. J., Barbaree,
J. M., Petrenko, V. A., and Chin, B. A. (2011). Ef-
fects of surface functionalization on the surface phage
coverage and the subsequent performance of phage-
immobilized magnetoelastic biosensors. Biosensors
and Bioelectronics, 26(5):2361 – 2367.
Horikawa, S., Chai, Y., Wikle, H. C., and Chin, B. A.
(2014a). Autonomous magnetoelastic biosentinels for
the detection and capture of invasive pathogens. ECS
Transactions, 64(1):149–155.
Horikawa, S., Zhao, R., Chai, Y., Wikle, H. C., and Chin,
B. A. (2014b). Self-propelled, phage-based magne-
toelastic biosentinels for detection of pathogens in
liquid. In SPIE Sensing Technology+ Applications,
pages 910805–910805. International Society for Op-
tics and Photonics.
Lakshmanan, R. (2008). Phage-Based Magnetoelastic Sen-
sor for the Detection of Salmonella typhimurium. PhD
thesis, Auburn University.
Li, S., Horikawa, S., Park, M., Chai, Y., Vodyanoy, V. J.,
and Chin, B. A. (2012). Amorphous metallic glass
biosensors. Intermetallics, 30(0):80 – 85.
Li, S., Li, Y., Chen, H., Horikawa, S., Shen, W., Simo-
nian, A., and Chin, B. A. (2010). Direct detection
of Salmonella typhimurium on fresh produce using
phage-based magnetoelastic biosensors. Biosensors
and Bioelectronics, 26(4):1313 – 1319.
Liang, C., Morshed, S., and Prorok, B. C. (2007). Cor-
rection for longitudinal mode vibration in thin slender
beams. Applied Physics Letters, 90:221912.
Scallan, E., Griffin, P. M., Angulo, F. J., Tauxe, R. V., and
Hoekstra, R. M. (2011a). Foodborne illness acquired
in the United States - unspecified agents. Emerging
Infectious Diseases, 17(1):16 – 22.
Scallan, E., Hoekstra, R. M., Angulo, F. J., Tauxe, R. V.,
Widdowson, M.-A., Roy, S. L., Jones, J. L., and Grif-
fin, P. M. (2011b). Foodborne illness acquired in the
United States - major pathogens. Emerging Infectious
Diseases, 17(1):7 – 15.
Scharff, R. L. (2012). Economic burden from health losses
due to foodborne illness in the United States. Journal
of food protection, 75(1):123 – 131.
Sorokulova, I. B., Olsen, E. V., Chen, I.-H., Fiebor, B., Bar-
baree, J. M., Vodyanoy, V. J., Chin, B. A., and Pe-
trenko, V. A. (2005). Landscape phage probes for
Salmonella typhimurium. Journal of Microbiological
Methods, 63(1):55 – 72.
WHO (2007). Food safety and foodborne illness. fact sheet
no. 237.
Direct Detection of Bacteria on Fresh Produce
53
