
In Vivo Experimental Detection of Inflammatory Process in Tissues
by Fluorescence Spectroscopy
Irina Guseva
1,2
, Dmitriy Rogatkin
1
, Polina Kulikova
1
and Dmitriy Kulikov
1
1
Moscow Regional Research & Clinical Institute "MONIKI" named after M.F. Vladimirskiy,
Shepkina str., Moscow, Russian Federation
2
National Research Nuclear University MEPhI, Kashirskoe highway, Moscow, Russian Federation
Key
words: Fluorescence, Non-invasive, Diagnostics, Inflammatory Process, In Vivo.
Abstract: Laser fluorescence spectroscopy (LFS) is widely used in medicine. Today, oncology and oncosurgery are
considered as the most promising fields of its application. It is known that cancerous tissues are able to
accumulate different porphyrins, both endogenous and exogenous, in enhanced amount due to increased
metabolism in cancerous cells. So, LFS can be used in vivo for detection of malignant tumours as well as for
real-time intraoperative imaging or diagnostics at a photodynamic therapy. One of the reason of the
enhanced accumulation of porphyrins in tissues is a chronic hypoxia. Therefore, it was hypothesized, that
LFS could also be used for diagnosis of local inflammation in tissues. Recently, some indirect data
confirming the hypothesis was obtained when observed inflammation due to invasion of external substances
into tissues. This study proves the hypothesis in a direct experiment with animals and laboratory tests.
Enhanced fluorescence intensity of the exogenous photosensitizer was found in inflamed tissues. The direct
association between intensity of the fluorescence, histological characteristics and blood test results was
shown. It was found that the registered fluorescence signal correlates with neutrophil counts in blood of
tested animals. It proves that LFS could be an effective tool for registration of local inflammation.
1 INTRODUCTION
Laser fluorescence spectroscopy (LFS) is currently
one of the promising methods for a non-invasive (in
vivo) characterisation of biological tissues and its
functional conditions (Johansson et al., 2008),
(Tuchin, 2002), (Mycek et al., 2003), (Rogatkin et
al., 2013). LFS is based on a registration of
fluorescence spectra and (or) fluorescence intensities
of endogenous or exogenous fluorophores on a
surface of the tested tissues. A great interest of
researchers to the method can be explained by its
advantages over other methods of assessment of soft
biological tissues. LFS differs from other methods
by noninvasive modality (minimally invasive, if
special preparations are used). It gives a possibility
of in vivo diagnosis in real time, and is safe for the
body (the method implies low-power laser light).
Among others areas, oncology and oncosurgery
are considered as the most promising fields of
application of noninvasive LFS in medicine of today
(Tuchin, 2002). It is known, that cancerous tissues
accumulate different porphyrins and its derivations,
both endogenous and exogenous, in enhanced
amount due to the increased metabolism in
malignant cells (Mycek et al., 2003). Therefore, LFS
can be used in vivo for a detection of malignant
tumors as well as for real-time intraoperative
imaging in oncosurgery or for a diagnostics at a
photodynamic therapy. A great number of medical
publications deal with the application of LFS for
early detection of malignancies in skin (Calin, et al
2013), oral mucosa (De Veld et al., 2005),
gastrointestinal tract (Duraipandian et al., 2012),
(Koizumi et al., 2013), and urogenital system (Stenzl
et al., 2011), (Karaoglu et al., 2014), as well as for
cancer diagnostics at a photodynamic therapy
(Andersson-Engels et al., 1995). In oncology LFS
can help surgeons to visually distinguish healthy
tissue from the cancerous one and to perform a
precise ablative process (Vahrmeijer et al., 2013).
Also, LFS can be used to identify sentinel lymphatic
nodes by providing their realtime intraoperative
imaging. Furthermore, it can be used to prevent
iatrogenic damage to vital structures, such as the
ureter or nerves (Handgraaf, 2014). There are many
Guseva, I., Rogatkin, D., Kulikova, P. and Kulikov, D.
In Vivo Experimental Detection of Inflammatory Process in Tissues by Fluorescence Spectroscopy.
DOI: 10.5220/0005659301390144
In Proceedings of the 9th International Joint Conference on Biomedical Engineering Systems and Technologies (BIOSTEC 2016) - Volume 1: BIODEVICES, pages 139-144
ISBN: 978-989-758-170-0
Copyright
c
2016 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
139

publications on the use of this method for
intraoperative imaging in the breast surgery,
gynecology, neurology and other medical specialties
(Handgraaf, 2014), (Tummers, 2014), (Sugie, 2013).
Application of LFS seems also to be very promising
in the robotic assisted surgery (Hellan,2014). All
these application of LFS are based on the fact of the
abnormal fluorescence of both endogenous and
exogenous fluorophores in tumorous tissues.
Meanwhile, it was shown previously, that one of
the reason of the enhanced accumulation of
porphyrins in tissues is a chronic hypoxia (Rogatkin
et al., 2009). Therefore, it can be hypothesized, that
LFS could also be used for diagnosis of a local
inflammation in tissues. Currently, the search of
noninvasive methods of local inflammation
diagnosis is an extremely actual task, especially in
the regenerative medicine, when local regenerative
processes are under investigations and control. Also,
it is important in oncology. It is well-known that a
lot of pathological changes in tumorous tissues, in
particular in cancerous ones, are accompanied by a
number of local and systemic inflammatory
responses (Diakos, 2014). What if the enhanced
accumulation of porphyrins in a tumour is caused
not only by malignat processes, but also by
inflammatory ones, and it is not specific for
cancerous cells? Today, there is a few data only
devoted to the differentiation of the fluorescence
spectra for a cancer and inflammation. So, this issue
requires further attention (Lv, 2015), (Zhang, 2013),
because of potential errors can exist in LFS due to
this phenomenon.
Generally, in clinical practice of today, a
leukogram response test is widely used for the
inflammation diagnosis. Leukocytosis, neutrophilia,
left shift of the leukogram, etc. are the frequent
blood hallmarks of inflammations (Marshall, 2006).
However, the leukogram is nonspecific indicator of
local inflammations. It is not able to specify the
location of the inflammatory process. Biopsy and
subsequent histological examination can accurate
and reliable detect of any local inflammations, but it
is an invasive method. There are publications on the
use of thermography as a noninvasive quantitative
imaging method for assessing the local inflammation
(Christensen, 2014), (Arfaoui, 2012). However, the
increase in temperature is only one of signs of local
inflammation and, therefore, may be considered as
an auxiliary method. The rise in temperature is not
pathognomonic for local inflammation and may
indicate a normal physiological reactions (enhanced
functional activity of muscles, heating of skin, etc.).
Another modern method for local inflammation
diagnosis is a scintigraphy (Love, 2013). However,
this method involves an introduction into the
organism of radioactive isotopes, so it is associated
with dangerous ionizing radiation exposure. Thus,
the search for new, noninvasive or minimally
invasive, and real-time instrumental methods for
diagnosis of a local inflammation is an extremely
important task.
Recently, some indirect data confirming the
hypothesis of enhanced accumulation of exogenous
fluorophores of an aluminum phthalocyanine series
in inflammed tissues were published (Petritskaya et
al., 2014). Enhanced fluorescence was observed at
local inflammation due to invasion of external
substances into tissues. But in the reffered study the
histological or a blood test confirmation of the
inflammation process wasn’t been done. The aim of
our current study was to confirm possibilities of LFS
to detect in vivo a local inflammation in tissues in a
direct experiment with animals and laboratory tests.
2 MATERIALS AND METHODS
The study was performed in white laboratory mice
(N=12) and was conducted in accordance with all
ethical principles formulated in the Declaration of
Helsinki on the care and use of animals in research
and the Regulations of the European Science
Association (86/609/ЕС).
Local inflammation was provoked as follows.
All procedures were carried out under general
anesthesia (Zoletil + Xylazine). An incision was
made in the lateral part of the inguinal fold, then
skin was separated from the fascia by blunt
dissection, and the underlying muscle was clamped
at a distance of 7 mm from the incision by a
Mosquito clamp. The size of the resulting zone of
injury was 3х3х3 mm (Figure 1). Then, the
photosensitizer “Photosens” based on aluminum
phthalocyanine was injected intraperitoneally in the
dose of 2 mg/kg. It is known, that the fluorescent
signal of this photosensitizer in tissues can be
detected during 4-5 weeks, so it is possible to use the
aluminum phthalocyanine based photosensitizer in
prolonged experiments without any additional
injections. To prevent side effects, experimental
animals were not exposed to direct sun radiation
during the experiment, so any phototoxic effects did
not affect the laboratory mice.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
140
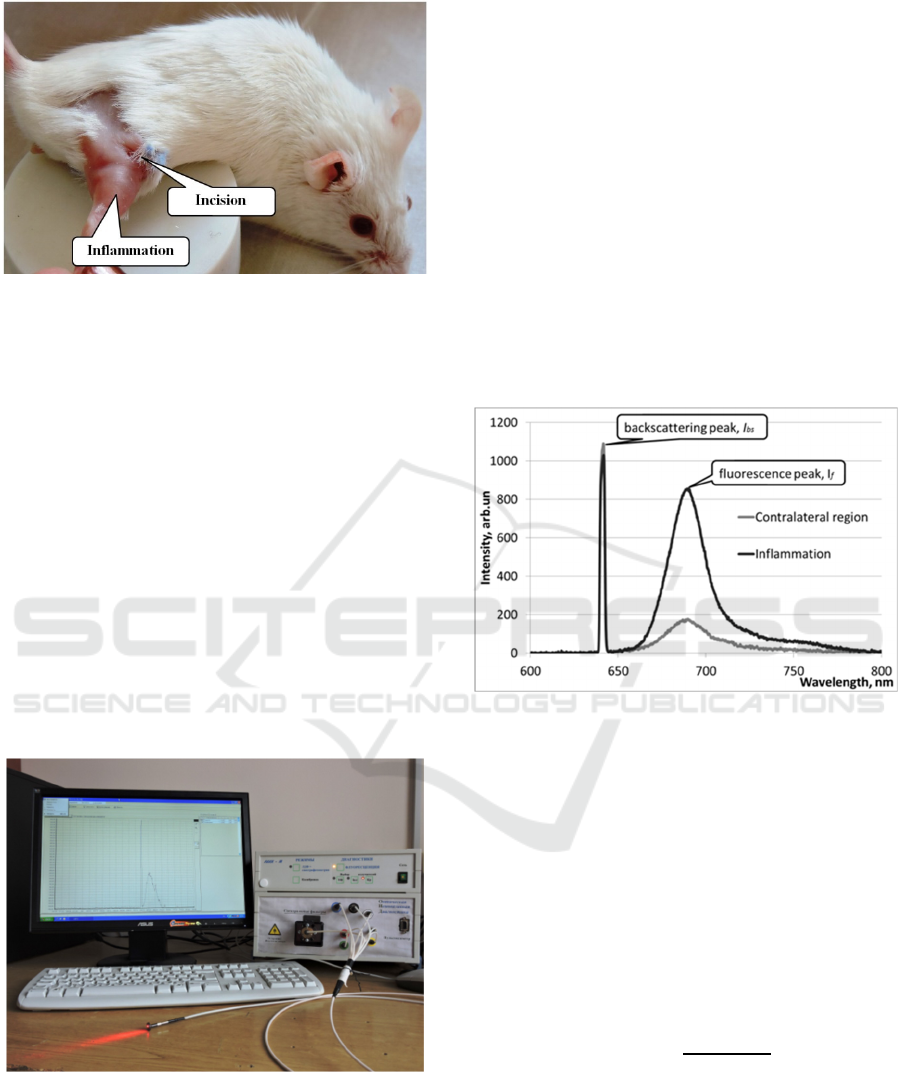
Figure 1: Position of the inflamed region relative to the
incision.
To confirm the fact of inflammation, hematology
tests as well as a conventional histological analysis
of the affected area of the hind limb of mice were
done prior the provocation and on Days 3 and 10
after the provocation of the local inflammation. For
this purpose, two mice every time points (Days 0, 3,
10) were withdrawn from the experiment. Since our
interest was only to confirm the presence of local
inflammation without assessment of any specialties
of the inflammation, it was sufficient to carry out
both histological examination and a blood test only
in these three points of time (Days 0, 3, 10) as the
most expressive points of the evolution of such
inflammation. In hematology test results we firstly
took into account the percentage ratio of neutrophils
(neutrophil counts) as the most informative
parameter at a local inflammation in animals.
Figure 2: The diagnostic system “LAKK-M”.
In all experiments, fluorescence was recorded in
vivo with the use of laser diagnostic system LAKK-
M in the “Fluorescence” operation regime. The
system is equipped with fiber optical probe (Figure
2). Excitation of tissue fluorescence was made in the
continuous wave (CW) mode at the wavelength 635
nm (a semi-conductor laser). Power of the laser
radiation on a distal end of the optical fiber probe
(on a surface of tissues) was around 5 mW.
Fluorescence intensity was measured at 670 nm – in
a maximum of the fluorescent spectrum of the used
photosensitizer “Photosens”. Subsequently, the
intensity at this wavelength will be called
“fluorescence intensity”.
Measurements of the fluorescence intensity in
animals were carry out before the provocation of the
local inflammation (before the injection of the
photosensitizer and on Days 2, 3, 6, 8, 10 and 16
after the provocation and the injection.
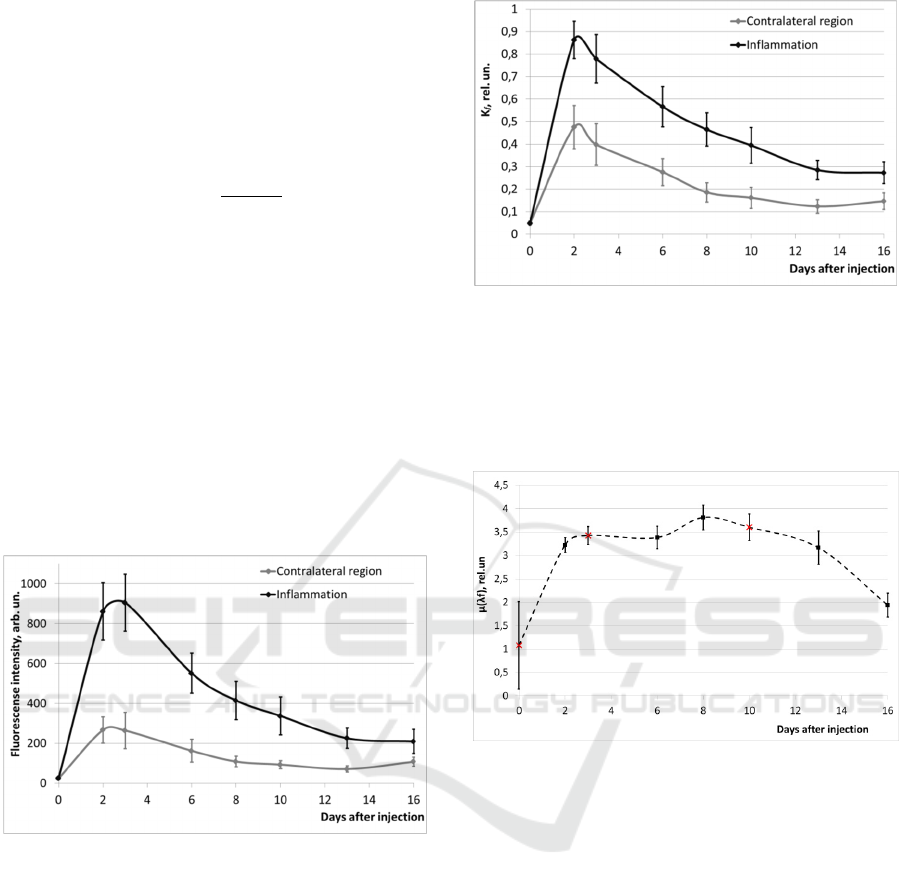
Examples of fluorescence spectra from inflamed
tissues and from a contralateral region are shown in
Figure 3.
Figure 3: Examples of fluorescence spectra from the
contralateral region and from inflamed tissues (3 days
after the injection of the photosensitizer).
To study the dynamics of photosensitizer
accumulation in inflamed tissues, the peak value of
the measured fluorescence intensities was tracked in
time. Also, to clarify the influence of the initial laser
radiation power as well as of local optical properties
of tissue on the registered intensities, we compared
the dynamics of the measured fluorescence
intensities and the dynamics of the coefficient of
fluorescence contrast K
f
(Rogatkin, et al 2013),
which calculated using the intensities as follows:
1
∙
∙
where K
f
is the coefficient of fluorescence contrast
(0<K
f
<2); I
f
is the maximum of the fluorescence
intensity; I
bs
is the measured intensity of the
backscattered radiation at the excitation wavelength;
β is an instrumental reducing coefficient (β≈1000 to
diminish I
bs
to a level which is comparable with the
level of I
f
). In this coefficient I
f
is normalized both
In Vivo Experimental Detection of Inflammatory Process in Tissues by Fluorescence Spectroscopy
141
by local optical properties of tissues and by a power
of excitation radiation, so it is less sensitive to theirs
changes.
To compare the fluorescence intensity from
inflammation and contralateral areas, an index of
inflammation intensity μ(λ
f
) was calculated as
follows:
where I
f
is the fluorescence intensity from the
inflamed area, I
f0
is the fluorescence intensity from
the contralateral region, and λ
f
is the fluorescence
wavelength (in our case λ
f
= 690 nm).
3 RESULTS AND DISCUSSION
All experimental results showed an enhanced
intensity of the fluorescence signal in the injured
tissues, compared to that in the contralateral region.
Figure 4 presents the averaged data for the group of
mice.
Figure 4: Dynamics of fluorescence intensities.
Earlier it was shown (Rogatkin et al., 1998), that
uncertainty of results of such measurements in the
laser fluorescence diagnostics amounts to 40% of the
measured value. Therefore, the experimentally
observed differences between signals from the intact
area and the inflamed tissues are significant. This
suggests that inflammatory processes can be in vivo
detected by LFS.
The dynamics of K
f
is shown in Figure 5. It is
easy to see that there are no fundamental differences
in the behavior of the curves in Figures 4 and 5. It
confirms, that the influence of the laser power
fluctuation or an influence of local optical properties
of tissues on registered intensities is small enough at
so high fluorescence of the photosensitizer used.
Figure 5: Dynamics of the coefficient of fluorescence
contrast.
Dynamics of the index of inflammation intensity
μ(λ
f
) versus days is shown in Figure 6. For clarity,
the time points of hematology and histology tests are
denoted by crosses.
Figure 6: Dynamics of the inflammation intensity index
μ(λ
f
).
All data of laboratory blood tests showed an
increase in the neutrophil counts (Figure 7) and a
relative increase in the band neutrophil counts
(Figure 8), i.e. a shift of the leukogram to the left.
These parameters indicate exactly the occurrence of
inflammatory process in organism.
Histological examination performed at Day 0
before the provocation of inflammation was normal.
Histological examination performed at Day 3 after
the provocation revealed an acute inflammatory
response in the injured muscle tissue, dermis and
subcutaneous adipose tissue, namely: there were
edema, leukocyte inflammatory infiltration of
tissues, and necrotic foci in muscle. Reduction of
inflammatory activity and some regenerative muscle
tissue changes were observed at Day 10. But the
inflammatory infiltrate, indicating a continuing
inflammatory process, remained in the dermis and
subcutaneous adipose tissue.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
142
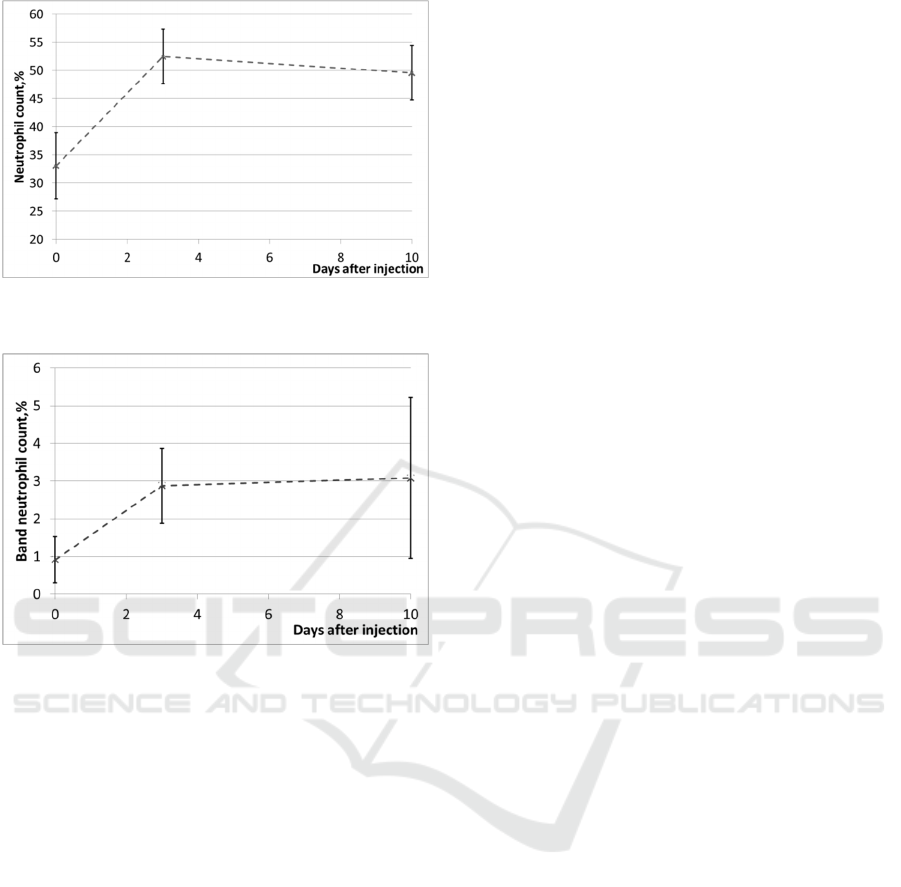
Figure 7: Changes of blood neutrophil counts in laboratory
mice.
Figure 8: Changes of blood band neutrophil counts in
laboratory mice.
Based on these results (Figures 7 and 8), it can
be assumed that the inflammation intensity index
μ(λ
f
) in inflammation regions correlates well with
blood neutrophil counts. The increase of the index
μ(λ
f
) on Day 3 corresponds to an acute local
inflammatory response, which was evident in both
hematology tests and at histological assessment. On
Day 10, the µ(λ
f
) remained high, correlating with
histologically confirmed persistence of the
inflammation in subcutaneous adipose tissue and
dermis, and with the increase in the blood neutrophil
counts.
4 CONCLUSIONS
The aim of the current study was to confirm
possibilities of LFS to detect in vivo a local
inflammation in tissues. In a direct experiment with
animals and confirmational laboratory tests it was
shown and proved that non-malignant inflamed
tissues can have an enhanced accumualtion of
exogenious photosensitizer and, therefore, can have
an enhanced superficial fluorescence of it like
several cancerous tissues have. Furthermore, it was
shown that the intensity of the fluorescent signal in
the inflamed tissues correlated with blood neutrophil
counts and was associated with changes in histology.
It is of special note, that this result is very
important both for an experimental research and for
a practical medicine. Many processes in a human
body are associated with development of local
inflammations. First of all, these are different
processes related to mechanical and thermal tissue
damage, to introduction of foreign agents into a
tissue, at a transplantation, for example. So, a
diagnosis of a local inflammation by LFS technique
in vivo can be used as a navigation method in
surgery and, also, as an intraoperative assessment of
tissue conditions at a regenerative surgery. These
data is important for the fluorescence diagnostics in
oncology, as well. It was shown that the exogenous
photosensitizer can be accumulated not only in
malignant cells, but also in the area of inflammation.
It makes us reassess the applicability limits of LFS
at a photodynamic therapy.
Radiation therapy is an effective and accepted
treatment modality in oncology. Various radiation-
induced reactions, including inflammation, could be
its side effects, which is the reasons why radiation
doses are often fractionated. However, fractionation
procedures are poorly individualized, and standard
radiation regimens are used due to a lack of
affordable instrumental method for assessment of
individual local inflammations. The proposed
technique could become such a method. So, in a
view of the foregoing, it can be assumed that this
method of diagnostics of a local inflammation may
have a broad clinical application.
REFERENCES
Andersson-Engels, S., Berg, R., Svanberg, K. and
Svanberg, S. (1995). Multi-colour fluorescence
imaging in connection with photodynamic therapy of
delta-amino levulinic acid (ALA) sensitised skin
malignancies. Bioimaging, 3.3, 134-143.
Arfaoui, A., Bouzid, M. A., Pron, H., Taiar, R., &
Polidori, G. (2012). Application of infrared
thermography as a diagnostic tool of knee
osteoarthritis. Journal of Thermal Science and
Technology, 7(1), 227-235.
Calin, M.A., Parasca, S.V., Savastru, R., Calin, M.R. and
Dontu, S. (2013). Optical techniques for the non-
invasive diagnosis of skin cancer. Journal of cancer
research and clinical oncology, 139(7), 1083-1104.
In Vivo Experimental Detection of Inflammatory Process in Tissues by Fluorescence Spectroscopy
143

Christensen, J., Matzen, L. H., Væth, M., Schou, S., &
Wenzel, A. (2014). Thermography as a quantitative
imaging method for assessing postoperative
inflammation. Dentomaxillofacial Radiology.
De Veld, D.C.G., Witjes, M.J.H., Sterenborg, H.J.C.M.
and Roodenburg, J.L.N. (2005).The status of in vivo
auto fluorescence spectroscopy and imaging for oral
oncology. Oral oncology, 41(2), 117-131.
Diakos, C. I., Charles, K. A., McMillan, D. C., & Clarke,
S. J. (2014). Cancer-related inflammation and
treatment effectiveness. The Lancet Oncology, 15(11),
e493-e503.
Duraipandian, S., Bergholt, M., Zheng, W., Ho, K., Teh,
M., Yeoh, K., So, J., Shabbir, A. and Huang, Z.
(2012).Real-time biomedical Raman spectroscopy for
in vivo, on-line gastric cancer diagnosis during clinical
endoscopic examination. Journal of Biomedical
Optics, 17 (8), 081418.
Handgraaf, H. J., Verbeek, F. P., Tummers, Q. R.,
Boogerd, L. S., van de Velde, C. J., Vahrmeijer, A. L.
and Gaarenstroom, K. N. (2014). Real-time near-
infrared fluorescence guided surgery in gynecologic
oncology: A review of the current state of the art.
Gynecologic oncology, 135(3), 606-613.
Hellan, M., Spinoglio, G., Pigazzi, A. and Lagares-
Garcia, J. A. (2014).The influence of fluorescence
imaging on the location of bowel transection during
robotic left-sided colorectal surgery. Surgical
endoscopy, 28(5), 1695-1702.
Johansson, A., Kromer, K., Sroka, R., and Stepp, H.
(2008).Clinical optical diagnostics – Status and
perspectives. Med Laser Appl, 23(4), 55–74.
Karaoglu, I., van der Heijden, A.G. and Alfred Witjes, J.
(2014).The role of urine markers, white light
cystoscopy and fluorescence cystoscopy in recurrence,
progression and follow-up of non-muscle invasive
bladder cancer. World J Urol., 32.3, 651-659.
Koizumi, N., et al. (2013). Detection of metastatic lymph
nodes using 5-aminolevulinic acid in patients with
gastric cancer. Annals of surgical oncology 20.11,
3541-3548.
Lv, M., Qin, F., Mao, L., Zhang, L., Lv, S., Jin, J., &
Zhang, Z. (2015). A study of diagnostic criteria
established for two oral mucous diseases by HMME-
fluorescence spectroscopy. Lasers in medical science,
30(8), 2151-2156.
Love, C., & Palestro, C. J. (2013, March). Radionuclide
imaging of inflammation and infection in the acute
care setting. In Seminars in nuclear medicine (Vol. 43,
No. 2, pp. 102-113). WB Saunders.
Marshall, A., Lichtman, T. J. K., Seligsohn, U.,
Kaushansky, K., & Prcha, J. T. (2006). Williams
Hematology, New York: McGrow-Hill Medical.
Mycek, M.A. and Pogue, B.W. (2003).Handbook of
biomedical fluorescence, New York: Marcel Dekker
Inc.
Petritskaya, E., Abaeva, L., Lapitan, D., Kulikov, D.,
Smirnova, O., Guseva, I., Rogatkin, D. (2014).
Detection of hypoxia and inflammatory processes in
tissues by fluorescence spectroscopy in vivo. 2014
International Conference Laser Optics. IEEE Press.
Rogatkin, D.A., Prisnyakova, O.A., Moiseeva, L.G. and
Cherkasov, A.S. (1998).Analysis of the accuracy of
clinical laser fluorescence diagnosis, Measurement
Techniques 41(7), 670-674.
Rogatkin, D.A., Bychenkov, O.A. and Lapaeva L.G.
(2009). The accuracy, reliability, and interpretation of
the results of in vivo laser fluorescence diagnosis in
the spectral range of the fluorescence of endogenous
porphyrins, J. Opt. Tech. 76(11), 708-713.
Rogatkin, D., Shumskiy, V., Tereshenko, S. and Polyakov,
P. (2013).Laser-based non-invasive spectrophotometry
- an overview of possible medical application. Photon
Lasers Med, 2(3), 225-240.
Stenzl, A., et al. (2011). Detection and clinical outcome of
urinary bladder cancer with 5aminolevulinic
acidinduced fluorescence cystoscopy. Cancer, 117.5,
938-947.
Sugie, T., Sawada, T., Tagaya, N., Kinoshita, T.,
Yamagami, K., Suwa, H. and Toi, M.
(2013).Comparison of the indocyanine green
fluorescence and blue dye methods in detection of
sentinel lymph nodes in early-stage breast cancer.
Annals of surgical oncology, 20(7), 2213-2218.
Tuchin, V.V. (2002). Handbook of optical biomedical
diagnostic. Bellingham: SPIE Press.
Tummers, Q. R. J. G., Verbeek, F. P. R., Schaafsma, B. E.,
Boonstra, M. C., van der Vorst, J. R., Liefers, G. J.
and Vahrmeijer, A. L. (2014). Real-time intraoperative
detection of breast cancer using near-infrared
fluorescence imaging and methylene blue. European
Journal of Surgical Oncology (EJSO), 40(7), 850-858.
Vahrmeijer, A. L., Hutteman, M., van der Vorst, J. R., van
de Velde, C. J. and Frangioni, J. V. (2013). Image-
guided cancer surgery using near-infrared
fluorescence. Nature Reviews Clinical Oncology,
10(9), 507-518.
Zhang, H., Fan, J., Wang, J., Dou, B., Zhou, F., Cao, J., ...
& Peng, X. (2013). Fluorescence Discrimination of
Cancer from Inflammation by Molecular Response to
COX-2 Enzymes. Journal of the American Chemical
Society, 135(46), 17469-17475.
BIODEVICES 2016 - 9th International Conference on Biomedical Electronics and Devices
144
