
Development of Techniques for the Fabrication of Micro- and
Nano-batteries for Biomedical Applications
Hayley Osman
1
, V. S. Sandeep Akkanapragada
1
, Jacob Conner
2
and Saibal Mitra
1
1
Dept. of Physics, Astronomy and Materials Science, Missouri State University,
901 S. National Avenue, Springfield, MO, U.S.A.
2
US Photonics Inc., 2241 Kearney Street, Springfield, MO, U.S.A.
Keywords: Microbatteries, Nanobatteries, Femtosecond Laser, iCVD, Biomedical Applications.
Abstract: Future bio-medical devices with dimensions in the nanoscale region will need independent energy sources
to power them. Lithium-ion micro- and nano-batteries are excellent candidates for these power sources. Our
proposed nanobattery design ensures that these batteries remain lightweight and safe with fast rechargeable
times. We have used femtosecond laser for precision machining. Intense electric fields produced by the laser
beam induces electrical breakdown due to avalanche ionization. For femtosecond pulses, this breakdown
threshold remains fairly deterministic thereby allowing the use of femtosecond lasers for micro- and nano-
machining. The nanobattery consisted of an anode, cathode and separator. The anode was made of graphite
or molybdenum oxide while the cathode was made of LiCoO
2
. The separator was a Kapton membrane with
an array of n x n holes micro- or nano-scale holes machined into it which were then filled with Li-based
electrolyte. For biomedical applications these batteries must be packaged with bio compatible polymers.
Initiated chemical vapor deposition is an attractive technique where polymeric films are deposited by
activating a mixture of gas of monomers and initiators. This solventless technique is substrate independent
and should lead to the deposition of biocompatible films that can be used to coat and package electronic
devices.
1 INTRODUCTION
As technology progresses there is an increasing
demand to shrink biomedical electronic devices to
the micro- and nano-scale. These nanoscale
biomedical devices will need bio-compatible energy
sources to provide power on demand. Thus,
development of micro- and nano-batteries is of great
interest to the biomedical instrumentation
community.
Ideally, a nanobattery is envisioned as a single
battery cell with nanoscale dimensions that can be
integrated on to a nanodevice. However, given the
current status of technology, it is difficult to
fabricate a single nanobattery with assured reliable
performance. Thus the first logical step to achieve
this goal is to develop a macrobattery with an array
of micro- or nano-cells. Each cell is itself a battery
and they are electrically connected in parallel so that
the total power drawn from this device is the sum of
power from this array of micro- or nano-cells. Once
this battery is fabricated then bio-compatible
coatings need to be developed to package the
nanobattery systems so that they may be implanted
safely in biological systems.
The nanobattery system developed by our group
also has the following advantages. While
conventional batteries are typically heavy and this
limits their use in a number of applications,
nanobatteries are lightweight and hence versatile.
Runaway thermal failure is eliminated since each
nanocell is electrically isolated from the rest of the
nanocells. Also, since the volume of each cell is
small, any thermal runaway reaction is contained in
a nanocell and will produce limited heat that the
battery can easily withstand.
Given their high energy density, Lithium ion
(Li
+
) based battery cells have become most accepted.
A survey of literature shows that there are numerous
papers that describe either the use of very thin films
of electrolyte materials to construct battery systems
or describe the potential of these films to be used in
batteries (Park et al., 2000a); (Park et al., 2000b);
(Dudney et al., 2000); (Humble et al., 2003);
127
Akkanapragada V., Conner J., Osman H. and Mitra S..
Development of Techniques for the Fabrication of Micro- and Nano-batteries for Biomedical Applications.
DOI: 10.5220/0004234601270131
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2013), pages 127-131
ISBN: 978-989-8565-34-1
Copyright
c
2013 SCITEPRESS (Science and Technology Publications, Lda.)

(Humble et al., 2001). However, recent papers by
number of researchers have described the
construction of micro- and nano-batteries with
diameters up to 200 nm across and 60μm deep
(Brazier et al., 2008); (Zhang et al., 2004); (Dewan
et al., 2003); (Stephan et al., 2003); (Vorrey and
Peters, 2003); (Layson et al., 2003). Individual
growth and use of nanoelectrodes using carbon
nanotubes were also described (Jogelkar et al.,
2004). However, these nanobatteries had some
serious drawbacks. The size and arrangement of
individual nanocells, which were fabricated porous
alumina, varied substantially. Only a small fraction
of the cells were electrically active and the outcome
was completely stochastic.
We have developed a laser machining technique
using a femtosecond laser that allowed us to
successfully fabricate array of nanocells in a Kapton
membrane. The cells are isolated and are connected,
in parallel, by the cathode and anode.
2 EXPERIMENTAL DETAILS
2.1 Development and Construction of
the Battery
All materials are susceptible to damage by a focused
laser beam when the induced electric field produced
by the laser beam is comparable to the Coulomb
field an electron sees in the proximity of the atomic
nucleus leading to the creation of an avalanche
process for the free electrons. This process also
occurs in transparent materials which become
opaque when the free electron density approaches
the critical density for that particular light. It is
important to note that this optical breakdown has a
non-linear dependence on intensity and this allows
for the damage to be restricted to the subdiffraction
limit by “thresholding” allowing the fabrication of
nanoscale features (Squier et al., 1991); (Jogelkar et
al., 2003). Though, optically induced dielectric
breakdown scales as t
1/2
, where t is the pulse width
for pulse durations longer than 10 ps, the damage
threshold remains fairly constant (and deterministic)
for shorter pulses (Stuart et al., 1996). For ultra-short
pulses, polarization of the beam also plays an
important part. For linear polarization, a machined
hole becomes a narrow groove in the direction of the
polarized beam. This effect was pronounced at low
pulse energy close to the threshold of the material
(Venkatakrishnan et al., 2002). At low energies, only
the central part of the beam has enough energy to
ablate the material and thus, in this energy regime,
polarization plays an important part. For laser pulses
with a Gaussian spatial beam profile, the diameter D
of the ablated area is given by
2
(1)
Here ω
0
is the beam radius and φ
0
and φ
th
are the
laser fluence and the threshold modification
fluences, respectively. The laser pulse energy E
pulse
is related to laser fluence by
2
(2)
Combining the above two expression, we have
(3)
where and are fitting parameters ( gives the
threshold energy). It is clear by controlling the
energy of the laser pulse the diameter of the ablated
area can be controlled.
The basic battery design is shown below in
Figure 1. The battery consists of three layers. The
middle layer is made of Kapton with an n x n array
of micro- or nano-cells machined into it. The bottom
and top layers are the two electrodes.
Figure 1: Basic design of a nanobattery. The middle layer
is laser-machined Kapton with an n x n array of nanocells.
The top and bottom layers are the electrodes.
In this work, we used commercially available
lithium cobalt oxide (LiCoO
2
) as the cathode and
molybdenum oxide (MoO
2
) as an anode. Lithium
hexafluorophosphate (LiPF
6
) was used as an
electrolyte. Details of the construction of these
micro- and nano-batteries are given elsewhere
(Conner and Mitra, 2011a; 2011b). In addition to the
micro- and nano-batteries, control batteries were
also constructed. Control batteries had the same
electrodes and electrolytes but polyolefin separators
were used instead of laser-machined Kapton
membrane.
Figure 2 shows the arrangement of micro-cells in
a laser-machined Kapton membrane.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
128
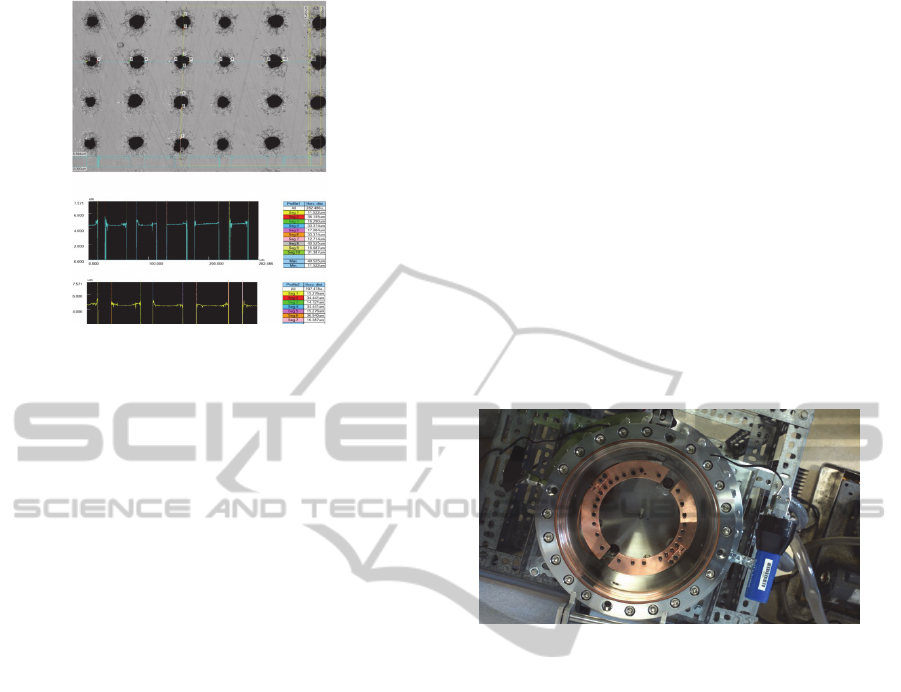
Figure 2: Array of cells machined in a Kapton membrane.
A 12 mm thick Kapton membrane was placed on a mask
and ablated with the laser. Note the near vertical cell
walls.
Once constructed, the current (I) versus potential
(V) was tested for these nanobatteries on an
electrochemical workstation CHI 660C. The open
circuit voltage (V
oc
) for each battery was measured
before each scan. For each battery, the first scan was
started from V
oc
and increased to 4.1 volts. The step
size was 0.001 volts and the scan rate was 0.01
volts/s. The potential for subsequent segments would
be cycled from 4.1 volts to 2.5 volts. Each battery
was cycled five times.
The performance of a micro-battery with Kapton
separator and LiCoO
2
and graphite and MoO
3
electrodes were analyzed and compared with the
control batteries. The performance of the
micro/nanobatteries is superior to the control
batteries with polyolefin separators. When a battery
is cycled through 2.5 volts and 4.1 volts, a certain
amount of charge the cycled charge is given by the
enclosed curve. In all cases, the micro-batteries
showed superior performance compared to the
control batteries. Taking into account the fact that
only 58% of the area in micro-batteries are active
compared to the control batteries, the performance
of micro- and nano-batteries is estimated to be at
least better by 40%. This is an important observation
for the use of micro- nano-batteries in nanoscale
devices for biomedical applications.
2.2 Challenges for Packaging
For use as a bio-medical power supply that can be
implanted in a biological system, the nanobatteries
need to be packaged in a safe and reliable way.
Given the small size of these devices, an attractive
packaging method is to use bio-compatible polymer
coatings. It is important to keep the overall size of
the system in the nanoscale region.
An attractive method is initiated chemical vapor
deposition (iCVD) of polymers. While CVD is a
mature technology, application of iCVD for
packaging of nanobatteries with polymer films
remains at a conceptual stage. In this process,
polymers are deposited from vapor phase monomers.
The reaction rate is greatly enhanced upon
introducing initiator molecules (Baxamusa et al.,
2009). Typically, a volatile initiator molecule is
mixed with the monomer and activated over a heated
filament in a vacuum chamber. iCVD is a one-step
film growth method which mimics the free-radical
chain growth polymerization. Figure 3 below shows
the top view of the iCVD reactor that is under
construction in our laboratory.
Figure 3: Top view of the iCVD reactor under
construction.
The reactor is stainless steel chamber with an
array of nickel-chrome filaments. The copper
electrodes allow the placing of evenly spaced
filaments across the substrate (which sits below the
filament). This geometry allows for the even
distribution of temperature. The filaments are
resistively heated and the temperature of the
filaments can be raised to 400
0
C. The substrate will
be placed about 2.5 cm from the filament array and
will be cooled actively to maintain a temperature of
50
0
C.The monomer and the initiator will be
premixed and introduced over the filaments. The
initiator molecules will decompose either on the
surface of the filaments or in the hot zone near the
filament. The iCVD method has several advantages.
First, the ability to conformally coat surfaces will
allow the coating of nanostructures and objects of
irregular shape. Second, the associated temperatures
needed for film deposition is low. This will allow
deposition on fabricated electron devices like
nanoscale batteries and biological systems without
causing damage.
Lastly, one can use a variety of commercially
available monomers to grow a variety of polymeric
DevelopmentofTechniquesfortheFabricationofMicro-andNano-batteriesforBiomedicalApplications
129

materials. There are a number of candidates for
biocompatible and biopassive surfaces. For example,
organosilicone coatings are electrically insulating
and biopassive.
Poly(trivinyltrimethylcyclotrisiloxane) (PV
3
D
3
)
films have been deposited by using iCVD
(O’Shaughnessy et al., 2006); (Murthy et al., 2002).
PV
3
D
3
has a highly crosslinked structure that makes
it insoluble in both polar and non-polar solvents.
This makes the structure also very stable. Another
possible monomer is Poly (tetrafluoroethylene)
(PTFE). It has been found that the rate of deposition
of PTFE films is dramatically increased with the
addition of perfluorooctane (PFOS) in the gaseous
mixture (Baxamusa et al., 2009). PTFE films can be
conformally coated. They are also hydrophobic and
biocompatible and are used in the medical device
industry and hence are expected to be a good
candidate material as a nanobattery packaging
system.
The iCVD films can be deposited rapidly and the
technology is scalable. Hence, iCVD is a very
attractive technique for packaging of nanobatteries
and any other nanoscale medical devices.
3 CONCLUDING REMARKS
In this work we report the fabrication of micro- and
nano-batteries using femtosecond laser machining.
Though the first batteries are array of n x n micro-
cells, laser machining is clearly a technology that
would allow the fabrication of a single nanobattery.
Though the field of nanobatteries is still in its
infancy, we have outlined methods that should lead
to the fabrication of an array of nanobatteries or
even a single nanobattery appropriately packaged in
biopassive and biocompatible coatings. This, then,
could be the first step to the development of power
sources for future nanodevices that can be directly
implanted in biological systems.
ACKNOWLEDGEMENTS
The authors would like to thank the National
Science Foundation and Missouri State University
for financial support.
REFERENCES
Baxamusa, S. H.; Im, S. G.; and Gleason, K., 2009.
“Initiated and oxidative chemical vapor deposition a
scalable method for conformal and functional polymer
films on real substrates”, Phys. Chem. Chem. Phys. 11,
5227 – 5240.
Brazier, A.; Dupont L.; Dantras-Laffont L.; Kuwata, N.;
Kawamura, J.; Tarascon, J.-M., 2008. “First cross-
section observation of an all solid-state lithium-ion
“nanobattery” by transmission electron microscopy”,
Chem. Mater. 20, 2352-2359.
Dewan, C. and Teeters, D., 2003. “Vanadia xerogel
nanocathodes used in lithium microbatteries”, J.
Power Sources 119-121, 310-315.
Dudney, N. J., 2000. “Addition of thin-film organic solid
electrolytes (Lipon) as protective film in lithium
batteries with liquid electrolyte”,“J. Power Sources
89, 176.
Humble, P. H. and Harb, J. N., 2003. “Optimization of
Nickel-Zinc microbatteries for hybrid powered
microsensor systems”, J. Electrochem. Soc. 150, 1182.
Humble, P. H.; Harb, J. N.; Lafolette, R., 2001.
“Microscopic nickel-zinc batteries for use in
autonomous microsystems”, J. Electrochem. Soc. 148,
1357.
Jogelkar, A. P.; Liu, H.-H.; Meyhofer, E.; Mourou, G.;
and Hunt, A. J., 2004 “Optics at Critical Intensity:
Applications to Nanomorphing”, PNAS, 101, 5856-
5861.
Jogelkar, A. P.; Liu, H.-H.; Spooner, G.; Meyhofer, E.;
Mourou, G.; and Hunt, A., 2003. J. Appl. Phys. B. 77,
23-30.
Layson, A. L.; Gadad, S.; and Teeters, D., 2003.
Electrochim. Acta 48, 2207.
Murthy, S. K.; Olsen, B. D. and Gleason, K. K., 2002.
“Initiation of cyclic vinylmethylsiloxane
polymerization in a hot-filament chemical vapor
deposition process”, Langmuir 18, 6424–6428.
O’Shaughnessy, W. S.; Gao, M. L. and Gleason, K. K.,
2006. “Ínitiated chemical vapor deposition of
trivilylmethylcyclotrisilozane for biomaterial
coatings”, Langmuir, 22, 7021–7026.
Park ,Y. J.; Kim, J. G.; Kim, M. K.; Kim, H. G.; Chung,
H. T.; Park, Y., 2000. “ Electrochemical properties of
LiMnO thin films: 24 suggestion of factors for
excellent rechargeability”, J. Power Sources 87, 69.
Park ,Y. J.; Park, K. S.; Kim, J. G.; Kim, M. K.; Kim, H.
G.; Chung, H. T., 2000. “Characterization of tin
oxide/LiMn
2
O
4
thin-film cell”, J. Power Sources 88,
250.
Squier, J.; Salin, F.; Mourou, G.; and Her, H. H., 1991.
“100-fs Pulse Generation and amplification in
Ti:Al
2
O
3
”, Opt. Lett. 16, 324-327.
Stephan, A. M. and Teeters, D., 2003. “Characterization of
PVdF-HFP polymer electrolytes confined in micor and
nanopores”, Electrochim. Acta 48, 2143.
Stuart, B. C.; Feit, M. D.; Herman, S., 1996. “Effect of
polarization on ultrashort pulsed laser ablation of thin
metal films”, J. Opt. Soc. Am, B 13, 459..
Venkatakrishnan, K.; Tan, B.; Stanley, P.; and Sivakumar,
N. R., 2002. “The effect of polarization on ultrashort
pulsed laser ablation of thin metal films”, J. Appl.
BIODEVICES2013-InternationalConferenceonBiomedicalElectronicsandDevices
130

Phys. 92, 1604-1607.
Vorrey, S. and Teeters, D., 2003. “Study of ion conduction
of polymer electrolytes confined in micro and
nanopores”, Electrochim. Acta 48, 2137.
Zhang, Z.; Dewan C.; Kothari, S; Mitra, S.; Teeters, D.,
2004. “Carbon nanotube synthesis, characteristics and
microbattery applications”, Mat. Sci. and Engg. B,
116, 293.
DevelopmentofTechniquesfortheFabricationofMicro-andNano-batteriesforBiomedicalApplications
131
