
SIMULATIVE PROGRAMMING OF A HYBRID WASHING OIL
SEPARATION SCHEME FOR PURE CHEMICALS
Lining Zhang
1,2
, Duo Zhao
3
, Peng Wu
1
, Jinsheng Sun
1
*, Lili Liu
1
, Yulan Ji
1
and Shuo Jiang
1
1
School of Chemical Engineering and Technology, Tianjin University, No. 92 Wei Jin Road, 300072, Tianjin, P.R. China
2
CNOOC Shandong Chemical Engineering Co., Ltd, No. 80 Lishan Road, Jinan, 250013, Shandong Province, P.R. China
3
Henan Shenma Nylon Chemical Co. Ltd, No.711 Jianshe Road, Pingdingshan, 467000, Henan Province, P.R. China
Keywords: Washing oil, Coupling separation technology, Distillation, Crystallization, Process simulation.
Abstract: Washing oil, one of the important distillation cut of coal tar, consists of a series of high value-added
components which can form azetropic and eutectic system, making it impossible to separate certain
components completely by normal distillation. In this work, a scheme coupling distillation with
crystallization to deal with this cut for pure chemicals is simulatively investigated before an industrial
design. By the aid of this research, a 25Kt/a pilot scale unit was successfully designed which is now running
with α-methyl-naphthalene, β-methyl-naphthalene, fluorene, dibenzofuran and acenaphthylene in high
purity as main products and naphthalene fraction, medium washing oil (dimethylnaphthalene) and heavy
washing oil as by-products.
1 INTRODUCTION
Washing oil is the distillation cut of coal tar between
230~300ºC, which consists of many organic
chemical materials, such as naphthalene, methyl-
naphthalene, dimethyl naphthalene, fluorene,
dibenzofuran, acenaphthylene etc (Wang, 2004).
Basically, there are two ways to produce these
substances of high purity, synthesis and separation.
The cost of the former is undoubtedly high, while
the physical separating way from washing oil is
simple and proved to be economical (Liang and Xue,
2008). Therefore, deep processing of this cut
through physical separative method becomes
practically significant (Song and Schobert, 1993).
On the other hand, washing oil was widely
available in coal based coking industry which is
usually applied in washing benzene from coal gas,
making this cut missed the opportunities for further
deep utilization for more value-added fine chemicals,
with the residue still remaining the above mentioned
bulk features, or becomes even better. The good
news is that the concern to separation options has
been given rise to with the increasing demanding for
fine chemicals, which can be extensively used in
pesticides, pharmaceuticals, dyes, synthetic
materials (Yang and Duan, 2006; González and
Gutierrez, 2008), as well as the increasing large-
scale of coal tar plant to offer more and more
resource of washing oil .
In the present work, a novel scheme coupling
distillation with crystallization for comprehensively
deep processing of washing oil is reported, and
subsequently simulatively researched and
programmed from the angle of industrial design,
with the recommended scheme then manifested to be
feasible through successfully running over 12
months of a 25Kt/a pilot scale unit. By the aid of this
research, this unit was designed with α-
methylnaphthalene, β-methylnaphthalene, fluorene,
dibenzofuran, and acenaphthylene as main product,
and the naphthalene, medium washing oil, and heavy
washing oil as byproducts.
2 MODELLA SCHEDULE
2.1 Crude Washing Oil Feedstock
The components of washing oil are listed in Table 1,
some of which with low content cannot be defined
by instrument analyses. One possibility for this
situation is to select substitutes for the undefined
components in the simulation calculation process,
through assigning corresponding selected substances
of different normal boiling points in accordance with
chromatograph peak sequence.
425
Zhang L., Zhao D., Wu P., Sun J., Liu L., Ji Y. and Jiang S..
SIMULATIVE PROGRAMMING OF A HYBRID WASHING OIL SEPARATION SCHEME FOR PURE CHEMICALS.
DOI: 10.5220/0003619404250428
In Proceedings of 1st International Conference on Simulation and Modeling Methodologies, Technologies and Applications (SIMULTECH-2011), pages
425-428
ISBN: 978-989-8425-78-2
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
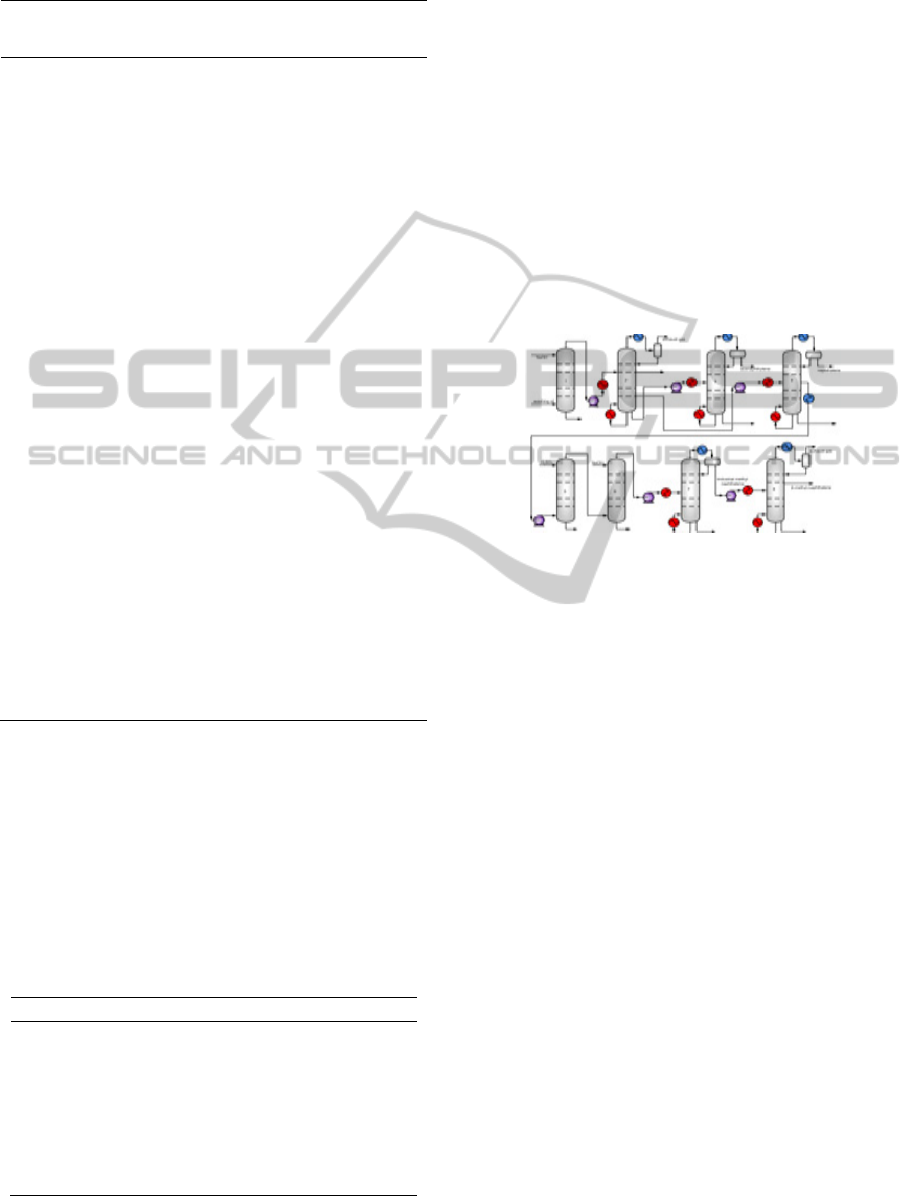
Table1: Components of original materials (washing oil)
and their characteristics.
No. Name
Boiling
Point
(ºC)
Molecular
Weight
kg/kmol
Mass
Fraction
(wt%)
1 Naphthalene 217.96 128.175 3.849
2 Quinoline 237.69 129.161 3.023
3 X-01 239.73 144.210 0.817
4 X-02 239.73 150.220 1.732
5 X-03 240.75 149.236 0.285
6 β-methylnaphthalene 241.05 142.202 21.153
7 α-methylnaphthalene 244.69 142.202 10.020
8 X-04 246.50 134.134 0.108
9 X-05 247.80 143.188 0.165
10 X-06 247.96 143.188 0.278
11 X-07 248.11 172.310 0.136
12 X-08 249.85 122.124 0.099
13 Biphenyl 255.00 154.211 4.589
14 X-09 258.05 170.211 1.497
15 2,6-
dimethylnaphthalene
262.00 156.227 3.244
16 X-10 264.85 220.354 0.093
17
1,3-
Dimethylnaphthalene/
1,4-
Dimethylnaphthalene
263.00 156.227 4.697
18 X-11 267.00 198.349 1.320
19 X-12 268.39 105.138 0.319
20 indole 253.00 117.15 1.396
21 Acenaphthylene 277.39 154.21 18.88
22 X-13 282.05 204.356 1.380
23 Dibenzofuran 284.71 168.195 9.491
24 X-14 284.85 152.15 0.336
25 X-15 286.00 196.29 0.337
26 X-16 293.15 183.252 0.508
27 X-17 293.85 166.177 0.277
28 Fluorene 297.29 166.222 7.624
29 X-18 300.78 143.188 0.341
30 X-19 306.09 143.188 0.972
31 X-20 336.85 192.26 0.443
32 X-21 346.00 179.221 0.594
As shown in Table 1, the boiling points of the
components in the mixture are so close. This implies
high challenges for distillation requiring high
purities of the distillates. Besides, many binary
azeotropics, e.g. indole-acenaphthylene, indole-
dimethylnaphthalene, together with some eutectics
which summarized in Table 2 are formed in washing
oil, which further increase the difficulty for
completely the separation of single component.
Table 2: The main eutectics of washing oil.
Eutectic
Melting Point (
℃)
Naphthalene/β-methylnaphthalene 26
Naphthalene/α-methylnaphthalene -34.6
Naphthalene/acenaphthylene 51
Naphthalene/fluorene 57
Naphthalene/indole 41.8
β-methylnaphthalene/α-
methylnaphthalene
-41
2.2 Flowsheet Description for Current
Deep Processing of Washing Oil
Currently, washing oil was only rough finished or
selectively separated for several components in
China. A number of processing technics have
already been reported, such as narrow fractions
separating method, azeotropic distillation, extractive
distillation, and combination of distillation and
washing (Wang, 2004). However, all of these
methods have extremely complex process with
limited product species. Reportedly, the most
recommended method was from the Chemical Co.,
Nippon Steel Corporation in Japan (Zhang and Yan,
2002). Their processing flowsheet is shown in
Figure 1.
Figure 1: The washing oil processing flowsheet of The
Chemical Co., Nippon Steel Corporation.
1- alkaline tower; 2-washed material distillation tower ; 3-
acenaphthylene tower; 4-naphthalene tower; 5-acid tower;
6-neutralizing tower; 7-methyl naphthalene tower; 8-β-
methylnaphthalene tower.
Washing oil is added to the alkaline tower after
mixed with naphthalene oil, and NaOH solution is
used as detergent to remove phenol. Then the
washed material is heated and pumped to the
distillation towers to separate low naphthalene oil,
medium oil, and residual oil in sequence. Low
naphthalene oil is used to recover benzene, residual
oil to obtain industrial acenaphthylene in the next
column, and medium oil is heated and then pumped
to naphthalene tower to distill industrial naphthalene,
methyl naphthalene, and residual oil. After that, the
methyl naphthalene is pumped to the acid tower to
remove pyridine and quinoline, and then neutralized
using NaOH in neutralizing tower. Afterwards,
industrial methyl naphthalene is obtained in methyl
naphthalene tower, and further it also can be refined
to β-methylnaphthalene. At the end, it can not only
obtain β-methylnaphthalene of high purity, but also
industrial naphthalene, industrial methyl naphthalene,
industrial fluorene and medium washing oil.
This technic can not only use to separate washing
oil, but also treat naphthalene oil and
methylnaphthalene oil, with large capacity. In the
SIMULTECH 2011 - 1st International Conference on Simulation and Modeling Methodologies, Technologies and
Applications
426

process, methylnaphthalene oil was drawn in gas
phase to avoid emulsification. As the five distillation
columns are packed with high efficient packing, all
the products are distilled to high purity, among
which the purity of β-methylnaphthalene reached
98wt%. With all the packed columns operated in
decompression, bottom temperatures are lowered
and demands of heating medium of high temperature
resisted. Besides, this process can take advantage of
self-produced steam and warm water to reduce the
energy consumption.
2.3 Novel Separation Process for
Washing Oil
To make full use of the washing oil resource, a novel
separation process was encouraged to present, as
shown in Figure 2. Crude washing oil is first fed into
naphthalene tower to obtain naphthalene cut. The
residual oil is pumped into methyl naphthalene
tower, where industrial methyl naphthalene can be
recovered as top product which is then driven to the
β-methylnaphthalene tower to produce β-
methylnaphthalene and α-methylnaphthalene
simultaneously. Moreover, the bottom α-
methylnaphthalene stream can exchange heat with
methylnaphthalene stream for sake of energy saving.
Residual methylnaphthalene oil is pumped into
acenaphthylene tower after mixed with crystalline
raffinate from acenaphthylene crystallizer to
distillate medium washing oil, crude acenaphthylene
and residual acenaphthylene oil. And then the crude
acenaphthylene is further refined by crystallization
to obtain industrial acenaphthalene. Residual
acenaphthalene oil is pumped into dibenzofuran
tower for dibenzofuran with the bottom residual
dibenzofuran oil driven into fluorene tower after
mixed with fluorene crystallizer raffinate. The side
product pumped to crystallization unit for industrial
fluorene.
In the original flowsheet, fluorene is reclaimed
from the top of T6, after crystallization, fluorene of
industrial grade is obtained, and the residual liquid
with a series of light components returns to the feed
stream of T6. Consequently, the light components
accumulated in the reflux and increased
continuously, resulting in decrease of fluorene
quality. As an improved process, the fluorene
fraction is drawn out as a side-product, the light
components is removed from the top of the column.
This is just the starting point of the coupled scheme
between ordinary distillation and crystallization. So
in this scheme, α-methyl-naphthalene, β-methyl-
naphthalene, fluorene, dibenzofuran, and
acenaphthylene are simultaneously obtained in high
purity, and the naphthalene fraction, medium
washing oil (dimethyl naphthalene), and heavy
washing oil can also be attained as by-product.
Figure 2: Flowsheet of distillation coupled with
crystallization and its on-site counterparts
T1-naphthalene tower; T2-methylnaphthalene tower; T3-
β-methylnaphthalene tower; T4- acenaphthylene tower;
T5- dibenzofuran tower; T6-fluorene tower; 7-
acenaphthylene crystallizer; 8-fluorene crystallizer
2.4 Simulative Design of Distillation
Coupled with Crystallization
Process
This novel process was simulatively analyzed with
SRK-modified Panag-Reid equation of state
(SRKM) and Grayson-Streed generalized
correlations (GS), which were empirically confirmed
by the co-authors in similar industrial practices to
describe the basic thermodynamic behavior of the
system appropriately.
SRKM equation is an
improvement for SRK equation to provide better
predictions of properties for multicomponent
systems:
{
}
ij
c
jiijiijijjiij
xxxkkkααα ))+/()((+)1()(=
2/1
(1)
And GS correlation is a modification of the Chao-
Seader correlation, which can be expressed as:
)(==
P
f
φ
γ
x
y
K
OL
i
i
i
i
i
i
(2)
10
)ln(+)ln(=)ln(
P
f
w
P
f
P
f
OL
i
OL
i
OL
i
(3)
Besides, the purity of acenaphthylene and
fluorene separated by crystallization was both set to
be 97%, and their yield were 75% and 60%
respectively according to the designers of the
crystallizers. So in the process simulation, the above
parameters are specified in the input procedures and
the quantity of circumfluence from the crystallizers
is calculated automatically.
In order to obtain simulative parameters that
agree as well as possible with the on-site
counterparts, hydraulic calculations of packings, and
the pressure drops of the distributers are taken into
account. That is, process simulation and structure
design rise alternately, with the results of process
SIMULATIVE PROGRAMMING OF A HYBRID WASHING OIL SEPARATION SCHEME FOR PURE CHEMICALS
427

calculation guiding the structure parameter design
and structure design, in return, amending the process
simulation. The operating parameters and the results
of process simulation are listed in Table 3 and 4.
Table 3: The operating data of six columns in simulation.
T1 T2 T3
Top Bottom Top Bottom Top Bottom
N
T
100 100 120
R 28 9 22
Mr 134 152 141 159
T(
℃)
85 266 85 280 110 251
P (kpa) 105 114 105 114 80 115
T4 T5 T6
Top Bottom Top Bottom Top Bottom
N
T
100 100 104
R 18 30 19
Mr 158 166 168 164 169 154
T (
℃)
85 295 85 304 85 320
P (kpa) 105 114 105 114 105 114
As shown, the purity of methylnaphthalene, β-
methylnaphthalene, fluorene, and acenaphthylene all
reach industrial grade. And actually the field date of
this 25Kt/a pilot scale unit indicated that the purity
of the 5 main products all go beyond 95wt%, which
fully accommodated the requirements of fine
chemicals.
Table 4: The components of main production in simulation.
No.
Methylnapht
halene
Acenaphthyle
ne
Fluorene
β-
methylnaphthale
ne
1 6.28E-10 0.0000 0.0000 9.25E-10
2 5.95E-03 9.15E-11 0.0000 8.7E-03
3 1.76E-04 3.14E-13 0.0000 2.59E-04
4 9.67E-03 1.57E-09 0.0000 0.0142
5 1.64E-03 3.52E-10 0.0000 2.42E-03
6 0.637 9.16E-07 0.0000 0.9201
7 0.306 8.18E-06 0.0000 0.0417
8 3.18E-03 1.26E-11 0.000 9.22E-05
9 2.64E-03 6.34E-06 0.0000 3.70E-03
10 5.71E-03 7.16E-06 0.0000 8.18E-03
11 7.77E-09 1.81E-17 0.0000 1.15E-08
12 2.95E-03 3.47E-12 0.0000 5.85E-04
13 3.31E-07 7.33E-04 0.0000 1.00E-16
14 1.27E-09 3.35E-04 0.0000 0.0000
15 4.89E-08 1.16E-03 0.0000 0.0000
16 9.24E-10 1.96E-03 0.0000 0.0000
17 7.21E-07 9.27E-13 0.0000 3.23E-16
18 3.29E-05 4.66E-04 0.0000 6.43E-20
19 0.0000 5.51E-05 0.0000 0.0000
20 0.0260 7.33E-11 0.0000 9.41E-08
21 0.0000 0.9695 4.07E-12 0.0000
22 9.75E-15 0.0182 0.0000 0.0000
23 1.46E-20 1.58E-03 1.68E-07 0.0000
24 2.02E-20 5.71E-03 1.36E-08 0.0000
25 0.0000 3.05E-05 0.0000 0.0000
26 0.0000 9.03E-08 1.15E-03 0.0000
27 0.0000 8.72E-09 1.47E-04 0.0000
28 0.0000 1.22E-04 0.9706 0.0000
29 0.0000 5.23E-06 0.0278 0.0000
30 0.0000 1.19E-07 2.77E-04 0.0000
31 0.0000 4.54E-05 1.44E-18 0.0000
32 0.0000 0.0000 2.44E-20 0.0000
3 CONCLUSIONS
A novel process based on distillation coupled with
crystallization for the separation washing oil to
reclaim high grade fine chemicals is presented in
this work, from first step simulative research to the
successful field fulfillment. It demonstrates that this
process can utilize washing oil more
comprehensively with up to 5 fine chemicals,
namely, α-methyl-naphthalene, β-methyl-
naphthalene, fluorene, dibenzofuran and
acenaphthylene as principal products, and
naphthalene fraction, medium washing oil (dimethyl
naphthalene), as well as heavy washing oil of
improved quality as byproducts. In addition, results
from a converged simulation revealed these main
products were in high purity, suggesting the
feasibility of this hybrid scheme, which was then
verified by field running data.
On this foundation, by the aid of high efficient
packing technology, a 25Kt/a pilot scale unit was
designed, and has already successfully run over 12
months.
REFERENCES
González, A. M. D., Gutierrez, B. C., Casal, B. M. D.,
2008. The use of solvents for purifying industrial
naphthalene from coal tar distilled oils. Fuel
Processing Technology.
Liang, X., Xue, Y., 2008. The abstraction of fluorene and
dibenzofuran from fas absorber oil. Thesis.
Song C., Schobert H. H., 1993. Opportunities for
developing specialty chemicals and advanced
materials from coals. Fuel Processing Technology.
Wang, F., 2004. Extraction of washing oil distillate from
tar and its application in fine chemistry industry. Coal
Chemical Industry.
Yang, R., Duan, R., 2006. Extraction, application and
prospect of the components of wash oil. Coal
Chemical Industry.
Zhang, X., Yan, H., 2002. Brief introduction to wash oil
processing technology of the Chemical Co., Nippon
Steel Corporation. Fuel & Chemical Processes.
SIMULTECH 2011 - 1st International Conference on Simulation and Modeling Methodologies, Technologies and
Applications
428
