
Features Extraction and Fuzzy Logic based
Classification for False Positives Reduction in
Mammographic Images
Arianna Mencattini, Giulia Rabottino, Marcello Salmeri, Roberto Lojacono and
Eleonora Tamilia
University of Rome Tor Vergata, Dept. of Electronic Engineering
Via del Politecnico 1, 00133 Rome, Italy
Abstract. Breast cancer is one of the most common neoplasms in women and
it is a leading cause of death worldwide. A proper screening procedure can help
an early diagnosis of the tumor so reducing the death risk. A suitable computer
aided detection system can help the radiologist to detect many subtle signs, nor-
mally missed during the screening phase, submitting to the radiologist’s attention
those regions that could contain an abnormality. However, one of the most critical
problem deals with a suitable tradeoff regarding the number of suspicious zones
to present to the radiologist and the capability of identifying the correct ones.
In this work, the classification of suspicious signs into normal tissue or massive
lesions has been faced in order to get a False Positive Reduction without notice-
ably affecting the number of True Positives.
1 Introduction
Breast cancer is one of the most devastating causes of death among women in the world
and mammography is still the most commonly used method for detecting breast cancer
at early stages. However, radiologists can miss a significant portion of abnormalities.
Some studies indicate that Computer Aided Detection systems (CADe) can provide a
second opinion to the radiologists and potentially decrease the missed detection rate [1].
A CADe system used in breast cancer screening programs is composed by two
main steps: the identification of suspicious regions and the false positives reduction [2]
(see Fig. 1). Algorithms for the False Positive Reduction (FPR) of suspicious signs of
disease, can work either with one view or with multiple views [3]. Tipically, the one-
view FPR is a two classes classification task in which each Region Of Interest (ROI)
can be classified as a mass or as normal breast tissue. A set of geometric and/or textural
features have to be extracted and selected to train the classifier. Alternatively, template
matching approaches can be used, comparing each extracted ROI with all the ROIs of a
certain database using similarity measures or features vectors.
In this paper, we propose an FPR procedure based on the extraction of many dif-
ferent geometrical and textural features, their selection, and the classification of de-
tected ROIs into normal or abnormal ones through a rule-based fuzzy inference system.
Mencattini A., Rabottino G., Salmeri M., Lojacono R. and Tamilia E..
Features Extraction and Fuzzy Logic based Classification for False Positives Reduction in Mammographic Images.
DOI: 10.5220/0003303500130025
In Proceedings of the 2nd International Workshop on Medical Image Analysis and Description for Diagnosis Systems (MIAD-2011), pages 13-25
ISBN: 978-989-8425-38-6
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
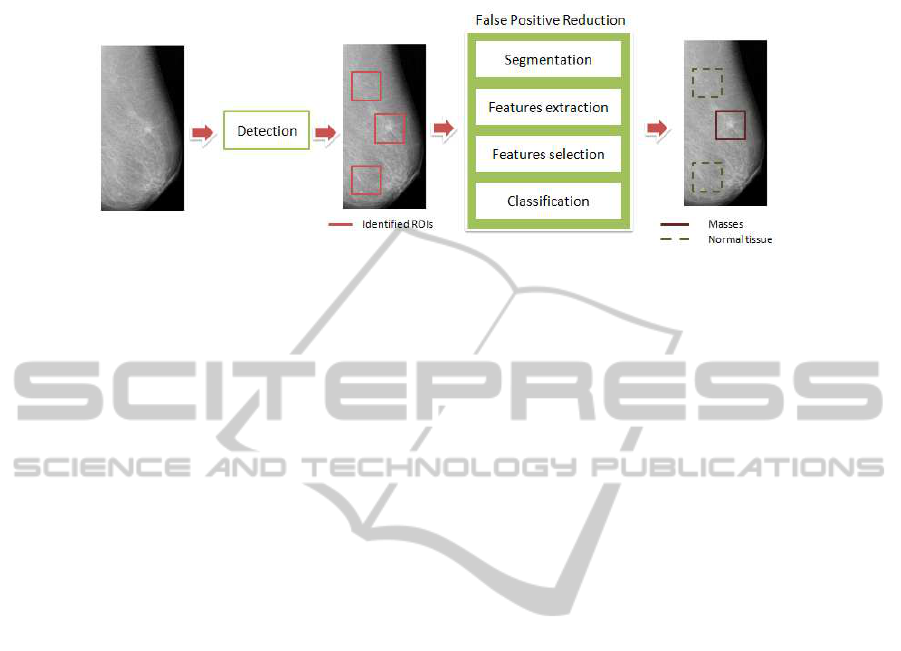
Fig.1. CADe block scheme.
According to BIRADs lexicon [4] there are four different signs of breast disease in
mammograms: masses, architectural distortion, calcifications, focal asymmetry.
– Masses are space occupying lesions seen in two different projections. They can
have: circumscribed margins well-defined or sharply-defined; indistinct margins ill
defined; spiculated margins, when the the lesion is characterized by lines radiating
from the margins of the mass.
– Architectural distortion appear when the normal architecture is distorted with no
definite mass visible. This includes spiculations radiating from a point, and focal
retraction or distortion at the edge of the parenchyma. Architectural distortion can
also be an associated finding.
– Focal asymmetry is a density that cannot be accurately described using the other
shapes. It is visible as asymmetry of tissue density with similar shape on two views,
but completely lacking borders and the conspicuity of a true mass. Additional imag-
ing may reveal a true mass or significant architectural distortion.
– Calcifications are tiny deposits of calcium in the breast. Malignant calcifications
are classified into: amorphous or indistinct calcifications often round or “flake”
shaped calcifications; coarse, heterogeneous calcifications, irregular calcifications
with varying sizes and shapes; fine, pleomorphic or branching calcifications, more
conspicuous than the amorphous forms, varying in sizes and shapes. Benign calci-
fications are usually larger than calcifications associated with malignancy, coarser,
often round with smooth margins and much more easily seen.
In particular, this study is devoted to the automatic massive lesions identification by
CADe systems.
2 Methods for the Performance Evaluation
All the images used in this study belong to the Digital Database for Screening Mam-
mography (DDSM) [5]. It contains 2275 studies, with two Cranio-Caudal (CC) views
and two Medio-Lateral Oblique (MLO) views of each breast. The digital database has
been obtained by digitalizing screen film mammographic images using four different
scanners devices at three different hospitals in South Florida, with a spatial resolution
14

in the range [42 − 50] µm and pixel resolution in the range [12 − 16] bpp. Although the
greatest request of the scientific community is at the moment to consider Direct Dig-
ital Databases, DDSM is the widest public mammographic images database available,
with the most relevant variety of cases, including masses, calcifications and architec-
tural distortions. Each study contains radiologist’s report of the identified lesions, if
present, including lesion type, position, biopsy proven assessment, boundary, subtlety,
etc., according to BIRADs lexicon third edition. Radiologist’s report can be used as the
ground-truth for the detection procedure, while it would not be enough for the diag-
nosis step, since for example, margins of masses are not drawn accurately. Having the
ground-truth, we can classify each ROI identified by the algorithm as a True Positive
(TP), a False Positive (FP), or a True Negative (TN) and compute the sensitivity of the
CADe and the number of False Positives per Image (FPpI). This task is recommended
to compare our algorithm to the others proposed in the literature.
3 Mass Identification
In the first step, a CADe system extracts, from the original mammogram, suspicious
regions on which the radiologists have to focus their attention. The method adopted
for the automatic identification of masses in the mammographic images, analyzes the
orientation of the gradient vectors in the image using circular support regions, to find
highly compact structures with a growing luminance towards their center. The steps of
this procedure are fully described in [6]. Here below, we only recall the basic ideas.
– Decimate the original image. Given the very large size of mammographic images
in DDSM, we reduce resolution to the range [400 − 500] µm. In this way, the
algorithm can still identify the smallest masses with a diameter of 3 mm, but allows
a fast computation.
– Segment the background. We isolate the breast region by implementing an active
contour procedure and histogram thresholding in order to avoid the automatic iden-
tification be applied to the film background regions.
– Grid the image. Applying the algorithm only on a 5-pixels step grid.
– Consider a circle with radius R around every point (i, j) of the grid and compute
on uniformly distributed N = 24 points on the circle (i.e., every 15 degrees) the
following quantity:
x
R
(i, j) =
1
N
X
k,lǫR
cos θ(k, l),
where θ(k, l) is the angle between a gradient vector in (k, l ) and the straight line
connecting the pixels at (i, j) and (k, l) (see Fig. 2 left). The term cos θ(k, l) rep-
resents a measure of the convergence of gradient vectors in the circle to the pixel
of interest (i, j). When x
R
(i, j) = 1 all the gradient vectors from the circle are
oriented toward the same point. This occurs when the iso-intensity lines are con-
centric. It occurs when the pixel (i, j) is near the center of a massive lesion as one
can see in Fig. 3. Figure 4 shows gradient vectors (blue lines) at every 5 pixels
and iso-intensity lines (red lines) superimposed to a ROI containing a mass and to
a ROI containing only normal tissue.
15
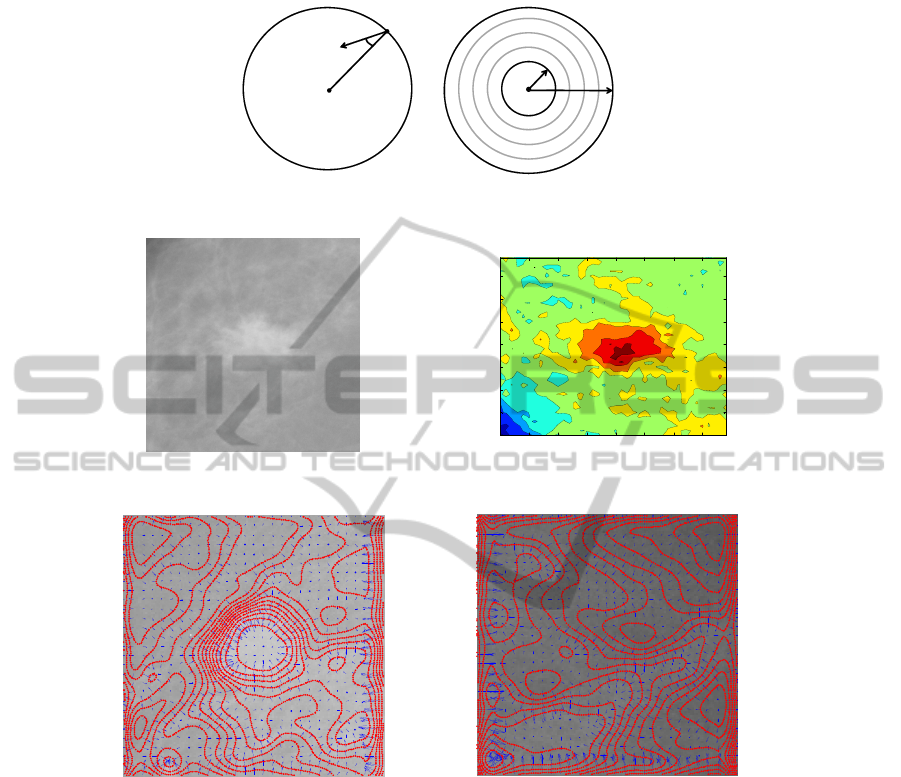
),( lk
),( ji
θ
gradient
vector
pixel of interest
),( ji
min
R
max
R
Fig.2. Definition of the quantities (left) and iteration for different radii (right).
100 200 300 400 500 600 700
100
200
300
400
500
600
700
Fig.3. A ROI containing a massive lesion (left) and the iso-intensity lines of the same ROI (right).
Fig.4. Two ROIs containing a mass (left) and not a mass (right) with isointensity lines and gra-
dient vectors superimposed.
– Repeat for different radii and compute, for every point (i, j) of the grid, the quan-
tity:
x(i, j) = mean
Rmin<R<Rmax
x
R
(i, j).
Because masses have different diameters, from 3 to 40 mm, the region of support
has to be adapted to all the possible sizes of the masses. In particular, we know that
the radius of typical masses is in the range [1.5 − 20] mm that for the considered
not decimated image corresponds to [30 − 400] pixels.
– Sort the results. In a preliminary phase, we show to the radiologist more than one
option. All pixels (i, j) which exceed a certain percentage p of the maximum value
16

of x(i, j) are presented to the radiologist and labeled in an ascending order: the
more suspicious structures have the bigger markers. So, we have to choose the
parameter p so that x(i, j) > p · ˆx where ˆx is the maximum value of x for every
i, j. Figure 5 shows two examples of the algorithm results: in the first case, the
algorithm finds only one mass that corresponds to the radiologist marker and in the
other case it finds 3 masses and the correct result is the mass labeled as the first. In
our study we use p = 0.80.
– Optimize. All the markers that fall into adjacent centers are grouped to have a more
readable result.
Fig.5. Two examples of the algorithm output.
After the automatic identification step, we only consider images where true positives
have been located by the algorithm, in order to separately assess the performance of
the FPR step. So, we consider the better resulting cases, containing 157 ROIs with TPs
and 312 ROIs with FPs. This setting provides an initial sensitivity equal to 1 and a
mean FPpI equal to 2.2. The aim of our study is to reduce the number of FPpI, while
preserving the sensitivity value the highest possible.
In order to introduce the reader to some of the problems encountered with the FPR
step, we report in Fig. 6 some of the ROIs containing a false positive.
Fig.6. Some examples of ROIs containing a false positive sign of disease.
17

4 Automatic Segmentation
After the identification algorithm locates suspicious signs in the mammogram, a ROI
of fixed dimension is extracted around the identified point. In the following, the ROI
is processed by a Fuzzy C-Means clustering algorithm to implement an intensity-based
segmentation with a number of intensity levels equal to 5. The reconstructed image is
then binarized considering as “foreground” only the largest group of adjacent pixels
belonging to the cluster with the highest luminance level, and as “background” the
remaining ones. Figure 7 shows some examples of the segmentation results for ROIs
containing a mass and ROIs with false positives.
Fig.7. Segmented ROIs containing a mass (left) and not a mass (right).
5 Features Extraction
The approach used in this work consists in testing a large set of features and then apply-
ing an automatic features selection algorithm in order to define a proper set of features
with respect to a given training set. After the segmentation of the mass boundary, we
have extracted the following features:
– Morphological features including area, circularity, eccentricity, roughness of the
contour, elongation;
– Law’s texture features [7]. Law proposed a method for classifying each pixel in
an image based upon measures of local texture energy. The texture energy features
represent the amounts of variation within a sliding window applied to several fil-
tered versions of the given image. These measures are computed by first applying
small convolution kernels to the image, and then performing a nonlinear window-
ing operation. The 2-D convolution kernels typically used for texture discrimina-
tion are generated from the following set of one-dimensional convolution kernels of
length 5: L5 = [1, 4, 6, 4, 1], E5 = [−1, −2, 0, 2, 1], S5 = [−1, 0, 2, 0, −1],
R5 = [1, −4, 6, −4, 1], W 5 = [−1, 2, 0, − 2, 1]. The operators listed above per-
form the detection of the following types of features: L5 local average (or level),
E5 edges, S5 spots, R5 ripples, W 5 waves. By combining in a nonlinear manner
the above filters, we obtain 14 Texture Energy Measures (TEM) images. The most
representatives are reported in Fig. 8 for a ROI containing an oriented pattern and
one with a mass. Finally, for each of the 14 images, the following parameters are
evaluated: mean, variance, kurtosis, and skewness.
18
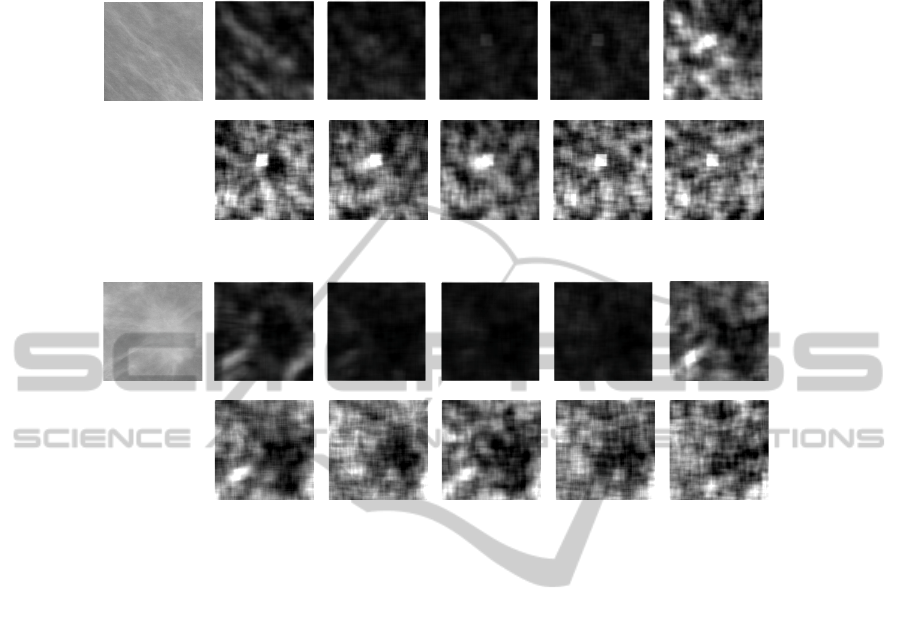
E5L5 S5L5 W5L5 R5L5
E5W5E5R5 S5W5 S5R5 W5R5
E5L5 S5L5 W5L5 R5L5 E5S5
E5W5 E5R5 S5W5 S5R5 W5R5
E5S5
Fig.8. Laws’ texture energy measure for a ROI with a mass (bottom) and one with an oriented
patter (up).
– Haralick texture features [8] are computed on the co-occurrence matrix extracted
from the original ROI, from the ROI transformed by nonlinear contrast enhance-
ment, from the ROIs transformedby the Ranklet Transform [9], and finally from the
ROI elaborated by Sobel filters at different sizes. In particular, the ranklet transform
is an orientation-selective, non-parametric and multi-resolution transform which
has already been successfully exploited in image classification tasks, specifically
face recognition in image frames [10]. The ranklet transform of an image involves
three phases: multiresolution, orientation-selective, and non-parametric analysis.
Fig. 9 shows some examples of the same ROIs above processed by Ranklet trans-
form, at three different resolution factors (4, 6, and 14).
6 Features Selection
At a preliminary step, more than 1000 features have been extracted and a ranking by
a specific selection criterion has been applied on this set. Features have been evalu-
ated one by one, in order to assign a score to each of them according to its relevance,
evaluated on a training set.
Three indexes have been computed in this study, leading to different working points
(i.e., different possible trades off among the ability of reducing false positives, while
preserving true positives):
19

Horizontal RES 4 Vertical RES 4 Diagonal RES 4
Horizontal RES 6 Vertical RES 6 Diagonal RES 6
Horizontal RES 14 Vertical RES 14 Diagonal RES 14
Horizontal RES 4 Vertical RES 4 Diagonal RES 4
Horizontal RES 6 Vertical RES 6 Diagonal RES 6
Horizontal RES 14 Vertical RES 14 Diagonal RES 14
Fig.9. Ranklet transform at three different resolutions of a ROI with a mass (bottom) and one
with an oriented pattern (up).
– the difference between the rate of ROIs correctly recognized as normal tissue in the
initial amount of false positives and the rate of wrongly recognized ROIs as normal
tissue in the initial amount of true positives:
IN D
1
=
T N
T N + F P
−
F N
T P + F N
;
– the difference between the improvement of the correctness and the decrease of the
sensitivity:
IN D
2
=
T P
T P + F P
−
sT P
sT P + sF P
+
T P
T P + F N
− 1
;
– how much the false positive reduction is stronger than the loss of false positives,
leading to an increasing of false negative ROIs:
IN D
3
= 1 −
F N
T N
;
Using the cited indexes,we denote the three differentranking vectors as RKC
1
, RKC
2
,
and RKC
3
.
As last step, it was necessary to find out the optimal number of features among
those ranked. Actually, the performance of the system can not be uniquely assessed,
because they depend on the radiologist’s needs and expectation. The radiologist, in fact,
could require the CADe system providing the minimum number of False Positives per
mammogram, that is the maximum reduction of the number of FPpI even in spite of a
20

reduction of sensitivity, in order to avoid too much false suggestions which could divert
away attention from the real mass. Otherwise, the radiologist may not require first of
all any losses of sensitivity, even at the expense of a low reduction of false positives. In
this case, the radiologist prefers receiving even many suggestions but always including
the real mass, when it is present. Section 8 will provide some numerical examples for
these options.
7 Fuzzy Classification
A Fuzzy Inference System (FIS) has been implemented and used for the classification
of ROIs into abnormal or normal breast tissue [11]. To decide an appropriate diagnosis
in one patient, we introduce three non-fuzzy sets, as explained in [11].
– The set of symptoms (corresponding to the set of features) S = {S
1
, S
2
, ..., S
n
}.
– The set of diagnosis D = {D
1
, D
2
, ..., D
p
} (where, in this case, p = 2 because of
the presence of two classes, “Cancer (C)” or “Normal (N)” that is ROI containing
a mass or ROI containing normal tissue).
– The set of patients P = {P
1
} (the set of ROIs cropped from the mammograms of
a patient).
The assignment of a diagnosis to the patient requires the evaluation of two distinct rela-
tions: the Patient-Symptom Relation (P S) where the different symptoms are evaluated
in the mammograms of the considered patient; the Symptom-Diagnosis Relation (SD)
where the importance of each symptom for the diagnosis (C or N) is evaluated. This
procedure is at a preliminary investigation stage, and at present it computes simply the
positive predictive value and the negative predictive values of each feature indepen-
dently, for each patient in the training set, leading to the so called incidence level of the
symptom to the diagnosis. Hence, the symptoms occurring in a set S are associated with
the diagnosis from set D (SD ) and further they are associated in turn with the patient P
(assuming that information about all symptoms in S is complete in the patient’s case),
in order to establish the final Patient-Diagnosis Relation (P D).
7.1 Fuzzy Inference Engine
A necessary step for the implementation of a FIS is the definition of a set of fuzzy
rules which relate the input values (the symptoms) to the output values (the diagnoses).
The fuzzy rules are defined according to the two above described relations and clinical
experience, according to [11]. In particular the following rules
if (symptom S
j
is a decisive feature for diagnosis B)
| {z }
SD relation
and (symptom S
j
is present in patient P )
| {z }
P S relation
then (diagnosis B is assign ed to patient P )
| {z }
P D relation
21

are defined, which consider only one symptom at a time. In general, for each symptom,
4 rules could be written which combine N and M diagnoses and low and high presence
of the symptom in the patient. However, the experience and the data lead to conclude
that, for a certain symptom S
j
, low means presence of mass and high means normal
tissue, or vice versa. Hence, only a subset of possible rules are implemented. In the
considered application, the output values are crisp (either N or M). This means that
the considered FIS is simplified with respect to the general case where also the output
variables are fuzzy sets. Hence, the defuzzification step is not required and, in order to
associate the final membership degrees to the two final diagnoses (N , M), the following
quantities can be considered [11]:
µ
N
=
P
N
opt
j=1
IL(S
N
j
) · µ
l/h
(S
j
)
P
N
opt
j=1
IL(S
N
j
)
µ
M
=
P
N
opt
j=1
IL(S
M
j
) · µ
l/h
(S
j
)
P
N
opt
j=1
IL(S
M
j
)
where N
opt
is the selected optimal number of features ranked according to one of the
three criterions shown above, and IL(S
N/M
j
) denotes the incidence of feature S
j
to
the diagnosis N/M. µ
N
provides the degree of not abnormality of the considered ROI,
while µ
M
provides the degree of abnormality of the same ROI. So, the outputs of the
fuzzy inference system are: the credibility degree that the ROI is a mass (µ
M
) and
the credibility degree that the same ROI is normal breast tissue (µ
N
). Differently from
standard classifiers, these two indexes are not complementary to the unity. These values
have to be compared to provide the final decision.
8 Results
For the performance evaluation of the fuzzy classifier, a leave-one-out cross-validation
technique has been adopted: the training set was composed of the entire dataset of ROIs
except one which is used as test. This procedure is repeated for each features vector
in the training set. The optimal number of features to be used is different according
to the ranking and to the selection criterion. In particular, using the same criterion for
both ranking and selection of the optimal number, we obtain NP
opt
= 90 for crite-
rion IND
1
, NP
opt
= 39 for criterion IND
2
, and NP
opt
= 27 for criterion IND
3
.
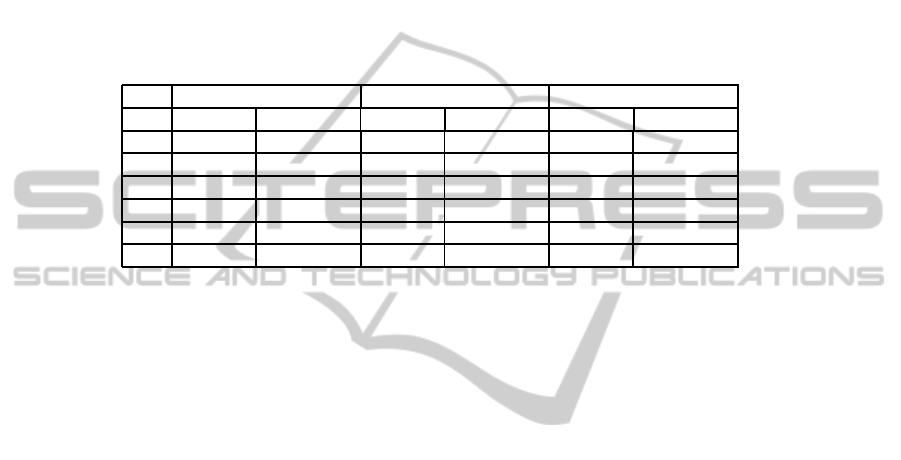
The results of the classification using the three methods are reported in Table 1, in the
columns identified with No Threshold. It means that the final decision is taken only by
comparing credibility degrees of abnormality and of not abnormality.
8.1 Uncertain Diagnosis: Certainty Threshold
The reported results exhibit a sensitivity always greater than 0.8 and a correctness
increased from 0.33 up to 0.5-0.7, with a number of FPpI that varies according to the
used criterion in the range [0.3 − 0.97]. Moreover, another important aspect has to be
considered. The fuzzy logic classifier assigns to each ROI under analysis two values
thus not conferring a single judgment of membership to a class. These membership
degrees have to be compared to make a decision, but this comparison can not be suf-
ficient. It is important to analyze the difference between µ
M
and µ
N
in order to take
22
into account the natural variability of these degrees. Hence a certainty threshold has
been empirically set to 0.15. This threshold has been set low enough in order to exclude
just the most evident doubtful cases. If the absolute value of (µ
M
− µ
N
) was less than
0.15, a conservative choice is to classify the ROI as abnormal. Thus, every doubtful
case can be further analyzed by the radiologist or by the computerized system. Table 1
shows the results obtained adopting this modified approach, in the columns identified
by Threshold. Using this threshold, the number of positives increases, thus causing the
Table 1. Results of classification with a certainty threshold equal to 0.15, compared with the
previous results without any threshold.
I N D
1
I N D
2
I N D
3
Threshold No Threshold Threshold No Threshold Threshold No Threshold
TP 147 126 157 153 157 156
FN 10 31 0 4 0 1
TN 210 264 183 198 105 159
FP 102 48 129 114 207 153
SENS 0.9363 0.8025 1 0.9745 1 0.9936
FPpI 0.65 0.30 0.82 0.72 1.31 0.97
increase in both True Positives and False Positives with respect to the No Threshold
case. At the same time, the working point shifts toward a greater sensitivity, with an
acceptable increase in F P pI. All these results prove that the features ranking/selection
algorithms, the fuzzy classifier, and the conservative setting achieved by the certainty
threshold perform well and can be easily adapted to different requirements of the radi-
ologists assigning to the CADe a good flexibility and adaptability to different clinical
scenarios.
9 Comparisons
We report also the most relevant results described in the literature concerning the False
Positive Reduction in the automatic detection of breast masses. In particular, Li et
al. [12] used a soft neural network decision classification. Angelini et al. [13] proposed
a support vector regression filtering approach. Masotti et al. [9] reduced false positives
via gray scale invariant ranklet texture features using a support vector machine clas-
sifier for discrimination. Tourassi et al. [14] used a template matching scheme based
on mutual information. Varela et al. [15] employed a neural network classifier to merge
different combination of features. All the results are compared in Table 2 in terms of the
True Positive Reduction (TPR) and the False Positive Reduction (FPR). The number of
ROIs used in the studies is also reported. We also insert our results obtained by criterion
IN D
2
.
10 Conclusions
In this work, we presented a study on the False Positives Reduction in the automatic
breast masses identification in mammographic images. We addressed the FPR step as
23

Table 2. Comparisons of different methods for false positive reduction.
TPR FPR ROIs
Li et al. 1% 56% 25
Angelini et al. 13% 38% 69
Tourassi et al. 10% 65% 1820
Varela et al. 22% 85% 120
Masotti et al. 0% 30% 884
Proposed method 0% 58% 469
a two classes classification problem, with the aim to assign to each suspicious ROI a
degree of abnormality and a degree of not abnormality, thus reducing the whole number
of ROIs to be presented to the radiologist. A large set of features have been extracted
from the ROIs identified by an automatic identification algorithm proposed by the au-
thors. Then, the selected features have been used to train a fuzzy classifier, properly
structured for medical applications. Different working points have been considered so
that the radiologist could choose the best tradeoff between sensitivity and false positive
per image, according to the clinical application.
References
1. M. Bazzocchi and F. Mazzarella, “CAD systems for mammography: a real opportunity? A
review of the literature,” http://www.springerlink.com/content/x3157r8u72196h45/
fulltext.pdf/, 2006.
2. A. Oliver, “A new approach to the classification of mammographic masses and normal breast
tissue,” in Proceedings of the 18th International Conference on Pattern Recognition, 2006,
vol. 4, pp. 707 – 710.
3. J. Wei, H-P. Chan, B. Sahiner, C. Zhou, and L. M. Hadjiiski, “Computer-aided detection
of breast masses on mammograms: Dual system approach with two-view analysis,” Med.
Phys., vol. 36, no. 10, pp. 4451 – 4460, 2009.
4. C. Balleyguier, S. Ayadi, K. Van Nguyen, D. Vanel, C. Dromain, and R. Sigal, “BIRADS
classification in mammography,” European Journal of Radiology, vol. 61, pp. 192–194,
2007.
5. University of South Florida, “DDSM: Digital database for screening mammography,”
http://marathon.csee.usf.edu/Mammography/Database.html, 2000.
6. A. Mencattini, G. Rabottino, M. Salmeri, and R. Lojacono, “Assessment of a breast masses
identification procedure using an iris detector,” IEEE Transactions on Instrumentation and
Measurement, in press.
7. K. I. Laws, “Laws’ texture measures,” http://www.ccs3.lanl.gov/ kelly/ZTRANSITION/
notebook/laws.shtml, 2001.
8. R. M. Haralick, “Statistical and structural approaches to texture,” Proceedings of the IEEE,
vol. 67, no. 5, pp. 786 – 804, 1979.
9. M. Masotti, N. Lanconelli, and R. Campanini, “Computer aided mass detection in mammog-
raphy: False positive reduction via gray scale invariant ranklet texture features,” Medical
Physics, vol. 36, no. 2, 2009.
10. F. Smeraldi, “Ranklets: Orientation selective non-parametric features applied to face de-
tection,” in 16th International Conference on Pattern Recognition, 2002, vol. 3, pp. 351 –
359.
24

11. E. Rakus-Andersson, Fuzzy and rough techniques in Medical Diagnosis and Medication,
Springer-Verlag, 2007.
12. L. Li, Y. Zheng, L. Zhang, and R. A. Clark, “False-positive reduction in cad mass detection
using a competitive classification strategy,” Medical Physics, vol. 28, 2001.
13. E. Angelini, R. Campanini, and A. Riccardi, “Support vector regression filtering
for reduction of false positives in a mass detection cad scheme: Preliminary results,”
http://amsacta.cib.unibo.it/archive/00000912/01/angelini05support.pdf, 2005.
14. G. D. Tourassi, R. Vargas-Vorecek, D. M. Catarious, and C. E. Floyd, “Computer assisted
detection of mammographic masses: a template matching scheme based on mutual informa-
tion,” Medical Physics, vol. 30, no. 8, pp. 2123 – 2130, 2003.
15. C. Varela, P. G. Tahoces, A. J. Mendez, M. Souto, and J. J. Vidal, “Computerized detection
of breast masses in mammograms,” Computers in Biology and Medicine, vol. 37, pp. 214 –
226, 2007.
25
