
ON VACCINATION CONTROLS FOR THE SEIR EPIDEMIC
MODEL WITH SUSCEPTIBLE PLUS IMMUNE
POPULATIONS TRACKING THE WHOLE POPULATION
M. De la Sen, S. Alonso-Quesada
Department of Electricity and Electronics, Faculty of Science and Technology, University of the Basque Country
Campus of Leioa, 48940-Leioa, Bizkaia, Spain
A. Ibeas
Departmento de Telecomunicación e Ingeniería de Sistemas, Escuela Técnica Superior de Ingeniería
Universitat Autònoma, Barcelona, Spain
Keywords: Epidemic models, Control, SEIR epidemic models, Tracking objective, Vaccination control, Positivity
stability.
Abstract: This paper presents a simple continuous-time linear vaccination-based control strategy for a SEIR
(susceptible plus infected plus infectious plus removed populations) propagation disease model. The model
takes into account the total population amounts as a refrain for the illness transmission since its increase
makes more difficult contacts among susceptible and infected. The control objective is the asymptotically
tracking the joint susceptible plus the removed-by-immunity population to the total population while
achieving simultaneously the remaining population (i.e. infected plus infectious) to asymptotically tend to
zero.
1 INTRODUCTION
Important control problems nowadays related to Life
Sciences are the control of ecological models like,
for instance, those of population evolution
(Beverton-Holt model, Hassell model, Ricker model
etc.) via the online adjustment of the species
environment carrying capacity, that of the
population growth or that of the regulated harvesting
quota as well as the disease propagation via
vaccination control. In a set of papers, several
variants and generalizations of the Beverton-Holt
model (standard time-invariant, time-varying
parameterized, generalized model or modified
generalized model) have been investigated at the
levels of stability, cycle-oscillatory behavior,
permanence and control through the manipulation of
the carrying capacity (De la Sen, 2008a, 2008b, De
la Sen and Alonso-Quesada, 2008a, 2008b, 2009).
The design of related control actions has been
proved to be important in those papers at the levels,
for instance, of aquaculture exploitation or plague
fighting. On the other hand, the literature about
epidemic mathematical models is exhaustive in
many books and papers. A non-exhaustive list of
references is given in this manuscript (Erturk and
Momani, 2008, Keeling and Rohani, 2008, Khan et
al., 2009, Mollison, 2003, Mukhopadhyay and
Battacharyya, 2007, Ortega et al., 2003, Song et al.,
2009, Yildirim and Cherruault, 2009, Zhang et al.,
2009). The sets of models include the most basic
ones (Keeling and Rohani, 2008, Mollison, 2003):
• SI models where not removed-by-immunity
population is assumed. i.e., only susceptible and
infected populations are assumed,
• SIR models, which include susceptible, infected
and removed-by-immunity populations, and
• SEIR models where the infected populations is
split into the “infected”, which incubate the
disease but do not still have any disease
symptoms, and the “infectious” or “infective”,
which do have the external disease symptoms.
Those models have also two major variants,
namely, the so-called “pseudo-mass action models”,
where the total population is not taken into account
165
De la Sen M., Alonso-Quesada S. and Ibeas A..
ON VACCINATION CONTROLS FOR THE SEIR EPIDEMIC MODEL WITH SUSCEPTIBLE PLUS IMMUNE POPULATIONS TRACKING THE WHOLE
POPULATION .
DOI: 10.5220/0003152901650172
In Proceedings of the International Conference on Bioinformatics Models, Methods and Algorithms (BIOINFORMATICS-2011), pages 165-172
ISBN: 978-989-8425-36-2
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

as a relevant disease contagious factor and the so-
called “true-mass action models”, where the total
population is more realistically considered as an
inverse factor of the disease transmission rates.
There are many variants of the above models, for
instance, including vaccination of different kinds:
constant (Yildirim and Cherruault, 2009), impulsive
(Song et al., 2009, Zhang et al., 2009), discrete-time
etc., incorporating point or distributed delays (Song
et al., 2009), oscillatory behaviors (Mukhopadhyay
and Battacharyya, 2007) and so on. In this paper, a
continuous-time vaccination control strategy is given
for a SEIR epidemic model which makes directly the
susceptible plus removed- by-immunity populations
to asymptotically track the whole population. It is
assumed that the total population remains uniformly
bounded through time while being nonnegative as
they are all the partial populations of susceptible,
infected, infectious and immune. Thus, the disease
transmission is not critical, and the SEIR-model is of
the above mentioned true-mass action type. Note
that although all the partial populations and the total
one are all nonnegative for all time in the real
problem under study, the property has to be
guaranteed for the mathematical SEIR-model (1)-(4)
as well.
2 SEIR EPIDEMIC MODEL
Let S(t) be the “susceptible” population of infection,
E(t) the “infected”, I(t ) the “infectious” population,
and R(t) the “removed by immunity” (or “immune”)
population at time t. Consider the SEIR-type
epidemic model:
()
S(t)I(t)
S(t) S(t) R(t) N(t) 1 V(t)
N(t)
=−μ +ω −β +ν −
(1)
S(t)I(t)
E(t) ( )E(t)
N(t)
=β − μ+σ
(2)
I(t) ( )I(t) E(t)=−μ+γ +σ
(3)
R(t) ( )R(t) (1 )I(t) N(t)V(t)=−μ+ω +γ −ρ +ν
(4)
subject to initial conditions
S(0) 0≥ , E(0) 0≥ ,
I(0) 0≥ and R(0) 0≥ under the vaccination
function
00
V:
++
→\\, with
{}
0
zz0
+
∈≥\ \ .
The vaccination control is either the vaccination
function itself or some appropriate four dimensional
vector depending on it defined “ad-hoc” for some
obtained equivalent representation of the SEIR-
model as a dynamic system. In the above SEIR-
model, N(t) is the total population, μ is the rate of
deaths from causes unrelated to the infection,
ω
is
the rate of losing immunity,
β
is the transmission
constant (with the total number of infections per
unity of time at time t being
S(t)I(t)
N
(t)
β ),
1
−
σ and
1
−
γ
are, respectively, the average durations of the
latent and infective periods. All the above
parameters are nonnegative. The parameter
ω
means the rate of immunity lost since it makes the
susceptible to increase and then the immune to
decrease. The usual simplified SEIR-model is
obtained with
ν
=μ and
0
ρ
=
. In that case,
[]
0
N(t) S(t) E(t) I(t) R(t)
N(t) S(t) E(t) I(t) R(t) 0 t
N(t) S(t) E(t) I(t) R(t) N(0) N 0
+
=+++
=μ − − − − = ∀ ∈
⇒+++==>
\
If
ν
>μ then the new-born lost of maternal
immunity is considered in the model. If
ν<μ then
there is a considered mortality incidence by external
causes to the illness. The parameter
(
]
0, 1ρ∈ is the
per-capita probability of dying from the infection. If
either
ν
≠μ and
0
ρ
=
or ν=μ and
0ρ≠
, and
otherwise,
()N(t)
I(t)
ν−μ
=
ργ
occurs eventually on a
set of zero measure only, then the total population
varies through time as obtained by correspondingly
summing up both sides of (1)-(4). Furthermore, (1)
and (4) and (2) and (3) might be separately summed
up to obtain the evolution dynamics of the separate
populations of joint susceptible and immune and
joint infected and infectious. This leads to:
N(t) ( )N(t) I(t)= ν−μ −ργ
(5)
[
]
()
S(t) R(t) S(t) R(t)
S(t)
1 I(t) N(t)
N(t)
+=−μ+
⎛⎞
+γ −ρ−β +ν
⎜⎟
⎝⎠
(6)
[]
S(t)
E(t) I(t) E(t) I(t) I(t)
N(t)
⎛⎞
+=−μ + −γ−β
⎜⎟
⎝⎠
(7)
Note that (5) is identically zero if
0ν−μ=ρ=
.
From (5)-(7), it follows that:
t
()t ()(t)
0
N(t) e N(0) e I( )d
ν−μ ν−μ −τ
=
−ργ τ τ
∫
(8)
[
]
t
t
(t )
0
S(t) R(t) e S(0) R(0)
S( )
e N() (1 ) I()d
N( )
−μ
−μ −τ
+= +
⎡
⎤
⎛⎞
τ
+
ν τ + γ −ρ −β τ τ
⎢
⎥
⎜⎟
τ
⎝⎠
⎣
⎦
∫
(9)
[
]
t
t
(t )
0
E(t) I(t) e E(0) I(0)
S( )
eI()d
N( )
−μ
−μ −τ
+= +
⎛⎞
τ
−
γ−β τ τ
⎜⎟
τ
⎝⎠
∫
(10)
BIOINFORMATICS 2011 - International Conference on Bioinformatics Models, Methods and Algorithms
166

In order to further solve (9), an integration by parts
is performed as follows:
[]
tt t
(t )
00 0
t
t
0
0
p
( )dq(t, ) p( )q(t, )d N( )e d
N( )q(t, ) q(t, )N( )d
−μ −τ
ττ=τττ≡τ τ
=τ τ− τττ
∫∫ ∫
∫
(11)
where:
[]
t
(t )
t
t
(t )
0
0
0
t
e
q(t) e d q(t, )
1e
q(t, t) q(t,0)
−μ −τ
−μ −τ
−μ
⎡⎤
τ= = τ
⎢⎥
μ
⎣⎦
−
==−
μ
∫
(12)
so that q(t, t) 1/
=μ,
t
q(t,0) e /
−μ
=μ and then,
using (5) in (11) yields:
[]
t
(t ) t
0
t
(t )
0
1
N( )e d N(t) e N(0)
1
e ( )N( ) I( ) d
−μ −τ −μ
−μ −τ
⎡⎤
ττ=−
⎣⎦
μ
−ν−μτ−ργττ
μ
∫
∫
(13)
which, after grouping identical terms, leads to:
t
(t )
0
t
t(t)
0
N( )e d
1
N(t) e N(0) e I( )d
−μ −τ
−μ −μ −τ
ττ
⎡⎤
=− +ργ ττ
⎢⎥
⎣⎦
ν
∫
∫
(14)
Thus, combining (9)-(10) and (14) yields:
[]
t
t
0
t
t
0
S(t) R(t) N(t) E(t) I(t)
S( )
e S(0) R(0) N(0) e I( )d
N( )
S( )
e E(0) I(0) e I( )d
N( )
−μ μτ
−μ μτ
+−=− +
⎡⎤
⎛⎞
τ
=+−+γ−βττ
⎢⎥
⎜⎟
τ
⎝⎠
⎣⎦
⎡⎤
⎛⎞
τ
=− + − γ−β τ τ
⎢⎥
⎜⎟
τ
⎝⎠
⎣⎦
∫
∫
(15)
3 VACCINATION CONTROL
If the control objective S(t) N(t) /=γ β for all time
is achieved with a positive vaccination control in
[]
0, 1 , it is proven below that the whole population
converges exponentially to the sum of the
susceptible population plus the immune population
while both the infectious and infective converge
exponentially to zero. This is theoretically the ideal
objective since the infection is collapsing as time
increases while the susceptible plus the immune
populations are approximately integrating the whole
population for large time. Other alternative objective
has been that the immune population be the whole
one but this is a more restrictive practical objective
since the whole susceptible population should
asymptotically track the immune one even those of
the susceptible who are not contacting the disease.
Theorem 1. Assume that
0β>γ≥
and that the
vaccination function is such that S(t) N(t) /=γ β
0
t
+
∀∈\ with a vaccination control in
[]
0, 1 for all
time. Then, the SEIR model (1)-(4) is positive for all
time. Furthermore,
[
]
[][]
tt
S(t) R(t) N(t) E(t) I(t)
e S(0) R(0) N(0) e E(0) I(0)
−μ −μ
+−=− +
=+−=−+
(16)
for all time what implies the following constraint for
the initial conditions:
[]
N(0)
S(0) E(0) I(0) R(0)
γγ
== ++
ββ−γ
(17)
As a result,
[
]
[]
t
t
t
R(t) N(t) S(t) e E(0) I(0)
N(t) e E(0) I(0)
N(t) e R(0) S(0) N(t)
−μ
−μ
−μ
=−− +
β−γ
=−+
β
⎡⎤
β−γ β−γ β−γ
=+− ≤
⎢⎥
βγβ
⎣⎦
(18)
0
t
+
∀∈\ . Then, R(t) N(t)
β−γ
→
β
as t →∞.
Furthermore, the following two limits exist:
{
}
{
}
tt
lim S(t) R(t) N(t) lim E(t) I(t) 0
→∞ →∞
+
−= +=
(19)
If, in addition,
0
ν
−μ=ρ=
then
{
}
{} {}
t
tt
N
(t) N(0) N lim S(t) R(t)
lim E(t) lim I(t) 0
→∞
→∞ →∞
=== +
==
(20)
Proof: The mathematical SEIR-model (1)-(4) is
positive since the vaccination control is in
[]
0, 1 for
all time so that no population takes negative values
at any time. On the other hand, (16) and (19) follow
directly from (15) and S(t) N(t) /=
γβ
for all time.
Finally, (20) follows from (16) and (19) since
0
ν
−μ=ρ=
implies
N
(t) 0≡
0
t
+
∀∈\ , i.e.,
N
(t) N(0)≡
0
t
+
∀∈\ from (5). ***
An associate stability result follows:
Theorem 2. Assume that
0ργ ≥
. Then, the
following properties hold:
ON VACCINATION CONTROLS FOR THE SEIR EPIDEMIC MODEL WITH SUSCEPTIBLE PLUS IMMUNE
POPULATIONS TRACKING THE WHOLE POPULATION
167

(i) The SEIR-model is globally stable if
0 ≤ν≤μ
and the vaccination law fulfils
[]
0
V: 0, 1
+
→\ .
(ii) If
S(t) N(t) /=
γβ
and
0ν>μ≥
then the
conditions
{}
()
0
t
with 0
N
(0) e I( )d , lim N(t) 0
∞
ν−μτ
→∞
μ<ν<μ+ργ ργ>
=ργ τ τ =
∫
are jointly necessary for global stability under
Theorem 1.
(iii) If
0ν>μ≥
and I(t) ( )N(t)=ν−μ ργ
00
t t (finite)
+
∀≥ ∈\ then global stability of the
SEIR-model (1)-(4) is guaranteed if
[]
0
V: 0, 1
+
→\ . If
0
ν
>μ≥
,
[
]
0
V: 0, 1
+
→\
and
I(t) ( )N(t)=ν−μ ργ is replaced with the
weaker condition
t
I(t) ( )N(t) o(e )
−α
−ν−μ ργ= for some
+
α∈\ then the SEIR-model (1)-(4) is globally
stable.
Proof:
(i)
If
0 ≤ν≤μ
and
0ργ ≥
then:
N
(t) ( )N(t) I(t) ( )N(t) 0=ν−μ −ργ ≤ν−μ ≤
0
t
+
∀∈\ so that N(t) N(0)≤<∞
0
t
+
∀∈\ . Since
the SEIR-model is positive if
[]
0
V: 0, 1
+
→\ then
all the populations are nonnegative and upper-
bounded by N(0).
(ii) On the other hand, the solution of (5) for any
initial conditions is:
t
()t ()
0
N
(t) e N(0) e I( )d
ν−μ − ν−μ τ
⎡⎤
=−ργττ
⎢⎥
⎣⎦
∫
which is uniformly bounded for all time only if
()
0
N
(0) e I( )d
∞
−ν−μτ
=ργ τ τ
∫
since
0ν>μ≥
. Also,
N(t) <∞
0
t
+
∀∈\ only if
N
(t) 0≤
on a non-
necessarily connected set of infinite Lebesgue
measure. Thus, there is a finite sufficiently large
finite time “t” such that:
[]
[]
[]
I(t) N(t) S(t) E(t) I(t) R(t)
1 I(t) S(t) E(t) R(t)
I(t) S(t) E(t) R(t)
ν−μ ν−μ
≥=+++
ργ ργ
⎛⎞
ν−μ ν−μ
⇔− ≥ + +
⎜⎟
ργ ργ
⎝⎠
ν−μ
⇔≥ + +
μ+ργ−ν
which requires the parametrical conditions
0
ρ
γ>
and
μ
<ν<μ+ργ. Since I(t) is of exponential order
of at most
−
μ from Theorem 1 [see (16)] then
S(t) E(t) R(t)++ is also of exponential of order of
at most
−
μ so that N(t) extinguishes exponentially
as they do all the populations of susceptible,
infected, infectious and immune.
(iii) If
I(t) N(t)
ν−μ
=
ργ
with ν>μ after some finite
time
0
t then
0
N(t) N(t )
=
<∞
0
tt∀≥ and the
SEIR-model is positive since
[
]
0
V: 0, 1
+
→\ .
Thus, global stability follows. If
t
I(t) ( )N(t) o(e )
−α
−ν−μ ργ= replaces the above
stronger condition
I(t) ( )N(t)
=
ν−μ ργ after a
finite time then
N
(t)
is of exponential order
−
α so
that
)t(N is uniformly bounded for all time and the
global stability still holds. ***
3.1 Control Law Synthesis
Note that the case
ν
>μ is not feasible in practice
for
0
ρ
γ=
since the population diverges. If
0
ρ
γ>
,
it requires a collapsing effect of the illness on the
population which is also unfeasible in practical
situations. It is now discussed how the vaccination
law is generated to keep simultaneously the SEIR-
model positivity plus the tracking objective of
Theorem 1 which requires positivity. The tracking
objective S(t) N(t) /
=
γβ for all time is equivalent
to any of the subsequent equivalent identities below:
[]
N
(t) N(t)/ E(t) I(t) R(t)
N
(t) E(t) I(t) R(t)
N
(t) E(t) I(t) R(t)
R(t) N(t) E(t) I(t)
=
γβ+++
⎛⎞
β−γ
⇔=++
⎜⎟
β
⎝⎠
β
⇔= ++
β−γ
β−γ
⇔= −−
β
(21)
which requires as necessary condition
0β>γ≥
.
Although unrelated to the physical problem at hand,
the necessary condition will be also accomplished
with
0
β
<
and
0
γ
≤
with S(t) N(t) /=γ β.
The solution of (4) matches (21) for all time if
and only if:
BIOINFORMATICS 2011 - International Conference on Bioinformatics Models, Methods and Algorithms
168

[]
[]
(
)
t
t
()t ()
0
R(t) N(t) E(t) I(t) N(t) e E(0) I(0)
e R(0) e (1 )I( ) N( )V( ) d
−μ
−μ+ω μ+ωτ
β−γ β−γ
=−−=−+
ββ
=+γ−ρτ+ντττ
∫
(22)
where (10), with S(t) N(t) /=γ β, has been used.
Define an everywhere time-differentiable
auxiliary function
0
h:
+
→\\ defined as:
[]
t
0
h(t) h(0) (1 )I( ) N( )V( ) d=+γ−ρτ+ντττ
∫
(23)
such that,
h(t) (1 )I(t) N(t)V(t)
1
V(t) h(t) (1 )I(t)
N(t)
=γ −ρ +ν
⎡⎤
⇔= −γ−ρ
⎣⎦
ν
(24)
for all time so that the last right-hand-side additive
term in (23) becomes after integration by parts:
t
()t ()
0
t
()t()t ()
0
eeh()d
e e h(t) h(0) ( ) e h( )d
−μ+ω μ+ωτ
−μ+ω μ+ω μ+ωτ
ττ
⎡⎤
=−−μ+ωττ
⎢⎥
⎣⎦
∫
∫
(25)
The replacement of (25) into (22) yields:
[]
[]
()t t
t
()
0
t
()t ()
0
e N(t) e E(0) I(0)
R(0) e (1 )I( ) N( )V( ) d
R(0) e h(t) h(0) ( ) e h( )d
μ+ω ω
μ+ω τ
μ+ω μ+ω τ
β−γ
−+
β
=+ γ−ρτ+ντττ
=+ −−μ+ω ττ
∫
∫
(26)
and equivalently:
[]
[]
[]
()
()t
t
()(t) t
0
t
()(t)
0
tt
h(t) N(t) e h(0) R(0)
( ) e h( )d e E(0) I(0)
N(t) ( ) e h( )d
e e h(0) R(0) E(0) I(0)
−μ+ω
− μ+ω −τ −μ
− μ+ω −τ
−μ −ω
β−γ
=+ −
β
+μ+ω τ τ− +
β−γ
=+μ+ω ττ
β
+−−−
∫
∫
(27)
generated from:
[]
[][]
()t t
t
2()(t)
0
h(t) ( )N(t) I(t)
( )e h(0) R(0) e E(0) I(0)
()e h()d()h(t)
−μ+ω −μ
−μ+ω −τ
β
−
γ
= ν−μ −ργ
β
−μ+ω − +μ +
− μ+ω τ τ+ μ+ω
∫
(28)
so that:
[][]
()t t
t
2()(t)
0
()( )
h(t) (1 )I(t) N(t) 1 I(t)
( )e h(0) R(0) e E(0) I(0)
( ) e h( )d ( )h(t)
−μ+ω −μ
− μ+ω −τ
⎛⎞
β−γ ν−μ γρ
−γ −ρ = +γ −
⎜⎟
ββ
⎝⎠
−μ+ω − +μ +
−μ+ω τ τ+μ+ω
∫
(29)
The vaccination law which ensures the positivity
of the mathematical SEIR-model (1)-(4) is generated
as follows:
[]
V(t) if V(t) 0, 1
V(t) 1 if V(t) 1
0 if V(t) 1
⎧
∈
⎪
⎪
=>
⎨
⎪
<
⎪
⎩
(30)
where:
h(t) (1 )I(t)
V(t)
N(t)
−γ −ρ
=
ν
(31)
Define the indicator function i(t) as follows:
[]
0 if V(t) 0, 1
i(t)
1 otherwise
⎧
∈
⎪
=
⎨
⎪
⎩
(32)
Then, one has instead of (15):
[
]
t
t
0
t
t
0
S(t) R(t) N(t) E(t) I(t)
S( )
e S(0) R(0) N(0) e I( )i( )d
N( )
S( )
e E(0) I(0) e I( )i( )d
N( )
−μ μτ
−μ μτ
+−=− +
⎡
⎤
⎛⎞
τ
=
+−+ γ−β τττ
⎢
⎥
⎜⎟
τ
⎝⎠
⎣
⎦
⎡⎤
⎛⎞
τ
=− + − γ−β τ τ τ
⎢⎥
⎜⎟
τ
⎝⎠
⎣⎦
∫
∫
(33)
which coincides with (15) for all time if the indicator
function is identically zero, that is, if h(t)
is such
that the auxiliary vaccination law (31) is in
[
]
0, 1
for all time. Also, one gets from (15) that:
t
(t )
0
S( )
N(t) S(t) R(t) e I( )i( )d
N( )
−μ −τ
⎛⎞
τ
−
−≤ε+ β −γτττ
⎜⎟
τ
⎝⎠
∫
(34)
1 N(0) S(0) R(0)
tT() ln
−−
⎛⎞
∀≥ ε
⎜⎟
με
⎝⎠
for any given
real 0
ε
> . The right-hand-side integral of (34) takes
into account the tracking deterioration if there is a
time interval of nonzero Lebesgue measure such that
V(t) V(t)≠
0
t
+
∀∈\ . The following result is
important to discuss stability when the vaccination
law
[
]
V(t) 0, 1∈ but it is not identically equal to
V(t) . In fact, the positivity part of Theorem 1 still
holds because of the SEIR-model is positive since
[
]
V(t) 0, 1∈
0
t
+
∀∈\ and the whole population
evolution is independent of the vaccination law
according to (5). However, the whole susceptible
plus immune does not asymptotically track the
whole population. In summary, one has:
Theorem 3. The vaccination law (28), (30)-(31)
makes the SEIR–model (1)-(4) positive and globally
stable under Theorem 2. Furthermore,
ON VACCINATION CONTROLS FOR THE SEIR EPIDEMIC MODEL WITH SUSCEPTIBLE PLUS IMMUNE
POPULATIONS TRACKING THE WHOLE POPULATION
169

{
}
t
t
(t )
0
t
lim N(t) S(t) R(t)
S( )
lim sup e I( )i( )d
N( )
→∞
−μ −τ
→∞
−−
⎡⎤
⎛⎞
τ
≤β−γτττ
⎢⎥
⎜⎟
τ
⎝⎠
⎣⎦
∫
***
A more practical vaccination law is defined as
follows:
{
}
{}
aux
min S(t), R(t) 0 and
V(t) if
V(t)
min E(t), I(t) 0
V (t) otherwise
⎧
>
⎧
⎪
⎪
⎨
=
≥
⎨
⎪
⎩
⎪
⎩
(35)
with
V(t) given by (31) and
aux
V (t) obtained from:
aux
1 if S(t) 0 and / or R(t) 0
V(t)
0 otherwise
==
⎧
=
⎨
⎩
(36)
Remark 1. The inclusion of the auxiliary function
aux
V (t) in (35) guarantees the non-negativity of the
susceptible and remove-by-immunity populations. In
this sense, note that large values of
V(t) could
eventually do negative S(t) or R(t) from (1)-(4).
This fact is avoided with such a construction of
V(t) since
aux
V(t) V (t)= at the time instants where
S(t) 0= and/or R(t) 0= guarantees that S(t) 0≥
and R(t) 0≥
0
t
+
∀∈\ . Moreover, the non-
negativity for S(t) and R(t) guarantees the non-
negativity of the infected and infectious populations
from (2) and (3). Finally, note that the construction
of the vaccination function (35)-(36) lets that E(t)
and I(t) reach zero, which is the ideal objective for
the eradication of the infection from the population.
In summary, such an alternative control law
guarantees the positivity property for the SEIR-
epidemic model by the proper construction of the
law. In this sense, the condition
[
]
0
V: 0, 1
+
→\ in
Theorem 1 is a sufficient, but a non-necessary,
condition to ensure the positivity of the system. ***
4 SIMULATION EXAMPLE
An example based on the rabbit hemorrhagic disease
in United Kingdom is considered to illustrate the
theoretical results presented in the paper. An initial
population of
N
(0) 1000= rabbits is used. Such an
epidemic can be described by the SEIR model (1)-
(4) with the parameter values:
0.01 per day (p. d.)μ= , 0.017 p. d.ν= ,
0.936 p. d.β= , 0.0333 p. d.ω= ,
0.9314ρ=
and
0.025 p. d.σ=γ= . Such values are commonly used
in the literature (Keeling and Rohani, 2008, White et
al., 2004). The main characteristic of such an
infection is its high mortality, note the value of the
probability of dying from the infection (
0.9314ρ=
)
close to 1. The initial conditions for the individual
populations are given by: S(0) 800= , E(0) 80
=
,
I(0) 50
=
and R(0) 70
=
.
The time evolution of the system in the free-
vaccination case, i.e. if V(t) 0=
0
t
+
∀∈\ is
displayed in Figure 1. The population of rabbits
disappears because of the high mortality of the
infection as it can be seen in such a figure. As a
consequence, a vaccination strategy has to be
applied if the persistence of the rabbits is required.
In this sense, Figure 2 displays the evolution of the
total, the susceptible and the removed-by-immunity
populations if the vaccination control law defined by
(28), (31), (35) and (36), with the initial condition
h(0) 0
=
, is applied. On the other hand, the time
evolution of the infected and the infectious
population with such a vaccination strategy is shown
in Figure 3. The total population of the rabbits
monotonically grows through time as it can be seen
from Figure 2. Moreover, the infected and infectious
population decrease to zero as time grows as it is
seen in Figure 3. In other words, the infection is
eradicated after a time interval and then, the
population of rabbits grows in a fast way, like it
occurs in absence of disease. Finally, the time
evolution of the vaccination function is displayed in
Figure 4.
0 200 400 600 800 1000 1200 1400 1600 1800 2000
0
200
400
600
800
1000
1200
Time (days)
Total and partial populations
N(t)
S(t)
E(t)
I(t)
R(t)
Figure 1: Time evolution of the total and individual
populations without vaccination.
BIOINFORMATICS 2011 - International Conference on Bioinformatics Models, Methods and Algorithms
170
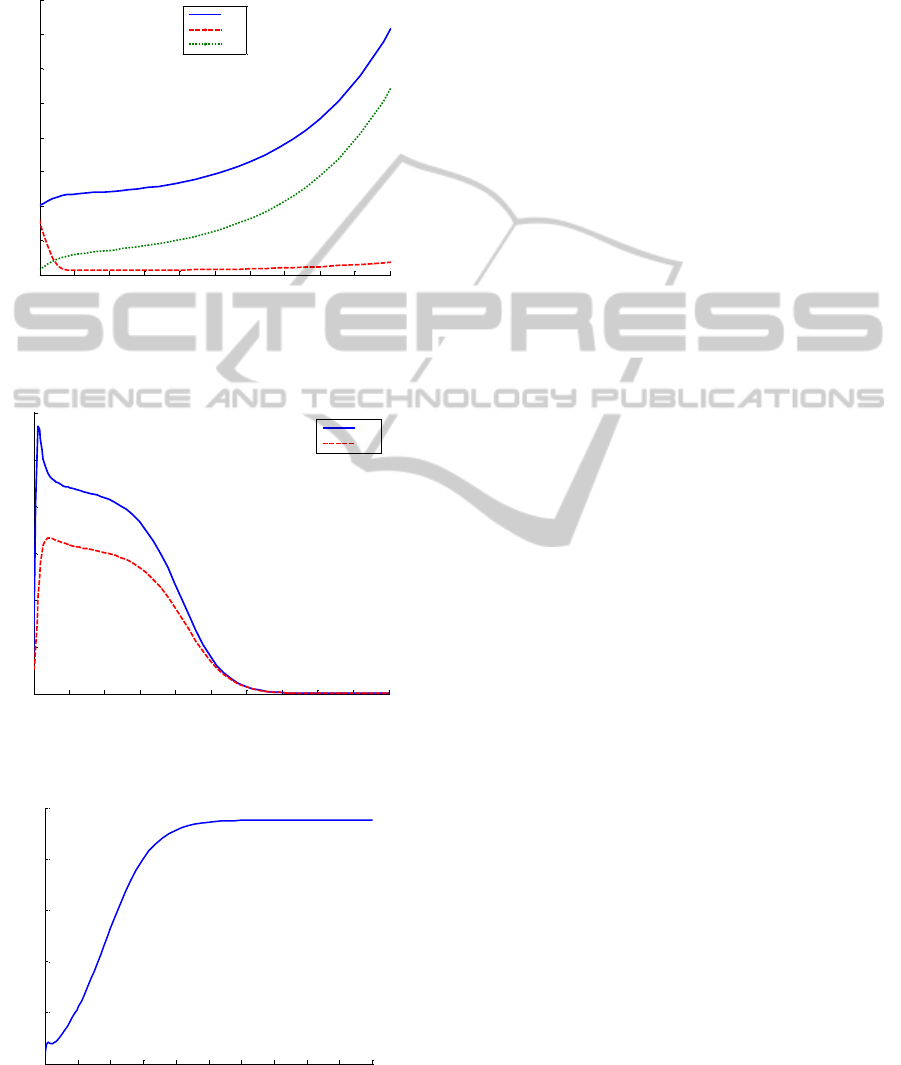
These simulation results point out the improvement
of the use of a vaccination strategy in order to
guarantee the suitable growth of a population against
a high mortality infectious disease.
0 50 100 150 200 250 300 350 400 450 500
0
500
1000
1500
2000
2500
3000
3500
4000
Time (day s )
Total, susceptible and remove-by-inmmunity populations
N(t)
S(t)
R(t)
Figure 2: Time evolution of the total, susceptible and
removed-by-immunity populations with the proposed
vaccination control law.
0 200 400 600 800 1000 1200 1400 1600 1800 2000
0
100
200
300
400
500
600
Time (days)
Infected and infectious populations
E(t)
I(t)
Figure 3: Time evolution of the infected and infectious
populations with the proposed vaccination control law.
0 200 400 600 800 1000 1200 1400 1600 1800 2000
0.5
1
1.5
2
2.5
3
Time (days)
Vaccination function
Figure 4: Time evolution of the vaccination associated to
the control law.
5 CONCLUSIONS
A vaccination control strategy has been presented to
eradicate the propagation of infectious diseases. The
SEIR mathematical model has been used to design a
control action via a vaccination strategy, which
modifies suitably the system dynamics in order to
get the disease eradication objective. The
performance of such a vaccination strategy has been
illustrated via some simulation results based on the
rabbit hemorrhagic disease. Such results show that
a
continuous-time vaccination through the population
could be carried out in order to eradicate the
epidemic
. Otherwise, the rabbits population
extinguishes due to the high mortality associated to
such an epidemic disease.
Future research is in progress to deal with more
general models to describe propagations of diseases.
Also, other types of control strategies based on
impulsive or discrete-time vaccinations are going to
be treated.
ACKNOWLEDGEMENTS
The authors thank to the Spanish Ministry of
Education by its support of this work through Grant
DPI2009-07197 and to the Basque Government by
its support through Grants IT378-10, SAIOTEK
SPE07UN04 and SAIOTEK SPE09UN12.
REFERENCES
De la Sen, M., 2008. About the properties of a modified
generalized Beveron-Holt equation in ecology models.
Discrete Dynamics in Nature and Society 2008,
Article ID 592950, 23 pages, doi:
10.1155/2008/592950.
De la Sen, M., 2008. The generalized Beverton-Holt
equation and the control of populations. Applied
Mathematical Modelling 32, pp. 2312-2328.
De la Sen, M., Alonso-Quesada, S., 2008. A control theory
point of view on Beverton-Holt equation in population
dynamics and some of its generalizations. Applied
Mathematics and Computation 199, pp. 464-481.
De la Sen, M., Alonso-Quesada, S., 2008. Model-
matching-based control of the Beverton-Holt equation
in Ecology. Discrete Dynamics in Nature and Society
2008, Article ID 793512, 21 pages, doi:
10.1155/2008/793512.
De la Sen, M., Alonso-Quesada, S., 2009. Control issues
for the Beverton-Holt equation in ecology by locally
monitoring the environment carrying capacity: Non-
adaptive and adaptive cases. Applied Mathematics and
ON VACCINATION CONTROLS FOR THE SEIR EPIDEMIC MODEL WITH SUSCEPTIBLE PLUS IMMUNE
POPULATIONS TRACKING THE WHOLE POPULATION
171

Computation 215, pp. 2616-2633.
Erturk, V. S., Momani, S., 2008. Solutions to the problem
of prey and predator and the epidemic model via
differential transform method, Kybernetes 37, pp.
1180-1188.
Keeling, M. J., Rohani, P., 2008. Modeling Infectious
Diseases in Humans and Animals, Princeton
University Press, Princeton and Oxford.
Khan, H., Mohapatra, R. N., Varajvelu, K., Liao, S. J.,
2009. The explicit series solution of SIR and SIS
epidemic models. Applied Mathematics and
Computation 215, pp. 653-669.
Mollison, D., 2003. Epidemic Models: Their Structure and
Relation to Data, Publications of the Newton Institute.
Cambridge University Press.
Mukhopadhyay, B., Battacharyya, R., 2007. Existence of
epidemic waves in a disease transmission model with
two-habitat population. International Journal of
Systems Science 38, pp. 699-707.
Ortega, N., Barros, L. C., Massad, E., 2003. Fuzzy gradual
rules in epidemiology. Kybernetes, 32, pp. 460-477.
Song, X. Y., Jiang, Y., Wei, H. M., 2009. Analysis of a
saturation incidence SVEIRS epidemic model with
pulse and two time delays. Applied Mathematics and
Computation 214, pp. 381-390.
White, P. J., Trout, R. C., Moss, S. R., Desai, A., Armesto,
M., Forrester N. L., Gould, E. A., Hudson, P. J., 2004.
Epidemiology of rabbit haemorrhagic disease virus in
the United Kingdom: evidence for seasonal
transmission by both virulent and avirulent modes of
infection. Epidemiology and Infection 132, pp. 555-
567.
Yildirim, A., Cherruault, Y., 2009. Anaytical approximate
solution of a SIR epidemic model with constant
vaccination strategy by homotopy perturbation
method. Kybernetes 38, pp. 1566-1575.
Zhang, T. L., Liu, J. L., Teng, Z. D., 2009. Dynamic
behaviour for a nonautonomous SIRS epidemic model
with distributed delays. Applied Mathematics and
Computation 214, pp. 624-631.
BIOINFORMATICS 2011 - International Conference on Bioinformatics Models, Methods and Algorithms
172
