
MRI-INDUCED SAR ON PACEMAKER LEADS
Numerical Simulations on Three Human Phantoms
Eugenio Mattei, Giovanni Calcagnini, Michele Triventi, Federica Censi and Pietro Bartolini
Italian National Institute of Health, Department of Technology and Health, Rome, Italy
Keywords: Magnetic Resonance Imaging, Pacemaker, Specific Absorption Rate, Human Visible Dataset.
Abstract: Numerical simulations were performed to evaluate the Specific Absorption Rate (SAR) induced at the tip of
a pacemaker (PM) implant by the 64 MHz radiofrequency (RF) field used in 1.5T Magnetic Resonance
Imaging (MRI) procedures. The analysis was performed by using a commercial FDTD software (SEMCAD
X, SPEAG, Switzerland) and aimed at the evaluation of the impact that the patient ‘s morphology has on the
induced local SAR at the implant tip. In particular three human phantoms were studied: a 34-year old man
model, a 26-year old woman, and a 6-year old boy. The three phantoms reproduce more than 70 tissues of
the human body with a resolution of 1 mm. Inside each phantoms, realistic implant configurations were
modelled, considering both left and right pectoral implants, and atrial and ventricular stimulations. The local
SAR values at the lead tip was compared for the three phantoms and sensible differences were observed:
with a RF excitation set to produce an whole-body average SAR of 2 W/kg without any implants, local SAR
values ranged from 641W/kg (woman model – right ventricular implant) to 3 W/kg (boy model – left atrium
implant). We also observed that, in general, ventricular implants showed a higher SAR compared to atrial
ones, as well as right pectoral implants compared to left ones. However, not always a higher implant area or
a longer lead path implied higher SAR at the tip, indicating the coupling mechanisms between the implant
and the RF field are likely to be more complex that the only area-dependent induction law.
1 INTRODUCTION
The number of Magnetic Resonance Imaging (MRI)
scans performed annually has increased dramatically
over the past few years. Parallel to the growth and
evolution of the MRI field, is the burgeoning
number of patients benefiting from implantable
cardiac systems including pacemaker (PM) and
implantable cardioverter/defibrillators (ICDs). The
combination of these two growing phenomena
results in an estimated 50-75% probability of a
patient being indicated for an MRI study over the
lifetime of their device; it has created an estimated
200,000 implanted patients who were denied the
MRI scan, and this numbers are likely to increase in
the future (Rougin et al, 2004). Given the rapid
expansion of technology in the fields of both MRI
and device arrhythmia management, there is
increasing interest in the issue of implantable device
safety in the MRI environment. For the purpose of
MRI, non-ferromagnetic material is available for
manufacturing of implantable devices. Considering
the impressive progress in the use of diamagnetic
material, the most important safety problem
associated with MRI and medical implantable
devices is the potential tissue heating induced by the
radiofrequency (RF) fields. In this filed, numerical
studies are crucial to extend the range of
experimental measurements and to correlate heating
results to those expected in humans. Simulations can
be used to model realistic patient geometries, to
deliver more information (e.g., 3D fields instead of
single measurement points) and to study individually
the impact of parameters such as tissue properties,
boundary conditions, etc. As the finite difference
time domain (FDTD) method has been a widely
used technique for characterization of RF heating, it
is an excellent candidate to render an accurate
estimate of the specific absorption rate (SAR), and
therefore heating, due to implantable devices during
MRI experiments.
The anatomical structure of the human body as
sets of minute elements (voxels) suitable to be
imported inside the FDTD environment have been
obtained from MRI scans, X-ray computed
tomography, or anatomical coloured images of the
135
Mattei E., Calcagnini G., Triventi M., censi F. and Bartolini P..
MRI-INDUCED SAR ON PACEMAKER LEADS - Numerical Simulations on Three Human Phantoms.
DOI: 10.5220/0003131501350139
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 135-139
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)

Visible Human Project (VHP). These data are today
largely available at a high resolution, so that almost
all the various human tissues can be taken into
account (Visible Human Dataset – VHD).
The purpose of this study is to numerically
compute the local SAR induced by a 64 MHz RF
coil used in MRI procedures at the lead tip of a PM
implant. Local SAR values were compared for three
human phantoms: a 34-year-old man model, a 26-
year-old woman, and a 6-year-old boy. Inside each
phantom, realistic implant configurations were
modelled; left and right pectoral implants, as well as
atrial and ventricular stimulations were taken into
account.
2 METHODS AND MATERIALS
The MRI RF coil model was developed using a
commercial FDTD (Finite Difference Time Domain)
solver (SEMCAD X, SPEAG, Zurich, Switzerland).
It represents a 16-rung low-pass birdcage coil (60
cm high, with an inner radius of 30 cm) which
reproduces the RF field generated during MRI
procedures (Figure 1).
Figure 1: FDTD model of the RF birdcage coil.
Two voltage sources were applied at one of the
two external rings of the coil, with a 90° shift both in
space and in time. Inside the coil, a uniform and
circularly polarized magnetic field was thus
generated. An external metal shield was used to limit
the RF field inside the birdcage coil.
The human phantoms were imported from “The
Virtual Family” package developed by the
Foundation for Research on Information Technology
in Society (IT’IS Foundation - Zurich, Switzerland ).
In particular we used:
• The 34-year-old man model;
• The 26-year-old woman;
• The 6-year-old boy model.
Each phantoms distinguishes more than 70 tissues
with a spatial resolution of 1 mm. The phantoms
were placed inside the RF coil and their position was
adjusted to have the coil isocenter at the lowest part
of the sternum (xiphoid process – Figure 2).
Figure 2: Human phantom (female) inside the birdcage
coil.
As PM implant, a bicameral stimulator was
modelled in the right and left pectoral regions. The
lead path was derived from RX images of patients
with a PM implant (Figure 3). Both for atrial and
ventricular stimulation, a unipolar lead was
modelled as a perfect electric conductor (PEC) thin
wire (radius = 0.5 mm) with a silicon insulation of
1.5 mm radius. At the end of the lead, a 1 mm bear
PEC tip was put in contact with the heart wall.
Figure 3: Human phantoms and PM implants.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
136

To obtain the required resolution at the implant,
a two-step approach has been employed: a first
simulation of the birdcage without the wire is
performed using a relatively coarse grid (≈15 million
cells, graded mesh). The fields are recorded on the
surface of a rectangular box around the implant, and
are used to excite a second simulation restricted to
the area where the PM and its lead are implanted
(≈20-30 million cells, graded mesh).
This two-step approach, which is based on
Huygens’ principle, allows to obtain a high
resolution around the implant and to properly model
the lead. To ensure the continuity of the inner
conductor of the lead and of its insulation a maximal
refinement at the lead (0.2-0.6 mm) was needed. The
same mesh parameters were used for the three
phantoms. In Figure 4 the graded mesh adopted in
the area of the implant and the resulting voxel are
overlaid to the hart and vessel of the VHD.
Figure 4: FDTD graded mesh in the area of the PM
implant: right pectoral implant (a) and left pectoral
implant (b).
Local SAR deposition was calculated from the
E-field estimated by the model. SAR was calculated
as described in the IEEE1528 standard (IEEE1528,
2006), over a 1mg mass.
A 1 mg mass was chosen as a trade-off between
a volume small enough to significantly account for
local SAR value, but big enough to prevent
misleading results due to computational errors.
3 RESULTS
Figure 5 shows the SAR distribution for the female
phantom with the PM in the right pectoral region,
exposed to the RF field of the birdcage coil.
Local hot-spots can be observed at the contact points
between the two lead tips and the heart wall, in the
atrium and in the ventricle. A similar distribution
was obtained also for the man and the boy
phantoms.
The local SAR values computed for the man, the
woman and the boy phantom are reported in Figure
6. The highest SAR was observed at the tip of the
right ventricular implant in the woman model (641
W/kg); on the other hand, the boy phantom is the
one associated to the lowest SAR values, for all the
implant configurations tested.
In general, the right pectoral positioning implies
higher SAR compared to left pectoral implants, as
well as ventricular stimulation compare to atrial one.
The only exception is represented by the left
atrial implant in the woman phantom, which showed
higher SAR values than the right atrial one.
In these simulations, the excitation signal applied to
the RF coil was not the same for the man, the
woman and the boy model, but was adjusted to
obtain an average whole-body SAR of 2 W/kg
(maximum value allowed during standard MRI
procedures) inside all the phantoms.
Figure 5: Example of the SAR distribution over the heart
surface resulting from the exposure of the implanted
phantom to the RF field generated by the birdcage coil.
The two hot spots represent the contact points between the
lead tips and the heart wall.
b
)
a)
MRI-INDUCED SAR ON PACEMAKER LEADS - Numerical Simulations on Three Human Phantoms
137
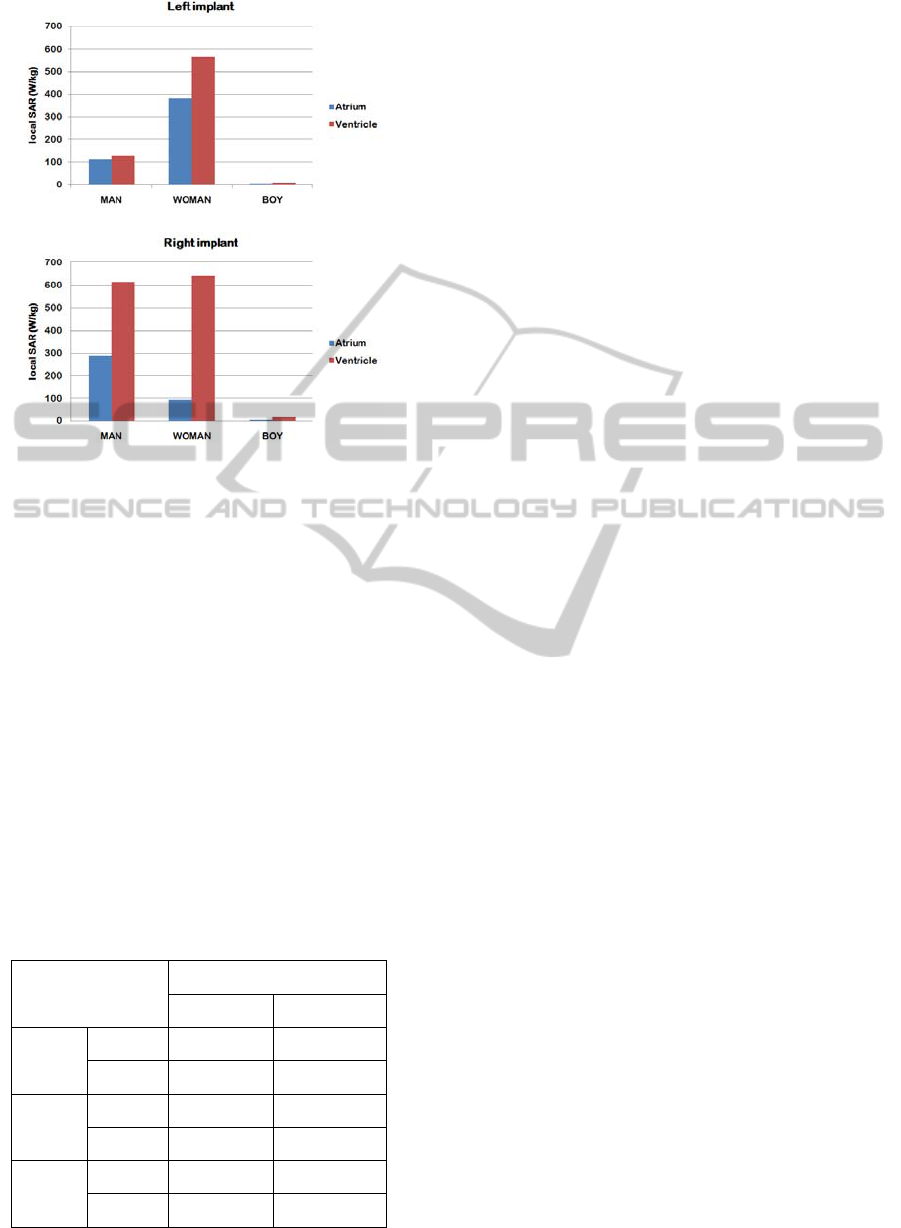
Figure 6: Local SAR values at the PM lead tip for the
man, woman and boy phantoms. SAR calculation was
averaged over 1 mg mass. The whole body SAR without
the implant was 2 W/kg in all the phantoms.
If the amplitude of the signal is not changed, the
average SAR significantly differs in the three
models: an excitation that produces an average
whole body SAR of 2 W/kg in the boy model, for
example, leads to an average SAR of 38.5 W/kg in
the man phantom, and of 25.9 in the woman
phantom.
In order to characterize the lead paths that a
realistic PM implant implies in different anatomical
structures and in different locations, we calculated
the lead length from the connection with the PM
chassis to the tip. The data reported in table 1 show
how a longer lead does not always imply a higher
SAR at the tip.
Table 1: Lead length for the configuration tested inside the
man, the woman and the boy phantoms.
Phantom type
Lead length (mm)
Atrium Ventricle
MAN
Left imp. 280 365
Right imp. 302 381
WOMAN
Left imp. 283 345
Right imp. 308 369
BOY
Left imp. 148 205
Right imp. 161 207
4 DISCUSSION
RF induced heating of biological tissue has long
been a concern for patients undergoing magnetic
resonance imaging (MRI). With regards to the MRI
induced heating on PM and ICD leads, a wide
database of experimental measurements is now
available in literature (Mattei et al, 2008; Nordbeck
et al, 2009). However, these studies use simplified
model of the human body, typically a rectangular
box phantom filled with an homogeneous gel, whose
physical properties are defined to closely match
those of biological tissues (ASTM F2182–02a).
Even when phantoms of more complex shapes are
used, they always assume a uniform behaviour of
human tissues and do not take into account the
realistic anatomical structure of the human body. In
addition, the large number of variables that take part
in the process may often result in a loss a general
validity, requiring additional efforts to perform
extensive and exhaustive measurements. Thus,
modellistic approaches based on numerical tools
might represent a useful mean, able to overcome
such limitations.
In this study we compared the RF-induced SAR
during MRI scans at the lead tip of a PM implanted
in human models that thoroughly reproduce the
anatomical structures of an adult male, an adult
woman and a boy. Realistic implant configurations
markedly differ for the three phantoms, in terms of
lead length, path, and area covered by the implant.
Thus, it is not surprising that also the local SAR
induced at the lead tip sensibly varies in the
simulations we performed. However, there are
several aspects that suggests how the coupling
mechanisms between the RF field and the PM
implant is much more complex than it may appear.
Several papers in the literature (Sommer et al,
2000, Rezai et al, 2005) chose a configuration of the
pacemaker lead in the coronal plane to achieve a
maximal magnetic induction area in order to
maximize the heating at the lead tip. We found that
the induced SAR is not always proportional to this
area. In particular, in the comparison between left
and right implant configurations, right implants
covered an area significantly smaller than the left
counterparts and the SAR is generally higher for the
former than the latter ones.
The lead length seems to be a parameter that
better correlates with the induced SAR (right
implant implies longer leads than left ones), but also
in this case, simulated data highlight some
exceptions: in particular, the highest SAR was
observed at the tip of the right ventricular implant in
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
138

the woman model, which has a shorter lead length
than the counterpart in the man model. Thus, there
should be other aspects related to the morphology of
the human phantom that can affect the amount of the
deposited power and consequently the tissue heating
at the lead tip; such aspects may involve the
particular path the lead follows inside the human
tissues and the electromagnetic field distribution
induced during MRI scans in the same regions.
5 CONCLUSIONS
The present numerical study shows how the
differences in terms of patient’s anatomy (different
gender and age) have an impact on the MRI induced
SAR, and consequently tissue heating, at the tip of
an implanted PM lead. It is justified by the changing
in lead path and electromagnetic field distribution
that different anatomical models imply. Our data
show also that the implant area or lead length are not
the only parameters that can affect the amount of
induced heating the implant tip, but more complex
coupling phenomena must be taken into account.
REFERENCES
ASTM F2182–02a – ‘‘Standard Test Method for
Measurement of Radio Frequency Induced Heating
Near Passive Implants During Magnetic Resonance
Imaging’’, ASTM International, 100 Barr Harbor
Drive, PO Box C700, West Conshohocken, PA,
19428–2959 USA
IEEE, IEEE1528, recommended practice for determining
the spatial-peak specific absorption rate (SAR) in the
human body due to wireless communications devices:
Measurement techniques, IEEE Standards Department
52(5), 1 2006
Mattei E, Triventi M, Calcagnini G, Censi F, Kainz W,
Mendoza G, Bassen HI, Bartolini P. Complexity of
MRI induced heating on metallic leads: experimental
measurements of 374 configurations Biomed Eng
Online. 2008 Mar 3;7:11
Nordbeck P, Weiss I, Ehses P, Ritter O, Warmuth M,
Fidler F, Herold V, Jakob PM, Ladd ME, Quick HH,
Bauer WR. Measuring RF-induced currents inside
implants: Impact of device configuration on MRI
safety of cardiac pacemaker leads. Magn Reson Med.
2009 Mar;61(3):570-8.
Rezai AR, Baker KB, Tkach JA, Phillips M, Hrdlicka G,
Sharan AD, Nyenhuis J, Ruggieri P, Shellock FG,
Henderson J. Is magnetic resonance imaging safe for
patients with neurostimulation systems used for deep
brain stimulation? Neurosurgery 2005
Nov;57(5):1056-62
Roguin A, Zviman MM, Meininger GR, Rodrigues ER,
Dickfeld TM, Bluemke DA, Lardo A, Berger RD,
Calkins H, Halperin HR. Modern pacemaker and
implantable cardioverter/defibrillator systems can be
magnetic resonance imaging safe: in vitro and in vivo
assessment of safety and function at 1.5 T.
Circulation. 2004 Aug 3;110(5):475-82.
Sommer T, Vahlhaus C, Lauck G, von Smekal A, Reinke
M, Hofer U, Block W, Traber F, Schneider C, Gieseke
J, Jung W, Schild H. MR imaging and cardiac
pacemakers: in-vitro evaluation and in-vivo studies in
51 patients at 0.5 T. Radiology 2000 Jun;215(3):869-
79
MRI-INDUCED SAR ON PACEMAKER LEADS - Numerical Simulations on Three Human Phantoms
139
