
DETECTION OF THE CYTOMEGALOVIRUS
A Mobile Device and a Disposable Cartridge for Detection at the Patient's Bed
Thomas Mangeat, Hichem Benalia, Christian Pieralli, Alain Rouleau, Wilfrid Boireau
Bruno Wacogne
FEMTO-ST Institute, UMR 6174, 16 Route de Gray, 25030 Besançon cedex, France
Jean-Sébastien Guerrini, Alain Coaquette, Georges Herbein, Lionel Pazart
Besançon University Hospital, 2 Place Saint Jacques, 25030 Besançon cedex, France
Christian Davrinche
INSERM U 563, Paul Sabatier University, Toulouse, France
Keywords: Biosensors, Embarked system, Cytomegalovirus, Immunofluorescence.
Abstract: Recently, cytomegalovirus (CMV) infection has become the most frequent cause of congenital infections.
The French Health Authority (HAS) is urging a diagnosis at birth for newborns. Since no screening device
is commercially available, a consortium has been established to set-up an original device. It consists of a
disposable cartridge containing the biological sample and the reactive liquids required for
immunofluorescencence detection on a functionalized surface. It also consists of a mobile reader used to
drive the fluids onto the biosensor and to ensure the optical measurement. Up to now, positive and negative
samples can be discriminated with a fluorescence intensity ratio of 3.
1 INTRODUCTION
Since the rubella vaccine was established, the
cytomegalovirus infection has become the most
frequent cause of congenital infections, particularly
in premature babies (prevalence between 2 and 10%
according to studies). Before deciding on the benefit
of screening in this population, the HAS (French
Health Authority) is urging "a study in newborns
(diagnosis at birth) with a long-term follow-up of
infected children” to be carried out. One of the
obstacles to carry out such a study lies in the
diagnostic means currently available (Demler-
Harrisson, 2009).
Congenital infections are the result of
transplacental transmission of CMV. Transmission
to the fetus may occur because of primary or
secondary maternal infection. The frequency of
intrauterine transmission following primary
infection during pregnancy is 30% to 40%,
compared with only 1% following secondary
infection (Stagno, 1986 – Raynor, 1993). Ten to
fifteen percent of congenitally infected infants will
have symptoms at birth, and 20% to 30% of them
will die (Raynor, 1993 – Nigro, 1999 – Pass, 2002).
Most of the congenitally infected infants (85–90%)
have no signs or symptoms at birth, but 5% to 15%
of them will develop sequelae such as sensorineural
hearing loss, delay of psychomotor development,
and visual impairment (Boppana, 1992 – Pultoo,
2000 – Lazzarotto, 2000).
Routine serologic screening for pregnant women
is rarely recommended by public health authorities
(Revello, 2002 – Yinon, 2010). The screening, if
done, should be performed at the beginning of
pregnancy or even prior to a planned pregnancy in
order to identify a seroconversion during pregnancy.
Instead of screening for pregnant women, some
authors recommend to screen babies at birth, but
reliable methods to screen newborns for congenital
cytomegalovirus (CMV) infection are still needed
(Fowler, 1999 – Boppana, 2010).
103
Mangeat T., Benalia H., Pieralli C., Rouleau A., Boireau W., Wacogne B., Guerrini J., Coaquette A., Herbein G., Pazart L. and Davrinche C..
DETECTION OF THE CYTOMEGALOVIRUS - A Mobile Device and a Disposable Cartridge for Detection at the Patient’s Bed.
DOI: 10.5220/0003120201030108
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2011), pages 103-108
ISBN: 978-989-8425-37-9
Copyright
c
2011 SCITEPRESS (Science and Technology Publications, Lda.)
The diagnosis of infection in newborns depends
on finding the virus in the different biological
liquids and more specifically urine which
concentrates the virus. Apart from CMV detection
kits for the laboratory, numerous lines of research
concern virus detection and Microsystems (µTAS,
MEMS, etc.) (Huikko, 2003 – Anderson, 2003).
These microsystems are generally dedicated to
detect genetic material after preparing samples, most
often by PCR or RT-PCR (Liao, 2005 – Park, 2004).
Concerning the biological fluid used and/or the type
of analysis, most examine blood cells or other types
of cells, which require virus extrusion operations of
the cells and a blood puncture for collecting
biological fluid. Indeed, a mobile device is required.
The microsystem presented here and developed
under the coordination of the FEMTO-ST Institute
in the framework of a 2006 ANR TecSan project,
approved by a microtechnics competitive cluster, is
an embedded detection device which uses a
microsystem including the functionalized surface for
CMV trapping (patent request submitted in
September 09). The detection and dosage of the viral
material are based on immunofluorescence
techniques, with materials and micromanufacturing
processes compatible with a low-cost industrial
production. Among the medical acts carried out at
birth, in particular in premature babies, gastric
aspiration allows a biological fluid combining foetal
urine (excreted as from the 5th month) with amniotic
fluid to be obtained easily. We therefore chose to
use this biological fluid as a screening medium.
In the next part of the paper, we describe the
immunofluorescence detection scheme as well as the
device we developed. The third part is devoted to the
bio-chemistry of the bio-sensor and to the first
experimental results concerning CMV detection.
Then a conclusion will be proposed to this work.
2 DESCRIPTION OF THE
DEVICE
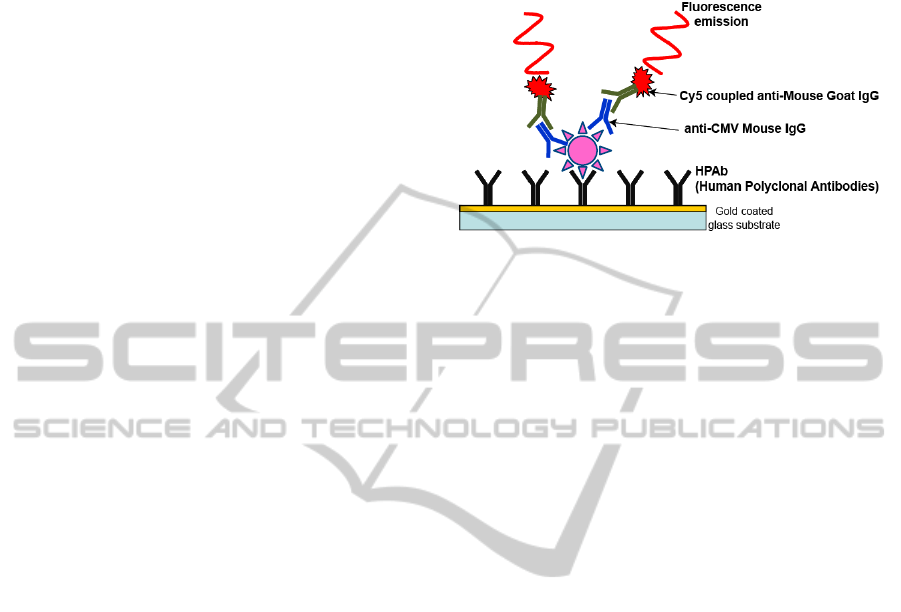
The detection relies on the use of an
immunofluorescence biosensor depicted in figure 1.
The biosensor surface is coated with CMV specific
antibodies. A biological sample is then applied onto
the surface of the biosensor. If CMV is present in the
biological sample, it is trapped onto the surface by
means of the antibodies. Then, after washing the
surface with buffer, a fluorescent probe is injected.
The latter consists of complementary Cy5 labelled
antibodies. Therefore, if CMV is present in the
sample, a fluorescent signal is detected.
Figure 1: Immunofluorescence detection of CMV.
This biosensor is integrated into a disposable
cartridge (figure 2). It contains all the fluids required
for the immunofluorescence reaction. In the figure,
we can see the gold coated functionalized surface as
well as different deformable balloons. Four balloons
are used. One contains the biological sample to be
tested, a second one contains the fluorescent probe, a
third one contains the buffer and the last one is used
for waste. In this example, the biological sample is
injected into the disposable cartridge with a
conventional syringe. Micro-channels are used to
drive the fluids from the balloons to the
functionalized window where the reaction takes
place.
Driving the fluid and detecting the possible
fluorescence signal is performed into the mobile
device, hereafter the reader, shown in figure 3. The
disposable cartridge is inserted into the reader
manually. Then the measurement starts. As
previously mentioned, the fluids are contained in
deformable balloons. Pistons are used to press the
balloons and put the fluids to movement. In our case,
we have 3 pistons: "sample piston", "buffer piston"
and "probe piston". The pistons motions, and
therefore the fluids flows, are driven thanks to a
computer program. The program also controls the
incubation times and washing duration. When the
biochemical reaction is finished, an ESE
fluorescence measurement unit is used to detect the
possible presence of CMV.
CM
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
104
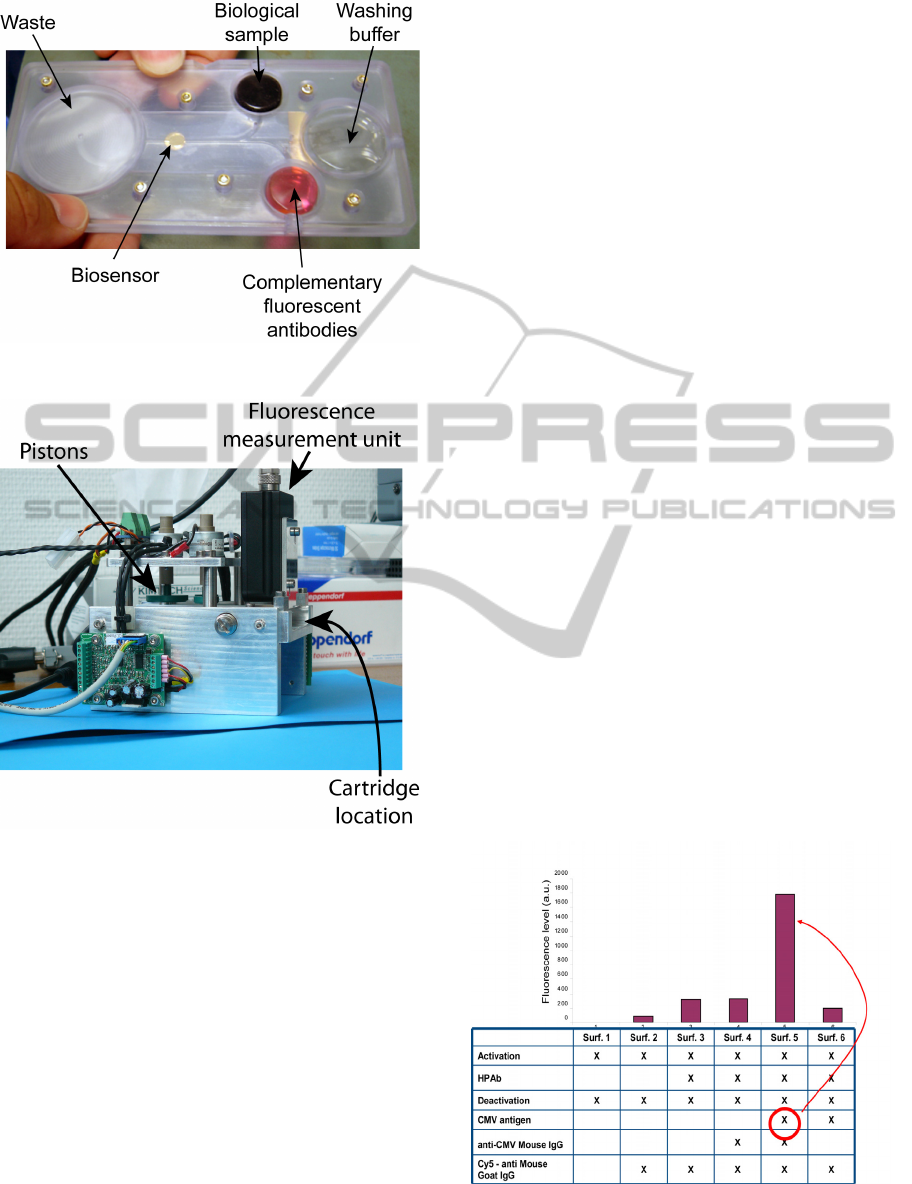
Figure 2: View of the disposable cartridge.
Figure 3: View of the reader/actuator.
It must be noted that the disposable cartridge was
designed using materials and micromanufacturing
processes compatible with a low-cost industrial
production.
3 IMMUNOFLUORESCENCE
DETECTION OF CMV
3.1 Bio-functionalization
Chips are incubated in a solution of 11-mercapto-1-
undecanol (97%) / 16-mercaptohexadecanoic acid
(3%) overnight at room temperature (RT).
Then, 40µl of EDC/NHS (1-ethyl-3-(3-
dimethylaminopropyl) carbodiimide hydrochloride/
N-hydroxysuccinimide) are added on each surface
and incubated during during 30 min at RT. This step
is necessary to activate C11/C16 layer.
The surfaces are then rinsed by 1X PBS
(phosphate buffer saline) and human polyclonal
antibodies (PAbH) are incubated on the chips during
1 hour at room temperature. To ensure optimal
grafting of the PAbH, antibodies are diluted in an
acetate buffer at 0,1mg/ml, pH 5.
Surfaces are then rinsed with 1X PBS and
C11/C16 layer is deactivated using 40µl
Ethanolamine-HCl (1 M pH 8.5) during 30 min à
RT. After a last rinsing by 1X PBS, biochips can be
used.
The fluorescent probe is composed of an anti-
CMV Mouse IgG coupled to an Cy5 - anti Mouse
Goat IgG.
3.2 Experimental Detection of CMV
Immunofluorescence detection of CMV antigen was
experimented in 3 steps.
In a first time, functionalized microscope slides
were used in order to test the biosensor alone. For
this, commercial CMV antigens were used as
biological samples. Six round gold surfaces were
deposited onto the slides and various biochemical
structures were tested as depicted in figure 4. It can
be seen from this figure that when the complete
antibodies-antigen combination is used, the
fluorescent signal is rather high. However, the
different fluids were applied by means of
conventional syringes and the measurements were
not perfectly reproducible.
Figure 4: Biosensor testing with antigen solutions.
DETECTION OF THE CYTOMEGALOVIRUS - A Mobile Device and a Disposable Cartridge for Detection at the
Patient's Bed
105
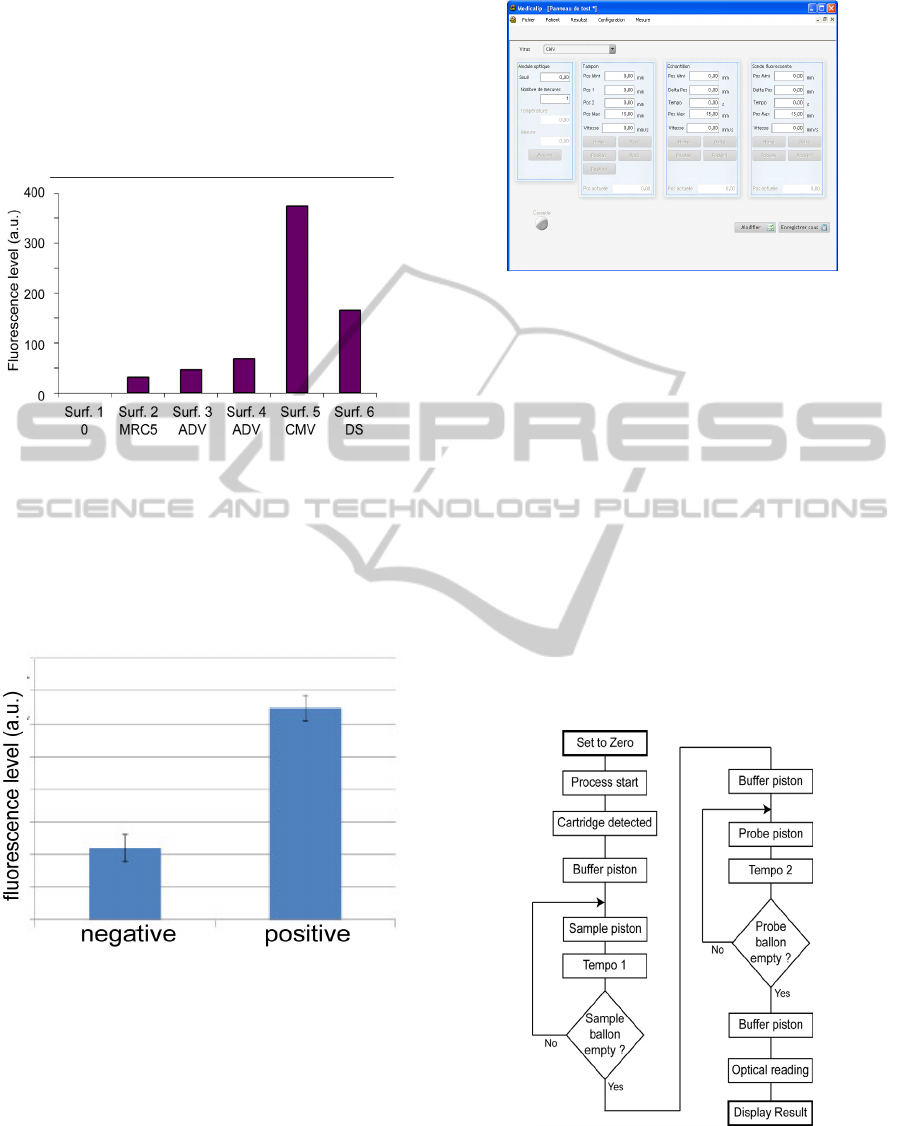
In a second time, we tested the specificity of the
sensor with various viral proteins obtained from
infected MRC5 cells. This experiment was done on
microscope slides. The result is shown on figure 5
where ADV, CMV and DS stand for adenovirus,
cytomegalovirus and commercial antigen
respectively.
Figure 5: Specificity of the bio-recognotion.
In a third time, disposable cartridges were used
together with the mobile reader. The idea was to test
the complete device with CMV infected cells. This
time, measurements show that the ratio between
positive and negative sample was of the order of 3 as
it can be observed from figure 6.
Figure 6: Complete device testing with CMV infected
cells.
4 DRIVING SOFTWARE AND
FURTHER DEVELOPMENTS
The device presented here is driven by means of a
computer. Fluids flows and optical detection can be
monitored by the computer. Figure 7 shows the
control window of the computer program.
Figure 7: Driving window of the computer program.
In order to enhance the immunofluorescence
reaction, fluids are driven as follows. First, the
biosensor surface is rinsed with the buffer. The
"sample piston" is actuated so that the reaction
chamber is filled. We wait a few minutes so that
viral proteins can be trapped onto the biosensor
surface. Then the "sample piston" is actuated again
in order to fill the reaction chamber with a new
sample volume. After a few minutes of incubation,
the process is iterated until the "sample balloon" is
empty. Rinsing the surface with the buffer is
performed in one go. Then, the "probe piston" is
actuated in the same manner as the "sample piston".
At the end, the biosensor's surface is rinsed with the
buffer and the fluorescence measurement is
performed. The automation of the measurement is
depicted in figure 8.
Figure 8: Control of the fluid flows and incubation times.
A measurement window is used to inform the
operator on the measurement step being processed.
It includes various parameters like the patient's
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
106
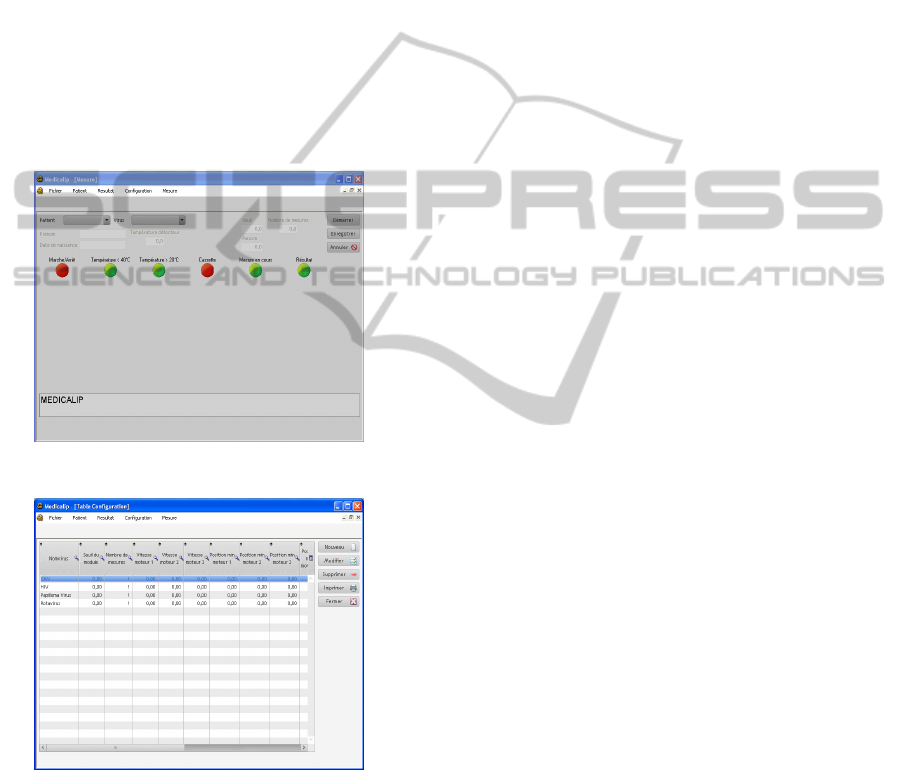
name, the name of the virus (we will comment later
on this aspect), the temperature controls and the
virus detection result (see figure 9).
One important aspect concerns the fact that all
the fluid required for the immunofluorescence
detection are contained in the disposable cartridge.
In this way, it is possible to fabricate specific
disposable cartridges for specific virus screening.
We may also envisage the detection of other kind of
biological entities. In fact, we plane to equip the
cartridges with bar codes that will be read by the
reader/actuator. The latter will then tune itself to the
right opto-fluidic parameters such as liquid flows,
incubation times and optical detection threshold. The
computer program offers the possibility to set-up
opto-fluidic parameters for each kind of virus as it
can be seen from figure 10.
Figure 9: Control window.
Figure 10: Setting the opto-fluidic parameters for each
specific virus.
Although this multi-virus arrangement is
particularly interesting in the cases where only one
mobile reader can be used, more specific application
may require a more compact and single virus
detection device. This is the case for CMV screening
for which the device will be used at birth in the care
room next to the delivery room. We are now
working on the ergonomic aspect of this specific
application.
The last issue that must be taken into account is
the time required for the test to be performed. Up to
now, a bit less than 1 hour is required from the
moment when the cartridge is inserted in the reader
and the moment when the result is displayed. It is
much more rapid than a diagnosis in a virology
laboratory (we mean taking into account the gastric
liquid sampling, the packaging of the sample, the
transportation to the virology laboratory and the
diagnosis). However, it can still be improved by
means of acoustic accelerating techniques we are
working on at the moment (Kardous, 2010).
5 CONCLUSIONS
In this conference, we have presented a mobile
device used to screen CMV at the newborn's bed.
Experimental results show a signal to noise ration of
about 3 which is enough for screening purposes. The
fact that all the required fluids are contained in a
stand alone disposable cartridge make the system
easy to transpose to the detection of various
pathology vectors. Our present work deals with the
study of such detections together with the set up of
an ergonomic biological sampling system that fulfill
the requirements of clinical use.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the support
of the French Agence Nationale de la Recherche.
REFERENCES
Anderson H., van den Berg A., Microfluidic, 2003,
Devices for cellonics: a review, Sens. and Act. B, Vol.
92, pp. 315-325.
Boppana S. B., Pass R. F., Britt W. J., Stagno S, Alford C.
A., 1992, Symptomatic congenital cytomegalovirus
infection: neonatal morbidity and mortality, Pediatr.
Infect. Dis. J. Vol. 11, pp. 93–99.
Boppana S. B., Ross S. A., Novak Z, Shimamura M, Tolan
Jr R. W., Palmer A. L., Ahmed A, Michaels M. G.,
Sánchez P. J., Bernstein D. L., Britt W. J., Fowler K.
B., 2010, Dried blood spot real-time polymerase chain
reaction assays to screen newborns for congenital
cytomegalovirus infection, JAMA. Vol.14, pp. 1375-
1382.
DETECTION OF THE CYTOMEGALOVIRUS - A Mobile Device and a Disposable Cartridge for Detection at the
Patient's Bed
107

Demmler-Harrisson G. L., 2009, Congenital
cytomegalovirus: public health action towards
awareness, prevention, and treatment, J. of Clin.
Virol., Vol. 46 Suppl 4, pp. S1-5.
Fowler K. B., Dahle A. J., Boppana S. B., Pass R. F.,
Newborn hearing screening: will children with hearing
loss caused by congenital cytomegalovirus infection
be missed?, 1999, J. Pediatr. Vol.135, pp. 60-64.
Huikko K., Kostiainen R., Kotiaho T., Introduction to
micro-analytical systems: bioanalytical and
pharmaceutical applications, 2003, Eur. J. of
Parmaceutical Sciences, Vol.20, pp.149-171.
Kardous F., Simon B., Yahiaoui R., Manceau J. F.,
Boireau W., Improving immunosensor performances
using acoustic mixer on droplet microarray, 2010,
Biosens. And Bioelec. In Press,
doi:10.1016/j.bios.2010.09.007
Lazzarotto T., Varani S., Guerra B., Nicolosi A., Lanari
M., Landini M. P., Prenatal indicators of congenital
cytomegalovirus infection, 2000, J. Pediatr. Vol.137,
pp. 90–95.
Liao C. S., Lee G. B., Liu H. S., Hsieh T. M., Luo C. H.,
Miniature RT-PCR system for diagnosis of RNA-
based viruses, 2005, Nucleic Acids Res. Vol. 33, pp.
e156.
Nigro G., Mazzocco M., Anceschi M. M., La Torre R.,
Antonelli G., Cosmi E. V., Prenatal diagnosis of fetal
cytomegalovirus infection after primary or recurrent
maternal infection, 1999, Obstet. Gynecol. Vol.94, pp.
909-914.
Park J. C., Park Y. S., Kim E. H., Oligonucleotide chip
composition for analyzing hepatitis c virus (hcv)
genotype and detecting method thereof, 2004, Patent
US2004170957.
Pass R. F., Cytomegalovirus infection, 2002 Pediatr. Rev.
Vol.23, pp. 163–170.
Pultoo A., Jankee H., Meetoo G., Pyndiah M. N., Khittoo
G., Detection of cytomegalovirus in urine of hearing-
impaired and mentally retarded children by PCR and
cell culture, 2000, J. Commun. Dis. Vol. 32, pp. 101–
108.
Raynor B. D., Cytomegalovirus infection in pregnancy,
1993, Semin. Perinatol. Vol.17, pp. 394-402.
Revello M. G., Gerna G., Diagnosis and management of
human cytomegalovirus infection in the mother, fetus,
and newborn infant, 2002, Clin. Microbiol. Rev. Vol.
15, pp. 680–715.
Stagno S., Pass R. F., Cloud G., Britt W. J., Henderson R.
E., Walton P. D., Veren D. A., Page F., Alford C. A.,
Primary cytomegalovirus infection in pregnancy.
Incidence, transmission to fetus, and clinical outcome,
1986, JAMA Vol. 256, pp. 1904–1908.
Yinon Y., Farine D., Yudin M. H., Gagnon R., Hudon L.,
Basso M., Bos H., Delisle M. F., Menticoglou S.,
Mundle W., Ouellet A., Pressey T., Roggensack A.,
Boucher M., Castillo E., Gruslin A., Money D. M.,
Murphy K., Ogilvie G., Paquet C., Van Eyk N., Van
Schalkwyk J., Cytomegalovirus infection in
pregnancy, 2010, J. Obstet. Gynaecol. Can. Vol. 32,
pp. 348-354.
BIODEVICES 2011 - International Conference on Biomedical Electronics and Devices
108
