
ROBUST EAR LOCATED HEART RATE MONITOR
L. Rossini, R. Vetter, C. Verjus, P. Theurillat, P. Renevey, M. Bertschi and J. Krauss
Swiss Center for Electronics and Microtechnology, Jaquet-Droz 1, Neuchˆatel, Switzerland
Keywords:
Ear located heart rate monitor, Strapless heart rate monitor, Ear clip, Earphone, Portable audio players.
Abstract:
We have developed a device for heart rate estimation with the optical sensing unit integrated in a classic
media player earphone. The sensing principle is based on an optical infrared measurement directly on the ear
lobe whereas the heart rate estimation is obtained utilizing robust model-based signal processing techniques
valid even for quasi periodic activities such as running. Nevertheless, the remaining problem related to these
strategies are statistically undetectable inter-beat during short term sporadic activities. In this paper, we present
a novel robust inter-beat discarding method based on an activity related modeling of the expected heart rate
dynamics that incorporates a simple cardiovascular model to reduce related inaccuracies in the heart rate
estimation. A validation protocol has been designed and 9 subjects were asked to carry out their daily normal
office activities for a time length ranging from 1 to 2 hours. A global absolute relative error mean of 0.9%
between the estimated heart rate and a reference device and a sensitivity above 90% demonstrate encouraging
performances of the proposed device.
1 INTRODUCTION
The advent of portable audio players is continuously
increasing the popularity of exercising with music.
Besides enjoying their favorite songs, people may find
themselves often more motivated toward the achieve-
ment of a given training load (Elliott et al., 2005;
Schie et al., 2008). However, to obtain a desired
training effect such as weight-loss or improvement of
the cardiovascularperformance, the exercising person
should observe and respect its personal heart rate tar-
get zones (Noakes, 2003). This requires supplemen-
tary equipment such as a heart rate monitor with its
associated chest-strap ECG sensing. Often, due to
the tightness of the strap, the heart rate monitor and
chest-strap sensor may be perceived by users as op-
pressive and may consequently diminish the exhila-
rating and motivating feeling provided by music. In
order to avoid this degradation, a device for heart rate
estimation based on a sensing unit directly located in
a classical audio player earphone has been presented
recently in (Celka et al., 2004; Verjus et al., 2003).
The sensing is based on an infrared measurement at
the ear cartilage whereas the signal processing is di-
rectly performed in the audio player unit where audi-
tory user feedback may be achieved. A critical aspect
of this approach is the signal processing. Adaptive
model-based enhancement of infrared signals is per-
formed to obtain a robust heart rate estimation even
for quasi periodic activities such as for example run-
ning. A remaining problem of this device is a reli-
able management of heart rate estimation during short
term sporadic activities. Indeed, in this case, model
based IR signal enhancement may not operate accu-
rately due to the convergencetimeof the enhancement
model (Haykin, 1991). Consequently, residual move-
ment related artifacts might induce erroneous inter-
beat detections that may not be detected with a sta-
tistical assessment (Vaseghi, 1996). In order to cope
with this problem, we have developed a reliable dis-
carding of erroneous inter-beat intervals based on an
activity related modeling of the expected heart rate
dynamics. The exploitation of previous biomedical
knowledge allowed us to develop a simple cardiovas-
cular model to reduce inaccuracies in the heart rate
estimations.
214
Rossini L., Vetter R., Verjus C., Theurillat P., Renevey P., Bertschi M. and Krauss J. (2009).
ROBUST EAR LOCATED HEART RATE MONITOR.
In Proceedings of the International Conference on Biomedical Electronics and Devices, pages 214-219
DOI: 10.5220/0001541002140219
Copyright
c
SciTePress

2 METHODS
2.1 Background
Optical probes for sensing biological tissue proper-
ties based on photoplestysmography(PPG) have been
widely used over the past years for the estimation of
cardiovascular parameters such as for example pulse
oximetry and heart rate (Webster, 1997), (Tremper
and Barker, 1989). Corruption of the PPG signal
arises from the influences of ambient light and subject
motion (Tremper and Barker, 1989) (Trivedi et al.,
1997). Processing of ambient light artifacts is not crit-
ical since the influence can be measured using multi-
plexing techniques and an artifact free PPG signal can
be restored using subtractive-type techniques (Trivedi
et al., 1997). Various methods for improving the PPG
technique during motion artifacts and low perfusion
of the tissue have been designed (Coetzee and Elg-
hazzawi, 2000). A very sound and robust approach
of motion artifacts removal in PPG measured sig-
nals has been recently addressed (Celka et al., 2004).
The parametric signal enhancement method exploits
the information contained in a motion reference sig-
nal generated by a two-dimensional accelerometer in
order to obtain a robust PPG heart rate estimation.
Very reliable heart rate estimations have been ob-
tained even under intense physical activity such as for
instance running. However, because of the slow con-
vergence of the enhancement algorithm, the heart rate
estimation may become erroneous during sporadic,
short, and transitory activities. The convergence time
of the enhancement algorithm is in the range of the
few seconds that are necessary to obtain sufficient ac-
curacy of the enhancement parameters.
2.2 Sensor and Processing Device
In this paper we propose a fully integrated heart rate
measurement device with sensing located at the ear
and providing reliable estimates of the heart rate that
are robust against short sporadic or transitory activi-
ties. This system is based on infrared optical measure-
ment of the sub-cutaneous blood flow by transillumi-
nation, together with an integrated two-dimensional
accelerometer (see Figure 1). The chosen optical
wavelength is 875 nm. The emitter is a light emit-
ting diode (LED) and the receiver is a photodetector
(PD). The light wave is sent trough the ear cartilage
and penetrates the skin and blood vessels to finally
reach the PD. The PD transforms the received light
intensity I(t) into a current that is then transformed
into a voltage. Subsequently, as shown in Figure 2, a
compensation of the ambient light is performed using
Figure 1: Ear located sensor device with portable process-
ing unit.
a subtractive multiplexing technique (Trivedi et al.,
1997).
The resulting signal IR(t) together with the two ana-
log acceleration signals a
1
(t) and a
2
(t) are then con-
ditioned through amplification and a 2nd order But-
terworth bandpass filter between 0.5 Hz and 3.5 Hz.
Finally, an analog-to-digital (ADC) conversion is per-
formed on these signals at a sampling frequency of
20 Hz. Signals are processed in the portable unit and
the resulting heart rate estimations are displayed to
the user. A direct auditory user feedback through the
sensing earphone is an attractive option for a future
development.
Figure 2: The optical concept and electronic signal con-
ditioning of the proposed ear located heart rate estimation
device.
2.3 Physical Principle
The principle of the proposed method resides in in-
jecting an optical infrared (IR) signal at the surface
of the body tissue and measuring the resulting op-
tical signal. This signal propagates through the ear
tissue where it is subject to modifications due to re-
flection, refraction, scattering and absorption. The
resulting signal is captured by one or multiple opti-
cal sensors distributed on the earphone. For the near
IR wavelength, the light propagation into the tissue
ROBUST EAR LOCATED HEART RATE MONITOR
215
is primarily governed by scattering and absorption
(Cheong et al., 1990). The Beer-Lambert equation
is generally used to describe the phenomenon of light
absorption in biological tissue (Coetzee and Elghaz-
zawi, 2000) relating the injected light I
i
to the output
light I
o
. However, motion artifacts affect the compo-
nents of the Beer-Lambert equation. Under this con-
ditions, the received intensity can be written in terms
of the major contributions:
I
o
(t) = I
i
(t) · γ
tissue
· γ
pulse
(t) · γ
motion
(t) (1)
where γ
tissue
is the static attenuation due to the tissue,
γ
pulse
(t) is the pulsatile component due to variations
in the sub-cutaneous blood flow, and γ
motion
(t) is the
contribution due to dynamic changes of the tissue in-
duced by movements of the head. The contribution
of γ
pulse
(t) in equation 1 is of pivotal interest for the
heart rate estimation. On the other hand, the time-
invariant term γ
tissue
is of no interest and can therefore
be removed using low-pass filtering. Adaptive sig-
nal processing enhancement techniques of the signal
I
o
(t) to cope with long term harmonic contribution of
γ
motion
(t) have been previously successfully presented
in (Celka et al., 2004). The technique developed dur-
ing the present activity addresses the problem of the
heart rate estimation during short and sporadic activi-
ties.
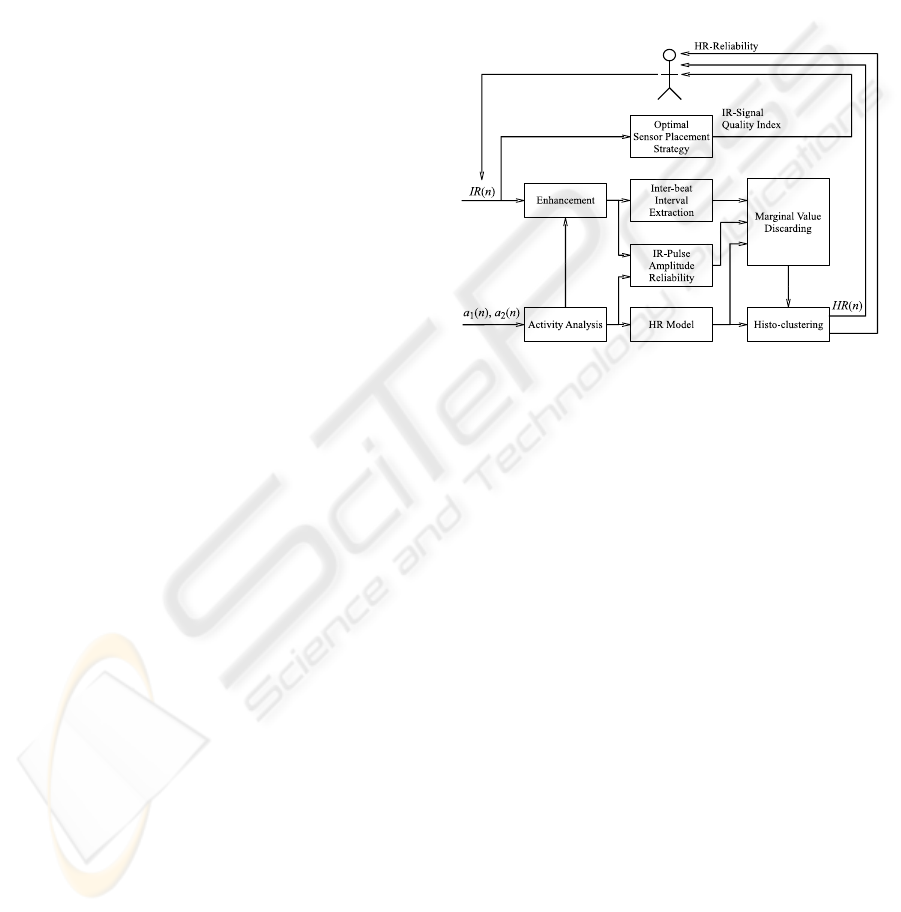
2.4 Proposed Algorithm
2.4.1 Concept
The concept of the proposed ear located heart rate
estimation device is shown in Figure 3. It mainly
consists of an enhancement of the motion corrupted
IR signals, an inter-beat interval (IBI) extraction on
the enhanced IR signal, a discarding of unreliable
IBIs and a final estimation of the most likely heart
rate through histogram clustering. The enhance-
ment method that receives one part of its parameters
from the activity analysis has been presented in detail
in (Renevey et al., 2001). The histogram clustering is
based on a standard method of statistical signal pro-
cessing (Gersho and Gray, 1992). The new features
that we introduce address the optimal sensor place-
ment strategy, the IR pulse-amplitude reliability as-
sessment, the activity analysis and the estimation of
a prior of heart rate using a simple model of the car-
diovascular dynamics. This prior estimation of heart
rate and its associated confidence intervals together
with the reliability index of the IR-pulse-amplitude
are then used to discard unreliable IBIs.
2.4.2 IR-Pulse-Amplitude Reliability
The reliability index of IR-pulse-amplitude describes
the likelihood that a given IBI has been extracted from
an IR signal with an amplitude that corresponds to
the expected amplitude of pulse contributions. It is
obtained by evaluating the probability that the instan-
taneous IR amplitude has been generated by a pro-
cess with mean µ
IR,ref
and standard deviation σ
IR,ref
,
which are continuously re-estimated during periods
without any movement activity.
Figure 3: The proposed heart rate estimation algorithm
based on motion artifact removal using accelerometer sig-
nals and discarding of unreliable inter-beat intervals using
activity related cardiovascular modeling.
2.4.3 Optimal Sensor Placement Strategy
Due to peripheral vasoconstriction and under-optimal
sensor placement, IR pulse contribution may be very
low. Therefore, we introduce an optimal sensor place-
ment procedure that evolves during the initialization
phase of the device:
• The signal amplitude is fed back to the user by
means of a quality index.
• As long as the quality index is below a given
threshold, the user is asked to adjust the sensor
placement to improve the signal quality.
We observed that this strategy leads to a sufficient
quality of the IR pulse contribution. In about 2 %
of the cases only, the subject was unable to properly
adjust the device in an appropriate position. This pro-
cedure could also be processed fully automatically if
a multi-sensor approach is used.
2.4.4 Activity based Model Heart Rate Model
The proposed ear located sensor is based on IR sig-
nals and as such is prone to movement related arti-
facts. During transitory periods when the enhance-
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
216

ment method cannot converge, these artifacts may in-
duce erroneous IBI detections. To discard such er-
roneous detections we improve the statistical robust-
ness of the proposed heart rate estimator by introduc-
ing a modeling of the expected heart rate based on
accelerometer measurements. The study and devel-
opment of cardiovascular models has attracted a wide
spreading interest in the scientific community(see (Le
et al., 2008) and references therein). In our appli-
cation, we are particularly interested in the descrip-
tion of the heart rate dynamics with respect to a given
input activity. Such an approach has been recently
proposed for cycling (Le et al., 2008). In particular,
the instantaneous heart rate is modeled as a weighted
function of its past value and the power generated by
the cyclist that is estimated using commercial devices.
Notice, however, that for activities like running and
walking the generated power output is not obviously
measurable. Nevertheless, during running and walk-
ing the exerciser’s power output is approximately re-
lated to his speed and a method for speed estimation
using core body located accelerometers has been re-
cently presented (Vetter et al., 2008). Even though
core body accelerometer measurements are not iden-
tical to ear located accelerometer measurement due to
slight head movements during each stride cycle, we
may assume that they are strongly related. We exploit
the method presented in (Vetter et al., 2008) to obtain
a rough measurement of the power output of a run-
ner P(n) as a function of the maximal eigenvalue of
stride-wise speed variations.
The heart rate model estimation HR
model
(n) at
sample n is a combination of the HR value before the
exercise HR
rest
(n) and a heart rate increase ∆HR(n)
HR
model
(n) = HR
rest
(n) + ∆HR(n) (2)
The dynamic behavior of the heart rate variation un-
der physical effort is described by
∆HR(n) = α∆HR(n− 1) + (1− α)HR
ATR
(n) (3)
where α determines the temporal behavior of our
model. Specifically, we use α
act
when the activity is
increasing or stationary and α
recov
during a recovery
phase. For this model, the evolution of the heart rate
is a function of both its past values and the innovation
term HR
ATR
(n). The innovation term combines the
power output of an aerobic effort P(n) with the differ-
ence between the cardiac anaerobic threshold HR
AT
and the heart rate at rest HR
rest
(n).
HR
ATR
(n) = α
P
[HR
AT
− HR
rest
(n)]P(n) (4)
The main goal of our model is to obtain a rough es-
timation of the heart rate in order to discard non-
plausible instantaneous estimations. In this sense,
to the various unknown parameters α
P
, α
act
, α
recov
and HR
AT
of our model, we apply commonly utilized
sport physiological values (Noakes, 2003). These
values are then updated during the use of the device
so as to correspond to a user’s specific profile.
2.4.5 HR Reliability
Sporadic motion artifacts augment the number of dis-
carded marginal values, reducing the reliability of the
estimated HR. For this reason, together with the esti-
mated HR, the user is provided with a HR reliability
index defined as
HR
rel
(n) =
N
IBI,∆T
(n)
∆T · µHR
∆T
(n)
(5)
where ∆T is the window length multiple of the sam-
pling time, N
IBI,∆T
(n) and µHR
∆T
(n) are respectively
the number of valid IBIs and the expected mean HR
at the sample n over the time span ∆T.
3 RESULTS
In order to validate the developed estimation tech-
nique, nine subjects were requested to follow an ad-
equate experimental protocol. Subjects were asked
to carry out their daily normal office work for a to-
tal time length comprised between 1 to 2 hours and
to carry out 2 to 3 high intensity activities lasting ap-
proximately 10 to 20 seconds. In addition to the de-
veloped ear located heart rate device, subjects were
equipped with a commercial chest-strap based heart
rate monitor (POLAR, RS800) employed as a refer-
ence.
To illustrate the performances of the proposed algo-
rithm, we present quantitative and qualitative results
comparing the heart rate estimated by the proposed
method to the POLAR reference. The most com-
monly applied quantitative assessments are based on
the Mean Absolute Relative Error (MARE) and Mean
Absolute Error (MAE):
MARE = 100
1
N
N
∑
n=1
ˆ
HR(n) − HR
polar
(n)
HR
polar
(n)
(6)
MAE =
1
N
N
∑
n=1
ˆ
HR(n) − HR
polar
(n)
(7)
Furthermore, we compute a mean reliability index
(R
HR
) associated to each measure. R
HR
is obtained
by adding all the HR reliabilities greater than 0.5 and
normalizing by the measurement length N, where HR
ROBUST EAR LOCATED HEART RATE MONITOR
217
reliabilities have been previously defined in equation
5.
R
HR
= 100
1
N
N
∑
n=1
(HR
rel
(n) > 0.5) (8)
The promising performances relative to the entire
database are presented in Table 1. Indeed, for the
whole database, the mean µ and standard deviation σ
associated to the MARE are 0.9 and 0.6 respectively.
It is important to observe that the objective of this
validation is to demonstrate the ability of our device
to accurately estimate the heart rate under baseline
resting conditions without concentrating on heart rate
variabilities (HRVs) (of the European Society of Car-
diology et al., 1996). For this reason, we filtered the
heart rate provided by the POLAR to eliminate HRVs
above 0.04 Hz. Therefore, HRVs associated to the
sympathetic and parasympathetic nervous system are
not retained in the validation.
Table 1: Mean Absolute Relative error (MARE), Mean Ab-
solute Error (MAE), and reliability for 9 subjects in baseline
conditions with intermittent sporadic activities.
Subject MARE [%] MAE [bpm] R
HR
[%]
1 0.4 0.3 98
2 0.3 0.6 95
3 1.2 1.0 92
4 1.2 0.8 94
5 0.6 0.8 94
6 1.4 1.0 90
7 0.7 0.5 99
8 2.8 2.4 93
9 1.6 0.9 93
µ 0.9 1.1 94
σ 0.6 0.8 2.8
Although the MARE and MAE provide an infor-
mation about the average estimation performance of
the developed device when compared to the POLAR
RS800, they both only partially describe the impres-
sion perceivedby the user. Indeed, the estimation per-
formance may be excellent for long periods despite
being erroneous for some short time intervals. There-
fore, to take into account this temporal variation, we
also apply an analysis of the specific threshold sensi-
tivity, namely, an assessment of the performance as
the percentage of time where the absolute value of
the error is lower than a given threshold κ. The spe-
cific threshold sensitivity of the algorithm is presented
in Table 2. The percentage of time where the error
in heart rate estimation is lower than the indicated
threshold κ highlights that low sensitivities are ob-
tained for a threshold of 1% and 1bpm respectively.
However, since the accuracy of the reference heart
rate monitor is about ±1%, the analysis may not be
Table 2: Percentage of time where the error in heart rate
estimation is lower than the indicated threshold κ .
κ
% bpm
Subject 1 3 5 1 3 5
1 92 99 100 95 100 100
2 90 95 97 93 97 99
3 56 88 96 62 90 97
4 68 89 95 76 92 97
5 74 94 98 82 97 99
6 48 85 94 62 91 97
7 77 98 100 84 99 100
8 33 68 83 46 80 91
9 62 83 92 72 92 97
µ 81 90 95 75 93 97
σ 19 10 5 16 6 3
of high statistical relevance. A validation with such
an accuracy should be performed using medical refer-
ence devices. In contrast, sensitivities for a threshold
of 3% and 5% are above or equal to 90%.
Figure 4 illustrates qualitative performances for
the first subject in the database. One can observe the
high accuracy of the proposed method. Notice that
at the beginning of the recording, the IR signal qual-
ity was insufficient to provide a valid heart rate esti-
mation because the user was unable to properly ad-
just the ear located sensor. However, once the sen-
sor placement strategy is successfully achieved, heart
rate estimation converged as confirmed by a MARE
of 0.4% and relative heart rate estimation error lower
than 1bpm for 99% of the time (see subject number 1
of Table 1 and Table 2).
In Figure 5 we depict the result relative to subject
8. A closer visual inspection on the data highlights
that there are mainly three segments where the results
of the POLAR and of our device diverged. To illus-
trate, we observe two segments at the beginning of the
recording where the heart rate estimated by POLAR
is about 170 bpm despite weak accelerometer signals
corroborating that the subject was in a resting posi-
tion. This unlikely estimation may be the result of
an incorrect manipulation of the chest strap reference
device such as for instance an insufficient humidifica-
tion. Finally, for the third segment at about 1300 sec-
onds where we notice a difference between the two
heart rate estimates there is an insufficient reliability
HR
rel(n)
of the heart rate provided by our device.
BIODEVICES 2009 - International Conference on Biomedical Electronics and Devices
218
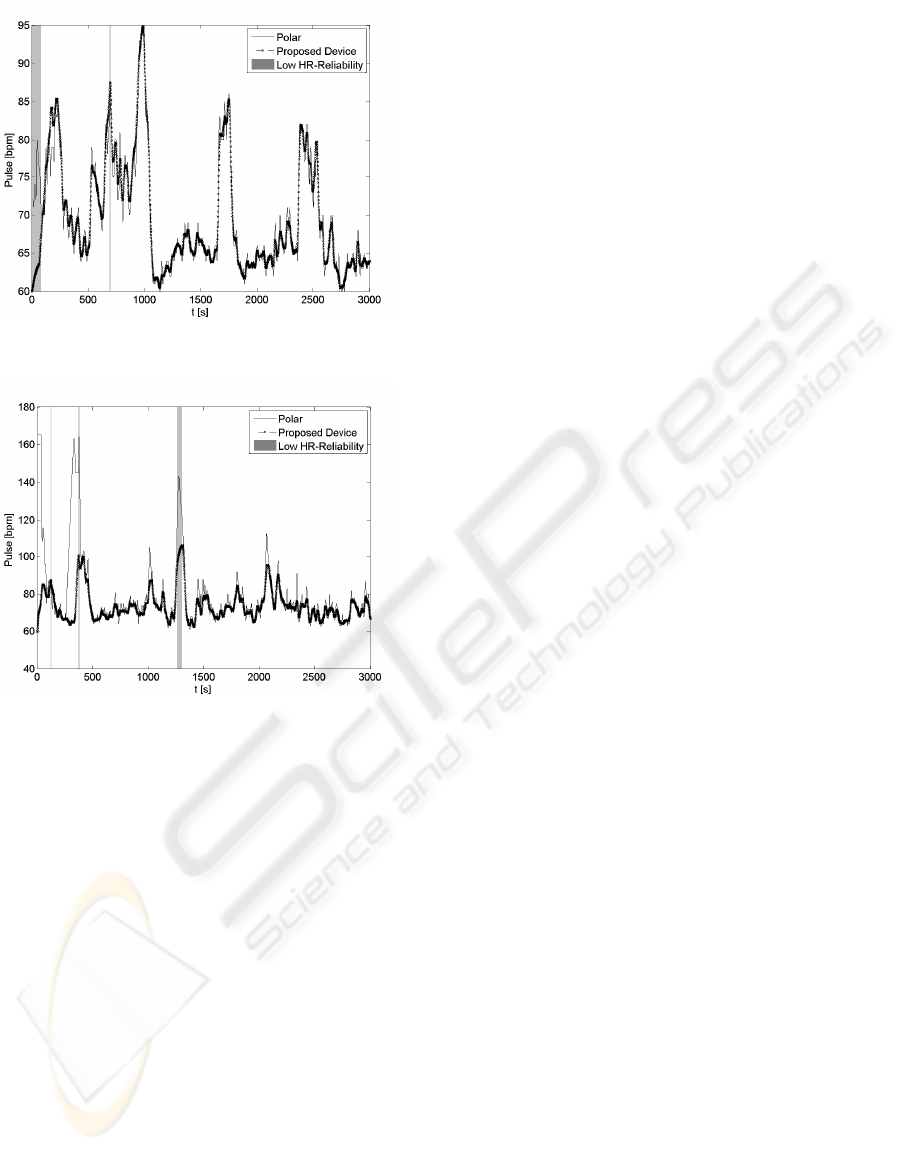
Figure 4: Heart rate estimation for subject 1 in baseline
condition with sporadic intermittent activities using the pro-
posed algorithm.
Figure 5: Heart rate estimation for subject 8 in baseline con-
dition with sporadic intermittent activities.
4 CONCLUSIONS
In this paper we have presented an innovative ap-
proach for heart rate estimation using infrared opti-
cal measurements of the tissue at the ear lobe. The
sensing device integrated in a classical audio player
earphone improves the user’s comfort with respect to
commonly used chest-strap based heart rate monitors.
The challenge connected to statistically undetectable
inter-beat during short term sporadic activities has
been approached using a novel robust inter-beat dis-
carding method. The latter incorporates a simple car-
diovascular dynamic model to reduce related inaccu-
racies in the heart rate estimation. Nine subjects were
asked to participate in the validation protocol of the
proposed device. The validation resulted in positive
performances with an absolute relative error mean of
0.9% and a threshold sensitivity above 90% relative
to the chest-strap heart reference monitor.
REFERENCES
Celka, P., Verjus, C., Vetter, R., Renevey, P., and Neuman,
V. (2004). Motion resistant earphone locatedinfrared
based heart rate measurement device. In IASTED Con-
ference on Biomedical Engineering (BioMED 2004),
pages 582–585.
Cheong, W.-F., Prahl, S., and Welch, A. (1990). A review of
the optical properties of bilogical tissues. IEEE Jour-
nal Quantum Electronic, 26:2166–2185.
Coetzee, F. M. and Elghazzawi, Z. (2000). Noise-resistant
oximetry using a synthetic reference signal. IEEE
Trans. on Biomedical Engineering, 47(8):1018–1026.
Elliott, D., Carr, S., and Omre, D. (2005). The effect of mo-
tivational music on sub-maximal exercise. European
Journal of Sport Medicine, 5:97–106.
Gersho, A. and Gray, R. (1992). Vector Quantization and
Signal Compression. Kluwer Academic Publishers.
Haykin, S. (1991). Adaptive Filter Theory. Prentice Hall.
Le, A., Jaitner, T., Tobias, F., and Litz, L. (2008).
A dynamic heart rate prediction model for train-
ing optimization in cycling. In The Engineer-
ing of Sport(ISEA2008), volume 1, pages 425–433.
Springer.
Noakes, T. (2003). Lore of Running. Ed. Champaign.
of the European Society of Cardiology, T. F., the North
American Society of Pacing, and Electrophysiology
(1996). Heart rate variability - standards of measure-
ment, physiological interpretation, and clinical use.
Circ., 93:1043–1063.
Renevey, P., Vetter, R., Krauss, J., Celka, P., and De-
peursinge, Y. (2001). Wrist-located pulse detectionus-
ing ir signals, activity and nonlinear artifact cancella-
tion. In IEEE EMBS Conference.
Schie, N., A.Stuart, Becker, P., and Rogers, G. (2008). Ef-
fect of music on sub-maximal cycling. South African
Journal of Sport Medicine, 20:28–31.
Tremper, K. and Barker, S. (1989). Pulse oximetry. Anes-
thesiology, 70:98–108.
Trivedi, N., Ghouri, A., Shah, N., Lai, E., and Barker, S.
(1997). Effect of motion, ambient light, and hypoper-
fusion on pulse oximeter function. Journal of Clinical
Anesthesia, 9:179–183.
Vaseghi, S. (1996). Advanced signal processing and digital
noise reduction. Wiley Teubner.
Verjus, C., Vetter, R., Celka, P., and Renevey, P. (2003).
Equipement portable destine a la mesure et/ou la
surveillance de la frequence cardiaque. In European
Patent EP 1 374 763, US Patent 7175601.
Vetter, R., Onillon, E., and Bertschi, M. (2008). Estimation
of a runner’s speed based on chest-belt integrated iner-
tial sensors. In The Engineering of Sport(ISEA2008),
volume 1, pages 151–159. Springer.
Webster, J. (1997). Design of pulse oximeters. IoP Publish-
ing.
ROBUST EAR LOCATED HEART RATE MONITOR
219
