
SUBSET SELECTION OF MYOELECTRIC CHANNELS
A Genetic Algorithm for Subset Selection of Myoelectric Channels for Patients
following TMR Surgery
Gernot Kvas
Otto Bock Healthcare GmbH, Vienna, Austria
Rosemarie Velik
Institute of Computer Technology, Vienna University, Vienna, Austria
Keywords:
Electromyogram, Pattern Recognition, Genetic Algorithm.
Abstract:
State of the art self powered prostheses make use of the surface myoelectric signal for motor control. With
increasing height of the amputation, control by residual muscles becomes less intuitive and physiologic. Tar-
geted muscle reinnervation (TMR), a surgery technique to increase the number of control sites available in
combination with multichannel surface electromyography allows for improved prosthesis control. This paper
presents a genetic algorithm that determines a channel subset with high classification accuracy out of a given
number of channels recorded from the reinnervated area of a patient.
1 INTRODUCTION
Current generations of self powered artificial limbs
for the upper extremity are controlled by electromyo-
graphic signals recorded from the surface of the skin
(Scott and Parker, 1988). Despite ongoing research
for more advanced neural interfaces (Navarro et al.,
2005), the surface myoelectric signal (MES) is cur-
rently the preferred way for estimating muscle activ-
ity of residual muscles. Commercially available pros-
theses systems are typically comprised of three logi-
cal building blocks, the pickup electrodes, a controller
and the mechanical arm itself. Two bipolar electrodes
are placed on synergist and antagonist muscles of the
residual limb where myoelectric activity is recorded,
amplified and fed to the controller for further process-
ing. The controller is responsible for translating mus-
cle activity in joint movement. A common method is
to rectify and low pass filter the myoelectric signal to
get an estimation for the mean absolute value (MAV).
1.1 Control Methods
Depending on the height of the amputation, available
prostheses systems may offer up to three active de-
grees of freedom. Control is usually achieved by hav-
ing as manystates as available degrees of freedom and
controlling each individual degree of freedom with
the same pair of electrodes at a time. State transi-
tion is achieved by applying a special signal, e. g.
a simultaneous contraction of synergist and antago-
nist muscles. The limited information of the mean
absolute value and a single pair of electrodes led to
research on pattern recognition methods to make use
of further information contained in the MES, as han-
dling more degrees of freedom becomes cumbersome
with the above mentioned method.
Features derived from the MES may be grouped in
time-domain, frequency domain and time-frequency
methods. Features calculated in the time domain are
e. g. waveform length, zero crossings and slope sign
changes, whereas time frequency methods may com-
prise the Fourier transform or wavelet transforms (En-
glehart et al., 1999). For each channel one or more
feature is calculated to form a feature vector. Feature
vectors recorded for each movement class are either
directly used for training a classifier or dimensional-
ity reduction (Chu et al., 2005) is applied to decorre-
late features. Different classification algorithms have
been deemed suitable for classification of myoelectric
signals (Huang et al., 2005).
222
Kvas G. and Velik R. (2009).
SUBSET SELECTION OF MYOELECTRIC CHANNELS - A Genetic Algorithm for Subset Selection of Myoelectric Channels for Patients Following TMR
Surgery.
In Proceedings of the International Conference on Bio-inspired Systems and Signal Processing, pages 222-226
DOI: 10.5220/0001433602220226
Copyright
c
SciTePress

1.2 Targeted Muscle Reinnervation
Classification algorithms allow for better discrimina-
tion of movements than traditional methods, but still
require enough control sites. For transradial amputa-
tions, sufficient muscles in the residual limb remain
to allow classification of different movements (Aji-
boye and Weir, 2005). For shoulder disarticulation
amputees however, no residual muscle from the arm
may be used for control sites, thus leading to non-
physiologic control of the prosthesis.
Kuiken et al. (Kuiken, 2006) identified this prob-
lem and developed the surgery method of targeted
muscle reinnervation (TMR) to create additional my-
oelectric control signals. After amputation, nerves
once supplying the limb remain unused. Similarly,
muscles serve no function due to the missing limb,
such as the m. pectoralis major which provides ad-
duction and anteversion of an intact arm. Targeted
muscle reinnervation surgically connects the nerves
of the missing limb to these otherwise unused mus-
cles in order to create additional myoelectric control
sites. Muscles serveas natural amplifiers for the nerve
signals and known methods of surface electromyogra-
phy can be used to record control signals.
1.3 Problem Setting
Whereas nerve muscle mappings in the residual mus-
cles are clearly known after amputation, the outcome
of targeted muscle reinnervation varies from subject
to subject. Zhou et. al (Zhou et al., 2005) used high
density recordings of 127 monopolar electrodes to
identify regions of activity. Huang et al. (Huang et
al., 2008 ) applied an algorithm based on the sequen-
tial forward search method (Somol et al., 1999) to find
a limited number of electrodes that contain most neu-
ral information.
This work proposes the application of a genetic al-
gorithm to find a channel subset that maximizes the
classification accuracy of a given number of chan-
nels. Previous work using principal component anal-
ysis (PCA) indicating that out of 24 bipolar elec-
trode channels, a much smaller number sufficed to
maintain high classification accuracy (Kvas and Ve-
lik, 2008). Bipolar electrodes were chosen, because
they are more clinically relevant as this type is used
for prosthesis control. The PCA based method would
act as a filter method and is thus computationally
cheap. This work extends the idea of subset selection
to a wrapper approach using a genetic algorithm for
improved classification accuracy. The genetic algo-
rithm presented utilizes the output from the classifier
trained on patient data as a fitness function to find the
near-optimal subset for a given number of channels.
A genetic algorithm was chosen over other heuristic
search algorithms due to easy mapping of the problem
domain to chromosom encoding and the possibility to
limit the search to a given number of channels using
“channel-constrained” genetic operators.
2 ALGORITHM
Genetic algorithms use principles of evolution to
solve amongst others optimisation problems. A ge-
netic algorithm operates on chromosomes, bit strings
with elements being either ’0’ and ’1’. Each chro-
mosome represents one possible solution within the
search space. A fitness function determines how well
a chromosome solves the problem at hand. Given
a population of chromosomes, a fitness score is as-
signed to each individual chromosome and genetic
operators are applied. Depending on preset rules,
chromosomes are subjected to crossover and muta-
tion, forming the base for a new generation of the
population.
The presented algorithm is based on these build-
ing blocks for genetic algorithms. Slight adaptions
are made to accommodate for the problem of channel
subset selection.
Chromosome Encoding. In each chromosome,
a bit ’1’ denotes an active channel, whereas ’0’
consequently denotes deactivation of this channel.
As the algorithm takes the number of channels n
to maximize classification accuracy as an input
argument, only n bits can be ’1’ at any given time for
the algorithm. This constraint requires modification
of the crossover and mutation operations.
Fitness Function. The fitness function is fundamen-
tal to each genetic algorithm and needs to be specific
for the given problem. In case for the channel subset
selection, the fitness function is defined as
fitness =
1
classes
classes
∑
i=1
featvec
correct,i
featvec
total,i
(1)
with featvec
correct
being the number of feature
vectors classified correctly out of all feature vectors
featvec
total
for a given class i.
Selection. Roulette wheel selection (De Jong, 1975)
was used to select parents for mating out of the entire
population.
Crossover. Several approaches to crossover exist,
most notably single point and multi point crossover.
SUBSET SELECTION OF MYOELECTRIC CHANNELS - A Genetic Algorithm for Subset Selection of Myoelectric
Channels for Patients Following TMR Surgery
223
For the algorithm at hand, neither of the above
method was implemented, but a simpler merge
crossover to meet the constraint of a constant number
of channels. Given two chromosomes, an offspring
was generated by merging both into a new chromo-
some. For the likely case that both parents are not
exact copies of each other, the resulting offspring has
more active channels than allowed by the constraint
of a given channel number. Consequently, channels
are deactivated at random until the constraint is met.
Mutation. Mutation is achieved by flipping a random
single bit of a chromosome. Again, to meet the
constraint of a fixed number of channels, a bit flipped
from 1 to 0 requires another random bit to be flipped
from 0 to 1 and vice versa.
Algorithm Pseudo Code
Algorithm 1: Pseudo Code.
input : Feature training set feat
train
Feature test set feat
test
Size of Subset n
Total Number of Channels tot
Crossover probability p
co
Mutation probability p
mu
Max number of iterations gen
max
Size of population pop
max
output: The subset yielding best performance
pop ←
InitPopulation(
pop
max
)
;
fit ←
Fitness(
feat
train
, feat
test
, pop
)
;
for gen
cur
← 1 to gen
max
do
parents ←
RouletteWheel(
fit, pop
)
;
foreach two parents par do
if
Random
> p
co
then
offspring ←
Crossover(
par
)
;
foreach offspring do
if
Random
> p
mu
then
offspring ←
Mutation(
offspring
)
;
fit ←
Fitness(
feat
train
, feat
test
,
offspring
)
;
Reinsertion(
pop, offspring, fit
)
;
Algorithm Run-Time. An exhaustive search to try
all subsets of size n within tot channels gives a total
of
invocations =
tot
n
(2)
subsets. For the worst case of 12 channels out
of 24, this would require 2704156 invocations of the
Fitness function. For the genetic algorithm however,
the maximum number of invocations is a function of
pop
max
, gen
max
and the number of parents nr
par
:
invocations
ga
= pop
max
+ gen
max
× nr
par
(3)
This can be easily seen, as for the initial run, the
fitness of every chromosome has to be determined.
For all subsequent runs, for every new generation a
maximum of nr
par
offspring are created, thus requir-
ing their fitness to be evaluated. For our problem
at hand, if the mean population fitness was within
0.01% of the maximum fitness, the algorithm would
exit early due to convergence. Table 1 shows the con-
vergence ratio for each of the datasets.
Table 1: Convergence Ratio.
Dataset Runs Converged Ratio
“A” 575 522 90.8%
“B” 575 503 87.5%
“C” 575 518 90.1%
3 METHODOLOGY
To test the suggested algorithm, surface myoelectric
signals from a patient who has undergone targeted
muscle reinnervation surgery were recorded. Surgery
was performed on the shoulder disarticulation side of
the patient at the Vienna General Hospital in Decem-
ber 2006 (Aszmann et al., 2008). This is so far the
only surgery performed in Europe, with further pa-
tients in the United States (Miller et al., 2008).
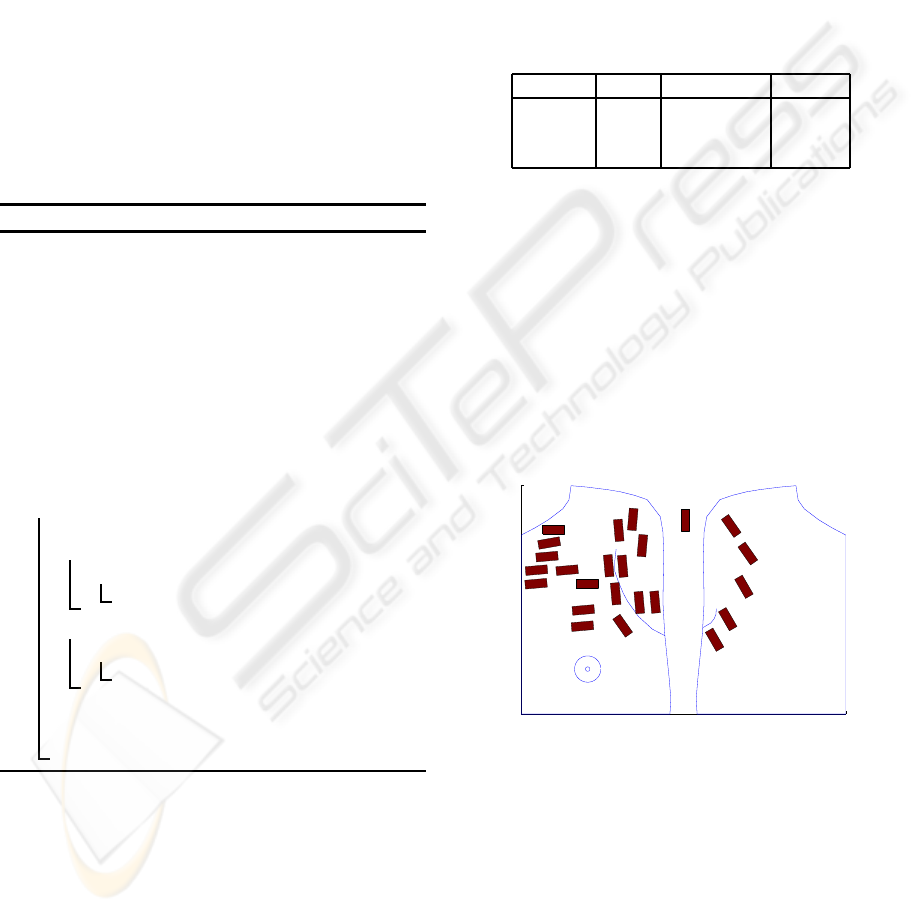
Anterior View Posterior View
Figure 1: Two dimensional sketch of the electrode place-
ment for 24 electrodes. The electrode floating between both
views of the body was placed on the m. deltoideus.
A total of 24 active bipolar electrodes where
placed on the patient following initial palpation of
muscle regions and additional measurements with ul-
trasound to determine muscle fiber orientation. Elec-
trodes where placed over reinnervated areas in the
chest and back region as indicated by Figure 1. Sig-
nals were amplified on the electrode with a gain
of 40 dB and further filtering and amplification was
achieved using a custom signal conditioning board.
BIOSIGNALS 2009 - International Conference on Bio-inspired Systems and Signal Processing
224
Signals were digitized using a National Instruments
USB-6259 board at a sample frequency of 3kHz.
Time domain features were calculated from non-
overlapping blocks of 512 samples. For each channel,
zero crossings, waveform length, slope sign changes
and the RMS for were calculated. Features were then
concatenated to form a feature vector. Classification
for determining fitness was carried out using a linear
discriminant analysis (LDA) classifier. As the genetic
algorithm requires a high number of training and clas-
sification invocations, a linear classifier is chosen due
to the lower computational requirements as opposed
to multilayer perceptrons or support vector machines.
The genetic algorithm was exercised on data
recorded over the last months. A session with the pa-
tient would typically involve a list of movements the
patient had to perform with the phantom arm. Each
movement would be demonstrated by the physiother-
apist. The patient would then try to perform the same
movement with the phantom arm. The patient was
instructed to hold each movement at convenient con-
traction strength for approximately 10 seconds.
4 EXPERIMENTAL RESULTS
To test the algorithm, data recorded from a single pa-
tient available for this study was used. Each session
consisted of 12 movements. No visual feedback was
given to the patient while performing the movements.
Table 2 showsthat number of available feature vectors
per session.
Table 2: Feature vectors.
Dataset Feature Vectors Classes
“A” 1599 12
“B” 1878 12
“C” 1686 12
The algorithm was initialized with a population
size of 50 with crossover and mutation probabilities
of 0.7 and 0.1, respectively. The maximum number
of generations was set to 500. Subsets for 1 to 23
of the total of 24 channels were calculated. For each
subset, nested 5-fold cross-validation was performed,
such that classification accuracy could be indepently
assessed from channel selection.
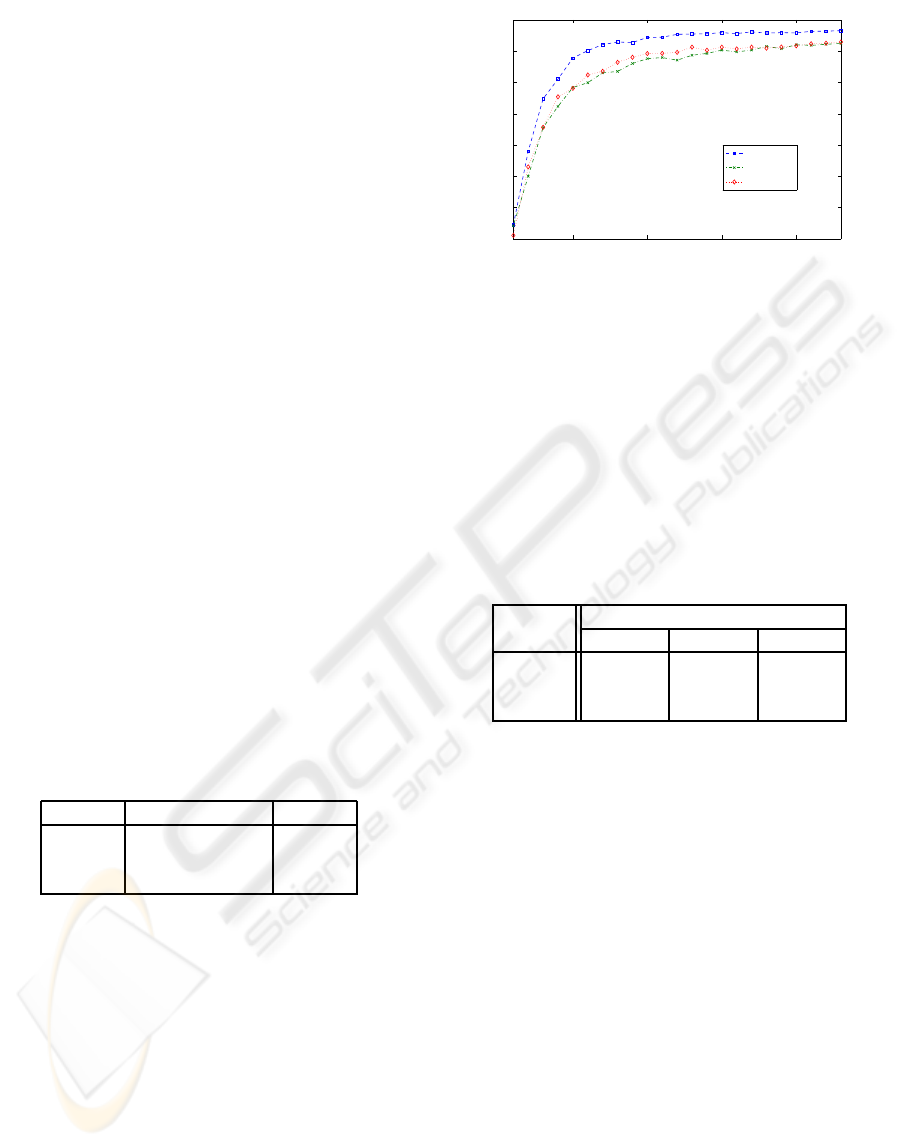
Figure 2 shows the classification accuracy for each
of the subsets determined by the genetic algorithm for
subset sizes of 1 to 23. Classification accuracy in-
creases from 34.4% for one channel to 96.57% for
23 channels for dataset A and from 34.14% for one
channel to 92.48% for 23 channels for dataset B.
The figure further shows that classification accuracy
5 10 15 20
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1
Channels
Classification Accuracy
Dataset A
Dataset B
Dataset C
Figure 2: Maximum classification accuracy for the fittest
channel subsets determined by the genetic algorithm for
datasets “A”, “B” and “C”.
is quickly increasing for the first half of the channels
(Dataset A: From 34.4% to 95.39 %), but on slightly
increasing for the second half of channel subsets. This
shows that high classification accuracy can be main-
tained with a substanstially smaller set of electrodes.
Table 3 gives an overview over classification accura-
cies for a selected number of channels.
Table 3: Classification accuracy for the specified number of
channels for each dataset.
Channels
Dataset 1 12 23
“A” 34.43% 95.39% 96.57%
“B” 34.14 % 87.12% 92.48%
“C” 31.03 % 89.93% 92.94%
Figure 3 shows the final configurations of 12 se-
lected electrodes averaged over 5 nested cross valida-
tion loops for dataset “A”. Here, darker electrodes in-
dicate multiple selections by each of the five subsets.
White electrodes indicate no selection. When com-
pared to the position of the nerve crafts, the electrode
configuration determined by the classifier is in accor-
dance with the expected outcome of the targeted mus-
cle reinnervation surgery for this patient. Electrodes
are selected in areas of high neural activity. Poste-
rior, electrodes are selected in areas with reinnerva-
tion by n. radialis and both non reinnervated parts of
the shoulder, accounting for muscle activity for cer-
tain shoulder-complex related muscle movements.
5 CONCLUSIONS
A genetic algorithm was used to find an near-optimal
subset of electrodes out of a larger number of chan-
nels. The results show that the number of electrodes
can be successfully reduced given the current setup
SUBSET SELECTION OF MYOELECTRIC CHANNELS - A Genetic Algorithm for Subset Selection of Myoelectric
Channels for Patients Following TMR Surgery
225

Anterior View Posterior View
Figure 3: Channels selected by the algorithm for 12
electrodes. Results have been averaged over five cross-
validation loops for the specified amount of channels. Not
all loop iterations have selected the same electrodes, but a
strong bias towards certain prefered channels can be ob-
served (Darker Electrodes).
of channels. The channels that are selected also map
very well to the areas where reinnervation was ex-
pected after surgery for this particular patient. How-
ever, more data from both this patient and additional
patients is needed to further confirm the results. Fu-
ture work will also be directed towards evaluating
these results using online realtime data.
This result is primarily important as an indication
for prosthesis fitting were a smaller number of sur-
face electrodes allows for easier fitting and far simpler
socket construction. A limited number of channels
also reduces hardware requirements and lessens the
computational burden on the myoelectric controller.
ACKNOWLEDGEMENTS
The authors would like to thank Univ.-Prof. Man-
fred Frey, Univ.-Prof. Oskar C. Aszmann, Univ.-Prof.
Tatjana Paternostro-Sluga (Vienna General Hospi-
tal) and physiotherapist Heidelinde Amon-Aspalter
(PhysioLeoben) for providing invaluable assistance
when working with the patient. The authors would
like to thank Dr. Roland Pawlik and Dr. Hubert Eg-
ger for project coordination and Dr. Hans Dietl for
making this research possible.
REFERENCES
Ajiboye, A.B. and Weir(2005). A Heuristic Fuzzy
Logic Approach to Emg Pattern Recognition for
Multifunctional Prosthesis Control. Neural Systems
and Rehabilitation Engineering, IEEE Transactions
on,13(3):280291.
Aszmann, O. C., Dietl, H., and Frey,M.(2008). Selective
nerve Transfers to Improve the Control of Myoelec-
trical Arm Prostheses. Handchirurgie Mikrochirurgie
Plas-tische Chirurgie,40(01):6065.
Chu, J.-U., Moon, I., and Mun, M.-S.(2005). Areal-time
emg pattern recognition based on linear-nonlinear fea-
ture projection for multifunction myoelectric hand.
In 9th International Conference on Rehabilitation
Robotics, pages 295298.
De Jong, K. A. (1975). An Analysis of the Behavior of a
Class of Genetic Adaptive Systems. PhD thesis.
Englehart, K., Hudgins, B., Parker, P. A., and Steven-
son, M.(1999). Classification of the myoelectric signal
using time-frequency based representations. Medical
Engineering & Physics, 21(6-7):431438.
Huang, H., Zhou, P., Li, G., and Kuiken, T.A.(2008). An
Analysis of Emg Electrode Configuration for Targeted
Muscle Reinnervation Based Neural Machine Inter-
face. Neural Systems and Rehabilitation Engineer-
ing,IEEE Transactions on,16(1):3745.
Huang, Y., Englehart, K. B., Hudgins, B., and Chan, A.
D. C.(2005). A gaussian mixture model based clas-
sification scheme for myoelectric control of powered
upper limb prostheses. Biomedical Engineering,IEEE
Transactions on,52(11):18011811.
Kuiken, T.(2006).Targeted reinnervation for improved
prosthetic function. Phys Med Rehabil Clin N
Am,17(1):113
Kvas, G. and Velik, R.(2008). A filter approach for myo-
electric channel selection. In Industrial Informatics,
2008. INDIN 2008. 6th IEEE International Confer-
ence on, pages 14371440.
Miller, L. A., Stubblefield, K. A., Lipschutz, R. D., Lock,
B. A., and Kuiken, T. A.(2008). Improved myoelec-
tric Prosthesis Control using Targeted reinnervation
Surgery:Acaseseries. Neural Systems and Rehabilita-
tion Engineering,IEEE Transactions on,16(1):4650.
Navarro, X., Krueger, T. B., Lago, N., Micera, S., Stieglitz,
T., and Dario, P.(2005). A critical review of interfaces
with the peripheral nervous system for the control of
neuroprostheses and hybrid bionic systems. Journal of
the Peripheral Nervous System,10(3):229258.
Scott, R. N. and Parker, P. A.(1988). Myoelectric prosthe-
ses: State of the Art. Journal of Medical Engineering
& Technology, 12(4):143151.
Somol, P., Pudil, P., Novovicova, J., and Paclik, P.(1999).
Adaptive Floating search Methods in feature Selec-
tion. Pattern Recogn. Lett.,20(11-13):11571163.
Zhou, P., Lowery, M. M., Dewald, J. P. A., and Kuiken,
T. A.(2005). Towards improved myoelectric prosthe-
sis control: High density surface emg recording after
targeted muscle reinnervation. pages 40644067.
BIOSIGNALS 2009 - International Conference on Bio-inspired Systems and Signal Processing
226
