
NOVEL SENSOR TECHNOLOGY INTEGRATION
FOR OUTCOME-BASED RISK ANALYSIS IN DIABETES
Mahesh Subramanian
1
, Edward C. Conley
1,2
,
Omer F. Rana
1
, Alex Hardisty
1
, Ali Shaikh Ali
1
Stephen Luzio
2
, David R. Owens
2
1
The Welsh e-Science Centre, Cardiff University School of Computer Science
5 The Parade, Roath, Cardiff, UK, CF24 3AA and
2
Diabetes Research Unit, Cardiff University School of Medicine
Academic Centre, Llandough Hospital, Penlan Road, Penarth, Cardiff CF64 2XX, UK
Steve Wright, Tim Donovan, Bharat Bedi, Dave Conway-Jones, David Vyvyan, Gillian Arnold
IBM United Kingdom Ltd, Hursley House, Hursley Park, Winchester, UK, SO21 2JN
Chris Creasey, Adrian Horgan, Tristram Cox
Smart Holograms, 291 Cambridge Science Park, Milton Road, Milton, Cambridge, UK, CB4 0WF
Rhys Waite
Zarlink Semiconductor Ltd, Phase 2 Mitel Business Park, Caldicot, Monmouthshire,UK, CF26 5YW
Keywords: Health informatics, home healthcare, biomedical sensor devices, mobility, wearable sensors, decision
support system, individualised risk analysis.
Abstract: Novel sensor-based continuous biomedical monitoring technologies have a major role in chronic disease
management for early detection and prevention of known adverse trends. In the future, a diversity of
physiological, biochemical and mechanical sensing principles will be available through sensor device
‘ecosystems’. In anticipation of these sensor-based ecosystems, we have developed Healthcare@Home
(HH) - a research-phase generic intervention-outcome monitoring framework. HH incorporates a closed-
loop intervention effect analysis engine to evaluate the relevance of measured (sensor) input variables to
system-defined outcomes. HH offers real-world sensor type validation by evaluating the degree to which
sensor-derived variables are relevant to the predicted outcome. This ‘index of relevance’ is essential where
clinical decision support applications depend on sensor inputs. HH can help determine system-integrated
cost-utility ratios of bespoke sensor families within defined applications – taking into account critical
factors like device robustness / reliability / reproducibility, mobility / interoperability, authentication /
security and scalability / usability. Through examples of hardware / software technologies incorporated in
the HH end-to-end monitoring system, this paper discusses aspects of novel sensor technology integration
for outcome-based risk analysis in diabetes.
1 INTRODUCTION
Continuous sensor-based monitoring technologies
are central to new models of ‘proactive’ health and
social care. In healthcare, ‘proactive’ implies a shift
away from ‘reactive’ care – i.e. an ‘illness-centric’
model where interventions are made following
presentation of symptoms or complications. The
‘proactive’ model embraces the World Health
Organisation’s (WHO) definition of health as “a
state of complete physical, mental and social well-
being and not merely the absence of disease or
infirmity” (WHO, 2007). To move towards this
visionary goal, individuals need realistic
119
Subramanian M., C. Conley E., F. Rana O., Hardisty A., Shaikh A., Luzio S., R. Owens D., Wright S., Donovan T., Bedi B., Conway-Jones D., Vyvyan D.,
Arnold G., Creasey C., Horgan A., Cox T. and Waite R. (2008).
NOVEL SENSOR TECHNOLOGY INTEGRATION FOR OUTCOME-BASED RISK ANALYSIS IN DIABETES.
In Proceedings of the First International Conference on Health Informatics, pages 119-126
Copyright
c
SciTePress

opportunities to improve and sustain their health and
quality of life thus contributing to their own well-
being. The availability of validated, relevant and
ubiquitous personal healthcare information to
minimise risk of predictable adverse events can
empower and incentivise individuals to adopt more
healthy lifestyles. Such technology can assist care
throughout the ‘patient path’ (Abidi, 2001;
Shnayder, 2005). Arising from these technology
developments are significant ethical issues - e.g. in
personal data protection and in establishing ethical
authority for personal data reuse. We will discuss
these issues in detail elsewhere in the context of our
Healthcare@Home (HH) project - a research-phase
generic intervention-outcome monitoring framework
that integrates sensor-based technology as part of a
disease early detection and prevention framework.
It is widely anticipated that future health
information systems (HIS) will need to move from
“institution-based” models to those that rely on ‘near
real time’ data integration close to the patient.
Interventions that use ethical risk stratification as
part of a personal data integration framework is a
priority in diabetes, where the number of affected
individuals is predicted to rise from c.135 million
people in 1995 to c.300 million in 2025 (King,
1998). All people with unmanaged diabetes are at
substantially increased risk of serious medical
complications such as retinopathy, kidney failure
and peripheral neuropathy requiring limb
amputation. As part of an individual’s personal
information management, the HH closed-loop model
uses sensor-based trends to compute and stratify risk
in a time frame and operational workflow that is
meaningful and in a format that can be utilised for
building decision support services (DSS). The DSS
model in HH is founded on requirements of the
Diabetes National Service Framework (NSF)
standards for Wales and associated integrated care
pathways (ICP). Section 2 summarises relevant
related work. Section 3 describes technical aspects
of the end-to-end HH system covering (1) smart
sensors; (2) biometric authentication; (3) ‘home hub’
and (4) server-side architecture. Section 4 discusses
concerns arising out of the project and possible
future work with a conclusion in Section 5.
2 RELATED WORK
A healthcare technology platform utilising sensor
devices can underpin comprehensive monitoring
services outside of the hospital environment. This
could support new ways of working that: (1) places
less reliance on frequent clinical visits – subject to
quality control / calibration safeguards and adequate
clinical ‘baseline’ data (2) can incentivise patients to
‘look after themselves’ with realistic (achievable)
personal guidelines within manageable episodes of
care; (3) allows team-based caseload sharing
between clinical visits to monitor progress and make
escalation procedures robust (4) provides for the
development of consistent risk prediction /
longitudinal ‘outcome recording’ methodologies that
are fit-for-purpose in scaleable evidence-based
models (Williams, 2003; Conley, 2007). Several
research projects address the issue of integrated care
through the use of ubiquitous computing devices.
SAPHIRE (Hein 2006) is concerned with
developing a healthcare monitoring and decision
support system for cardiovascular disorders, assisted
by wireless sensor devices in home settings.
(Clemensen 2004) applied pervasive computing
devices to the treatment / monitoring of diabetic foot
ulcers. The CODEBLUE project (Lorincz, 2004) is
typical of sensor device applications in medical
emergency scenarios. In this project, micro-scale
sensor devices (motes) (Crossbow, 2007) have been
used to continuously monitor and wirelessly transmit
vital sign data (e.g. heart rate and oxygen saturation
data) to a data hub for processing. The CART
(continuous automated real-time triage) system,
developed by Advanced Health and Disaster Aid
Network (AID-N) builds on the work carried out by
the CODEBLUE team. A wearable tag has been
developed which performs the following functions:
triage, status display, vital signs monitoring, location
tracking, information display and alarm signalling
(Gao, 2006). The Smart and Aware Pervasive
Healthcare Environment (SAPHE, 2007) supports
telecare and lifestyle monitoring paradigms for early
detection and prevention of adverse events i.e. for
intervention before they become critical or life-
threatening. Several remote healthcare monitoring
systems are that currently use proprietary device
information systems (e.g. Honeywell, American
Telecare and AMD Telemedicine). Many more
device families are expected to be developed in
coming years conforming to global standards being
established by the Continua Healthcare Alliance
companies (Continua, 2007).
The HH system’s conceptual driver is enabling
‘near real time’ risk analysis for early detection and
prevention of disease. A Web Services-based
platform to ‘push’ or ‘pull’ individual’s health-
related data along the patient path is being
HEALTHINF 2008 - International Conference on Health Informatics
120

configured in a manner that will reduce
transcriptional errors. The end-to-end framework
(Figure 1, see below) employs a collection of
clinical hubs, mobile devices and / or dedicated
home-based network servers to one or more data
analysis engines.
3 END-TO-END FRAMEWORK
Figure 1 illustrates the conceptual design of an ‘end-
to-end’ framework adopted in the HH project. The
framework allows data capture from both wearable
sensors and specialist hand-held instruments with
wireless data transfer capability. Through a
messaging fabric and / or dedicated integration
application, raw data used in the risk analysis
modules may originate from a wide variety of
sources and device types (e.g. electronic forms,
physiological monitors, retinopathy cameras,
scanners, clinical chemistry or nucleic acid
sequencing instrumentation). The data is integrated
using a schema indicated as “QUID” (QUantitative
Individualised Data integration) in the figure. In the
context of an ‘initial assessment’ workflow, the
types of diagnostic test to be performed are specified
by a clinical registration procedure to be described
elsewhere. The registration procedure has specific
functions to ensure compliance to ethical protocols
and informed consent procedures for re-use of data
while verifying patient identity (see below). HH has
adopted the Diabetes Continuing Care Reference
(DCCR) dataset as the basis of its schema).
Subsequent risk analysis of baseline data is
undertaken in the context of a “disease model” - a
quantitative research-based predictive framework
that indicates which risk variables are most relevant
to system-specified outcomes. In this regard, HH has
been conceived as a comprehensive healthcare
outcomes evidence-based learning platform.
3.1 Smart Sensors
In monitoring applications, the most common
physiological analytes are blood gases (e.g. carbon
dioxide, oxygen), blood electrolytes (e.g. potassium,
sodium, and chloride), blood glucose, creatinine,
urea, pH, cholesterol, bilirubin and proteins (e.g.
albumin). The relative significance of values
depends on the type of investigative scenario and
progression status of disease. In diabetes, the
measurement of glucose concentration at an
appropriate frequency is routine in short-term
prevention of hyper- and hypo-glycaemic events and
in long-term prevention of complications.
Automated measurements can be enabled to support
continuous monitoring. Conventionally, glucose
monitoring is performed using ‘finger-stick’ devices.
These provide only intermittent measurements and
rely on patient compliance. Next-generation glucose
Figure 1: HH end-to-end framework.
NOVEL SENSOR TECHNOLOGY INTEGRATION FOR OUTCOME-BASED RISK ANALYSIS IN DIABETES
121
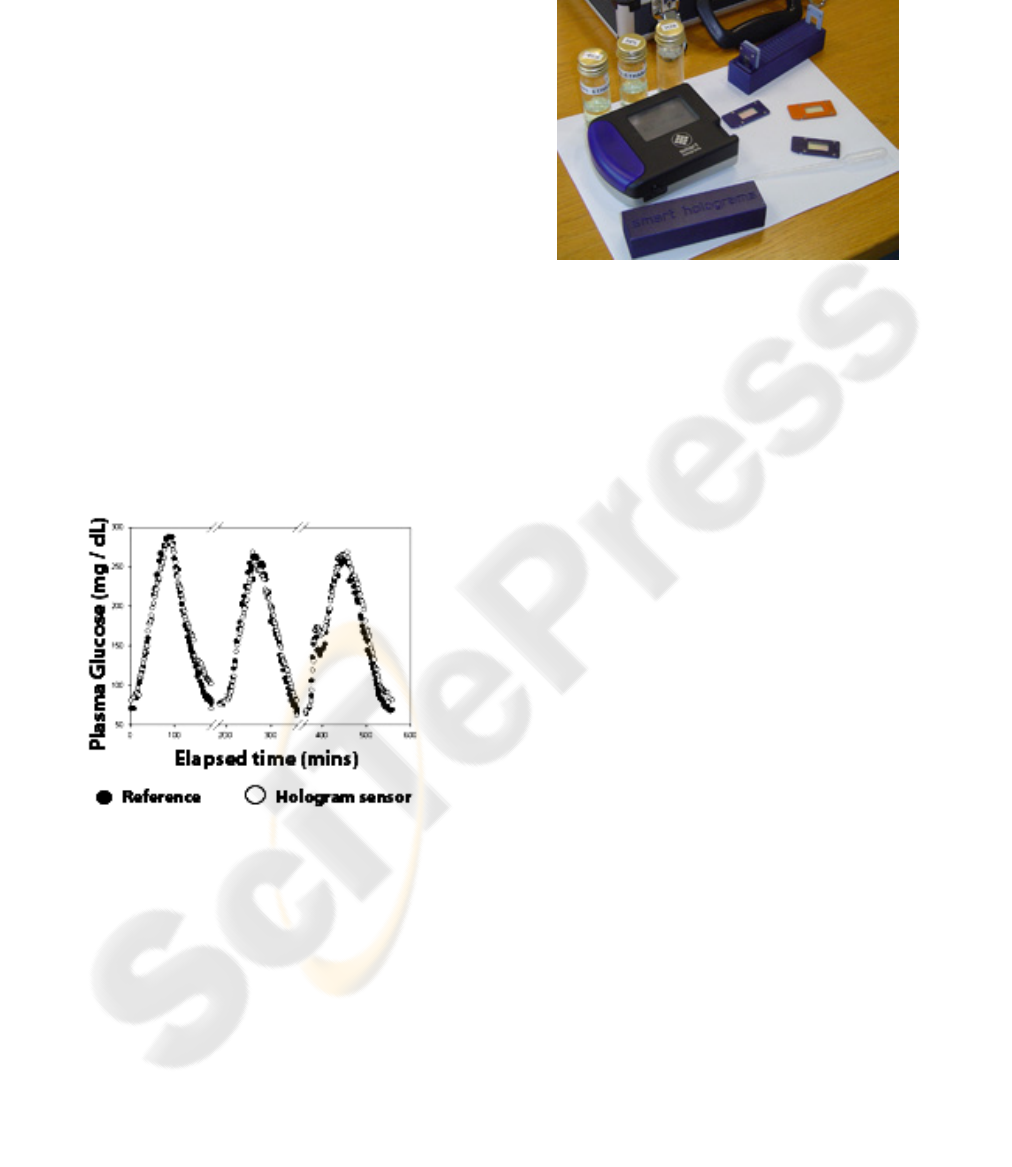
monitoring devices aim to provide real-time
continuous measurements by non- or minimally-
invasive means. One such sensor designed for
integration into the HH system uses glucose-
responsive holograms based on thin-film polymers
incorporating phenylboronic acid receptors.
Selective binding of glucose to the receptors induces
swelling or contraction of the film. This physical
change in turn causes the spacing between
holographic fringes within the film to increase or
decrease, thereby modulating the colour of the light
diffracted according to Bragg’s Law.
To obtain repeatable quantitative measurements
(figure 2), the colour of the hologram can be read
with a portable optical reader equipped with
Bluetooth®. These sensors exhibit long-term
chemical and physical stability, enabling
measurements over long time periods without
evidence of hysteresis. A further advantage is the
ability of holographic analyte sensors to be
incorporated into a multitude of formats (e.g.
catheters, contact lenses, implants), thereby offering
increased patient choice.
The prototype hand-held device (figure 3) has a
touch-screen user interface. The reader employs a
bespoke spectrometer optical sub-assembly,
combined with analogue to digital converters
(ADCs). These ADCs are read by a microprocessor
which provides a calibrated output of wavelength
and the corresponding concentration of the analyte
being measured. This is achieved by use of look up
tables or calibrated polynomial curve fit data.
Temporal data trends can be calculated on-device or
data can be sent via Bluetooth® to a HH web service
to enable downstream decision support.
3.2 Biometric Authentication
The HH system design has evaluated scenarios
where people entering data might be easily confused
by similar names or as different members of the
same family. A number of protocols for
disambiguation and absolute patient identification
can be incorporated, including biometrics and / or
smartcards. Biometric variables can be classified
either as physiological (e.g., derived from a
fingerprint, face or iris scan) or behavioural (e.g.,
speech recognition) (Biometrics, 2007).
The HH system requires technology options that
are cost-effective, fast and accurate. Based on these
criteria, biometric identification used robust
fingerprint recognition technology (‘2’ in figure 1)
(Wilson 2003). For flexibility, the biometrics device
was designed to be “loosely-coupled” with the
sensor and the home hub modules (refer to section
3.3). This permitted different packaging options
with integration of new sensor types without
substantial additional engineering cost. The hub is
used to tag the biometric ID to the incoming sensor
data in order to form an association between a
patient identity and a data reading. Communication
between sensor, home hub and the biometrics
module is via Bluetooth®, using a protocol specified
by IBM, Zarlink and Smart Holograms.
In its current operational mode, the biometric
device (figure 4) used in the HH system saves the
scanned fingerprint of a user in local memory, and
the individual is prompted to assign an “ID” specific
to that fingerprint. That ID is then transmitted via
Figure 2: Real-time measurement of blood glucose using a
holographic sensor compared to measurements made off-
line and post experiment by a traditional reference
method.
Figure 3: Smart Holograms prototype Hologram reader
with integrated Bluetooth
®
connectivity.
HEALTHINF 2008 - International Conference on Health Informatics
122

Bluetooth® to the data hub to be tagged within an
electronic patient record. This “enrolment” process
can be used prior to sensor readings to validate
identity. A delay-free smartcard that has high end-
user acceptance (e.g. contact-less ‘wave & pay’
cards) can also be used in appropriate circumstances
e.g. to enable inter-service access along the patient
path. Identification of the patient by means of their
enrolled ID sends that ID to the patient-proximal
hub (in the clinical data ‘baseline data’ operational
hub or the patient’s own home hub, or mobile hub).
The home hub can associate the subsequent sensor
reading with the patient ID. This is the
“identification” process. The design of the device
ensures security of patient information. All scanned
fingerprints are stored in the memory of the
biometric device. No fingerprint scans are
transmitted. All patient-identifiable information can
be encrypted at source, in transmission and storage.
If a hacker were to capture the wireless
transmissions the data would have no meaning.
3.3 Home Hub
The function of the ‘home hub’ (‘3’ in figure 1) is to
collect and collate the data from sensor(s) and the
biometric and smartcard / reader device(s) and to co-
transmit these via an appropriate communication
channel to the remotely-located server. The home
hub sits at the centre of the data collection and
transmission capability of the system, where all
devices - sensors, authentication module and server
connect through common interfaces. Various
physical realizations of the hub are possible. It can,
for example, be a mobile device such as a standard
mobile phone (we have demonstrated functionality
on a Sony Ericsson P910 phone - figure 5) that can
provide near real-time data connectivity. The hub
can also be deployed as a fixed ‘wired’ hub using,
for example, Ethernet connectivity. In a clinical
environment this also provides near real-time data
connectivity. For home use, hubs can be configured
to upload data periodically e.g. once or twice a day.
Figure 5: Mobile phone personal data hub by IBM.
Sensor devices currently connect to the hub
through adapters specific to the type of interface
required. Adapters are device transmission protocol
specific and can be developed by any manufacturer
wanting to provide connectivity of their devices to
this infrastructure. In HH, the medical devices use
Bluetooth®, although the architecture allows for this
to be any available. Zigbee has some power
consumption advantages over Bluetooth
®
(Zigbee,
2007).
We have developed adapters for a variety of
devices that can be used in conjunction with the
demonstrator system; namely for: (1) weighing
scales; (2) blood pressure cuff; (3) pulse oximeter;
(4) glucose meter. In addition to adapters for sensor
devices, an adapter is also required to interface the
hub to an appropriate application server. This step is
also protocol-specific and in practice a range of
adapters may be needed according to specific
application scenarios.
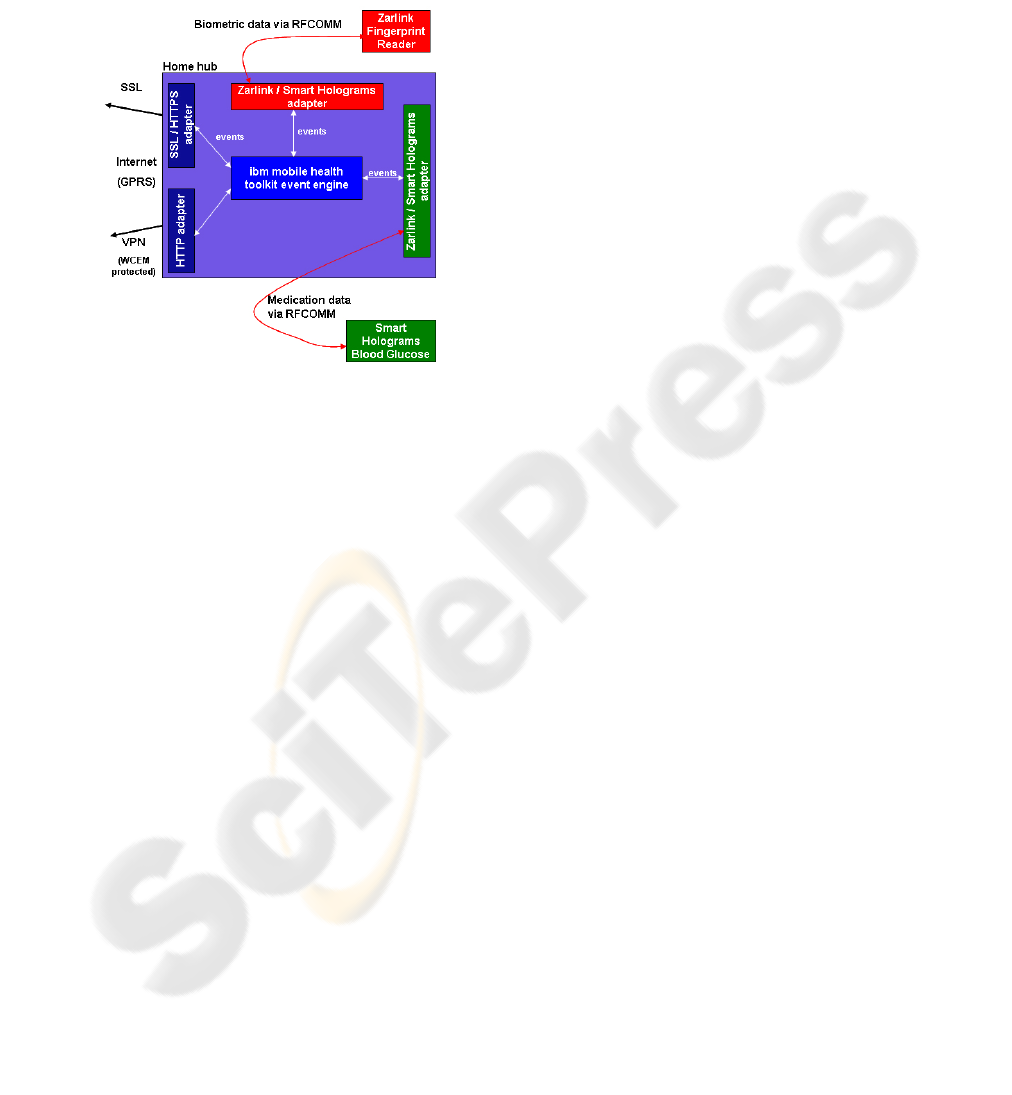
The hub architecture (figure 6) consists of an
event engine that behaves much like a broker, in that
it can receive events from one adapter and passes
these onto another adapter. In the simplest operation,
the event engine receives events from a single sensor
device (for example, weighing scales) and sends this
event to the adapter that transmits these events to an
application server. The current implementation of
this architecture uses the IBM Personal Care
Connect toolkit (Blount, 2007) and is based around a
number of standard technologies. These include:
• Java 2, Micro Edition, Mobile Information
Device Profile, Connection-Limited Device
Configuration (J2ME, MIDP, CLDC) or OSGI
for fixed hub (Java ME, 2007).
Figure 4: Fingerprint scanner by Zarlink.
NOVEL SENSOR TECHNOLOGY INTEGRATION FOR OUTCOME-BASED RISK ANALYSIS IN DIABETES
123
• JSR-82 to allow Java to interface to Bluetooth®
(assuming Bluetooth-enabled medical devices)
(JSR 82, 2007).
• Bluetooth® or mobile connectivity via GSM /
GPRS for wireless hubs.
• Ethernet, ISDN, etc. for fixed / wired hubs.
The hub design supports multiple adapters – key
to creation of an open-hub platform. Each adapter is
specific to a hub family, which is characterised by
the following attributes: (i) device-to-hub
communication, (ii) event data representation, (iii)
event serialisation, and (iv) hub-to-server
interaction. Different hub families can use different
event classes, communication protocols, and
serialisation schemes. A hub family may be
optimised to handle a specific set of biomedical
sensor types. Each adapter handles communication
with a family of hubs by (i) defining a protocol, (ii)
de-serialising the data forwarded by the hub, and
(iii) extracting event data from the event after
instantiating the events. Adapters also convert
proprietary data events into a consistent format – i.e.
act as a definable interface. It is expected that HH
will migrate to industry recommendations of
consortia such as the Continua Alliance (Continua,
2007). The hub functionality is currently
implemented in Java and is deployable to a mobile
phone as a MID-let or to a fixed hub as a set of
OSGI bundles.
In the current research demonstrator, sensor data
can be obtained from devices and stored on the hub
prior to transfer by a standard HTTP protocol. A
HTTP ‘Post’ agent uses stable storage to cache
events that cannot be sent immediately and then
forwards them when the next connection to the
server is established. Data sent to the server is
secured using the Secure Sockets Layer (now
referred to as Transport Layer Security) (SSL, TLS).
Other options include: Access Point Node (APN)
and Virtual Private Network (VPN).
Secure Sockets Layer: SSL is commonly used
within internet applications to provide secure client
to web server connections.
Access Point Node: The Access Point Node (or
Name) is the definition of the internet connection on
a GPRS mobile device that provides the route the
data will take from the GPRS device to access other
networks such as the Internet. All APN’s are defined
within the mobile device with a username and
password. The APN concept assumes the existence
of GPRS support nodes that exist between a mobile
device (using a GSM or UMTS service) and a server
providing IP-based access to the Internet. By
obtaining a private APN (with its own unique
credentials) it is possible to create a private
connection back to the HH servers. The HH
demonstrator has been successfully tested with APN.
Virtual Private Network: A VPN effectively
creates a private network by creating a secure
‘tunnel’ through the existing IP network. The HH
demonstrator has successfully used the IBM VPN
product WebSphere Everyplace Connection
Manager (WECM).
A sub-class of specialised functions that mimic
adapters exist to create functions internal to the hub.
A number of these have been implemented within
the current demonstrator. For example, an “Audio
Alert Agent” makes pre-configured audio alerts in
response to certain events like the reception of data
from a device or successful transmission of an event
to the server. The “User Display Agent” enables the
display of status information and provides input
capability for the user.
3.4 Server-side Architecture
The server-side architecture comprises of a ‘QUID’
and ‘QUIRA’ component (figure 7 and ‘4’ in figure
1) and has been described in detail elsewhere
(Subramanian, 2006, Shaikh-Ali, 2007, Conley,
2007).
The QUID component focuses on delivering data
collection, data storage, process execution and portal
Figure 6: Hub technical overview.
HEALTHINF 2008 - International Conference on Health Informatics
124

infrastructures to support users of the system
including clinicians, patients and researcher roles.
The development of QUID has been guided by the
requirements of Integrated Care Pathways (ICPs) for
diabetes (ICP, 2007). The QUID component
collects, validates and stores data streams from hubs
along the patient path. The interface for data
presentation / collection emulates workflows in real-
world care pathways. This ‘end-user familiarity’
design feature is strengthened by presentation of the
sensor data in a timeline-based (longitudinal)
layered manner, make the data meaningful. We
have used OpenLaszlo (Laszlo, 2007) to display
charts in an intuitive manner. Similarly, the patient
portal permits access to personal data supporting the
self-management paradigm
The QUIRA (Quantitative Individualised Risk
Analysis) component comprises a risk analysis
engine performing various operations identifying
signals in the longitudinal data stream, alerting the
care team to fulfilment of pre-defined risk criteria.
QUIRA represents ongoing research we will report
on in the future.
4 CONCERNS & FUTURE WORK
“Pervasive” or “ubiquitous” computing covers a
range of research topics, including distributed
computing, mobile computing, sensor networks,
communications, artificial intelligence, and human-
computer interaction. It is an emerging field of
research, and as such has many unresolved issues –
notably in areas like security, usability, privacy and
ethics amongst others. In the framework described in
this paper, ubiquitous computing devices demand
secure transmission of data to the server, in turn
demanding encryption mechanisms that defeat
purposeful or accidental ‘eavesdropping’. Device
miniaturisation assists the resolution of powering
issues. Significant ethical and privacy issues remain,
and it is axiomatic that a comprehensive informed
consenting process needs to be developed that is fit-
for-the defined purpose of disease early detection
and prevention. Informed consent needs to be
properly structured in the recording workflow.
There is currently no widely-accepted standard
protocol for the device-to-server data transmission
and / or format / structure for data being transferred
between devices. We expect interoperability
between devices from different vendors to be a key
focus within the scope of the Continua Healthcare
Alliance (Continua, 2007). Irrespective of global
technical standards adopted, methodological
standardisation of data acquisition needs to be
defined in order for patients and carers to reap the
benefits of interoperable systems.
The HH project has to date employed non-
invasive sensors. However we anticipate significant
developments in coming years in the area of
invasive (implantable) sensors. Implantable sensors
will likely be micro-miniaturised devices that can be
implanted into a patient’s body to enable relaying of
health-critical signals on a semi-continuous basis.
5 CONCLUSIONS
System-based management of chronic conditions is
essential to improve healthcare outcomes.
Conventional models of healthcare provision lack
capacity to continuously monitor physiological data
.
Figure 7: Physical implementation of the conceptual design shown in Figure1.
NOVEL SENSOR TECHNOLOGY INTEGRATION FOR OUTCOME-BASED RISK ANALYSIS IN DIABETES
125

combined with life event ‘timelining’. In such an
information system, healthcare can be provided in a
‘patient-centric’ model that maximises healthcare
resources.
ACKNOWLEDGEMENTS
This project has been funded by the Inter Enterprise
Computing Theme of the UK Department of Trade
and Industry (DTI)-led Technology Programme, for
which we are grateful. ECC and DRO are grateful
for support by the Wales Office of Research and
Development for Health and Social Care, Wales
Assembly Government.
REFERENCES
Abidi, S.S.R, 2001, “An Intelligent Tele-Healthcare
Environment Offering Person-Centric and Wellness-
Maintenance Services”, Journal of Medical Systems,
Vol. 25, No.3.
Biometrics, a, date visited: July 2007, link visited:
http://en.wikipedia.org/wiki/Biometrics
Blount, M., Batra, V.M., Capella, A.N., Ebling, M.R.,
Jerome, W.F., Martin, S. M., Nidd, M., and Wright,
S.P., 2007, “Remote health-care monitoring using
Personal Care Connect”, Volume 46, Information-
Based Medicine, IBM Systems Journal.
Conley, E.C., Owens, D.R. Luzio, S., Subramanian, M.,
Shaikh Ali, S., Hardisty, A., Rana, O. (2007)
“Simultaneous Trend Analysis for Evaluating
Outcomes in Patient-Centred Health Monitoring
Services”, Springer Journal of Healthcare
Management Science, Submitted.
Clemensen, J., Larsen, S. B., and Bardram, J., 2004,
“Developing Pervasive e-Health for Moving Experts
from Hospital to Home”, Proceedings of the IADIS e-
Society Conference, pp.441-448, Avilla, Spain.
Common User Interface, June 2007, link visited:
https://www.cui.nhs.uk/Pages/NHSCommonUserInterf
ace.aspx
Continua Healthcare Alliance, June 2007, link visited:
www.continuaalliance.org
Crossbow, June 2007, link visited: www.xbow.com
FIPS, 2002. “Security Requirements for Cryptographic
Modules”, link accessed
http://csrc.nist.gov/cryptval/140-2.htm
Gao, T., Massey, T., Sharp, J., Bishop, W., Bernstein, D.,
and Alm, A.., September 2006 “Integration of Triage
and Biomedical Devices for Continuous, Real-Time,
Automated Patient Monitoring”. Proceedings of the
IEEE Medical Devices and Biosensors Conference
(IEEE MDBS 2006), Boston, MA..
Hein, A., Nee, O., Willemsen, D., Scheffold, T., Dogac,
A., Laleci, G., 2006, “SAPHIRE - Intelligent
Healthcare Monitoring based on Semantic
Interoperability Platform - The Homecare Scenario”,
1st European Conference on eHealth (ECEH’06),
Fribourg, Switzerland.
Java ME: Mobile Information Device Profile (MIDP), date
visited: July 2007, link visited:
http://java.sun.com/products/midp/
JSR 82: Java APIs for Bluetooth, link visited:
http://www.jcp.org/en/jsr/detail?id=82
King, H., Aubert, R., Herman, W., 1998, “Global burden
of diabetes, 1995-2025: prevalence, numerical
estimates, and projections” Journal: Diabetes
Care,Volume: 21 Issue: 9, pp 1414-31.
Lorincz, K., Malan, M., Fulford-Jones, T., Nawoj, A.,
Clavel, A., Shnayder, V., Mainland, , S., Welsh, M.,
Oct-Dec 2004, “Sensor Networks for Emergency
Response: Challenges and Opportunities”, In IEEE
Pervasive Computing, Special Issue on Pervasive
Computing for First Response.
Laszlo, date visited: July 2007, link visited:
http://www.laszlosystems.com/.
ICP, Diabetes NSF (Wales) Standards, link accessed:
www.wales.nhs.uk/sites3/home.cfm?orgid=440
Shnayder, V., Chen, B., Lorincz, K., Jones, T., & Welsh,
M., 2005, “Sensor Networks for Medical Care”,
Technical Report TR-08-05, Division of Engineering
and Applied Sciences, Harvard University.
Shaikh-Ali, A., Rana, O.F. Hardisty, A., Subramanian, M.,
Luzio, S., Owens, D.R. and Conley, E.C., October
2007, "Portal Technologies for Patient-centred
Integrated Care", European Conference on eHealth,
Oldenburg, Germany.
SAPHE project, July 2007, link visited:
http://ubimon.doc.ic.ac.uk/saphe/m338.html,
Subramanian, M., Shaikh-Ali, A., Rana, O.F. Hardisty, A.
and Conley, E.C., October 2006, "Healthcare@Home:
Research Models for atient-Centred Healthcare
Services", John Vincent Atanasoff International
Symposium on Modern Computing, Sofia, Bulgaria.
IEEE Computer Society Press.
WHO, World Health Organisation, date visited: July 2007,
link visited: http://www.who.int/en/
Wilson, C., Hicklin, R.A., Korves, H., Ulery, B., Zoepfl,
M., Bone, M., Grother, P., Micheals, R., Otto, S.,
Watson, C., 2003, “Fingerprint Vendor Technology
Evaluation”, National Institute of Standards and
Technology, Mitretek Systems, NAVSEA Crane
Division
Zigbee, date visited: July 2007, link visited:
http://www.zigbee.org/en/index.asp
HEALTHINF 2008 - International Conference on Health Informatics
126
