
Thyroid Classification in Ultraound by Deep Multimodal Learning
Jiong Shao, Hanshuo Xing, Mengyin Li and Xinglong Wu
School of Computer Sciences and Engineering, Wuhan Institute of Technology, Wuhan, China
Keywords: Deep Learning, Multimodal Learning, LSTM, Thyroid Cancer.
Abstract: Purpose: Biopsy results are the gold standard for testing the benignity and malignancy of thyroid cancer, but
they also brings the problems of overdiagnosis and overtreatment. This is a challenging task to avoid these
two problems while ensuring diagnostic accuracy and efficiency. In this paper, we use deep learning
multimodal models to assist physicians in diagnosis and improve diagnostic accuracy.
Methods: This paper presents a multimodal deep learning model to assist physicians in the diagnosis of
thyroid tumor. The model uses ultrasound images of the patient, geometric features of the lesion site, and
clinical information to fuse modeling, with clinical information as the first modality, geometric features of
the images as the second modality, and medical images as the third modality. The results are compared with
other single-modal models to analyze and validate the performance of the multimodal model.
Results: For the dataset used, the multimodal model had an accuracy of 0.884, precision of 0.865, recall of
0.859, and F1 of 0.862, the Area Under Curve (AUC) of the multimodal mode was 0.933, the AUC of the
ResNet50 was 0.639, the AUC of the InceptionResnetV2 was 0.612, the AUC of the Densenet121 was 0.654,
and the AUC of the EfficientNetB3 was 0.649.
Conclusion: The multimodal model has high accuracy, sensitivity, and specificity in distinguishing benign
and malignant thyroid tumors, and its performance is significantly better than the four single-modal deep
learning classification models used for comparison. The proposed method is therefore valuable and is
expected to help clinicians diagnose thyroid cancer efficiently.
1 INTRODUCTION
Thyroid cancer is one of the common malignancies
we encounter with our life (Durante et al., 2018),
and its incidence is increasing worldwide. According
to the latest national cancer statistics released by the
National Cancer Center in February 2022
(Changfa et al., 2022), thyroid cancer ranked
seventh in incidence, with 50,000 new cases of
cancer in men and 170,000 in women, who remain
the most prevalent group. Thyroid cancer has
received widespread attention because of the
youthfulness of the incidence population and the
increasing incidence rate year by year.
In medicine, thyroid tumors are mainly
discovered by ultrasonic diagnosis (Zhaohui et al.,
2010), which is used to detect thyroid nodules. If a
nodule is found to be abnormal, a biopsy by
puncture of cells (CHEN & JIANG, 2017) is
required. Puncture biopsy is the gold standard for
diagnosing benign and malignant thyroid tumors
(Qingwen et al., 2017), but because of the
prevalence of thyroid masses in daily life, direct
biopsy can again lead to overdiagnosis and
overtreatment. In order to reduce this, the current
popular diagnostic method is mainly diagnosed by
the physician's observation of the patient's
ultrasound images before deciding whether a biopsy
is needed However, the diagnosis by the naked eye
of a professional doctor is not only time-consuming
but also inaccurate and subject to false
detection.Thus the use of deep learning methods to
assist physicians in diagnosis became gradually
popular (Fujita, 2020).In recent years, deep learning
techniques had been successful in several areas of
medical- assisted diagnosis (Juan-Xiu et al., 2018) ,
and their features such as end-to-end and automatic
learning (Jeong et al., 2019) also provide new
solutions for assisted diagnosis (Guang-Yuan et al.,
2018). At the same time deep learning for text, audio
and video data analysis has also achieved a lot of
results (Sun et al., 2022).we propose a joint deep
learning model using data onto multiple modalities
for modeling, that is, a multimodal (Ngiam et al.,
216
Shao, J., Xing, H., Li, M. and Wu, X.
Thyroid Classification in Ultraound by Deep Multimodal Learning.
DOI: 10.5220/0011918100003612
In Proceedings of the 3rd Inter national Symposium on Automation, Information and Computing (ISAIC 2022), pages 216-223
ISBN: 978-989-758-622-4; ISSN: 2975-9463
Copyright
c
2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

2011) approach to enhance the model..
In this paper, we use the data on three different
modalities of patient's ultrasound images, clinical
information, and image geometric features of
modeling to achieve benign and malignant
classification of thyroid tumors, aiming to assist
physicians in diagnosis and improve the efficiency
and accuracy of diagnosis.
2 RELATED WORK
2.1 Research on Medical-Assisted
Diagnosis by Single-Modal Deep
Learning
Zhu et al. used VGG-16T for the diagnosis of benign
and malignant thyroid tumors (YiCheng et al.,
2021), with additional BN and Drop-out layers in
addition to the fully connected layer, and performed
a 10-fold cross-validation with high sensitivity,
specificity, and accuracy of the results.. Peng et al.
proposed ThyNet for the classification of thyroid
tumors (Peng et al., 2021), ThyNet was constructed
using a combination of three networks. Comparing
the results obtained by this method of professional
physicians, the results are superior.Li et al.
implemented a deep CNN model on thyroid tumor
diagnosis based on ultrasound images (Li et al.,
2019), where the team used a DCNN model for
training. The method achieved better results, with
diagnostic performance essentially equal in
sensitivity and higher specificity compared to
experienced imaging physicians.
The Classification of thyroid cancer using a
single-modality deep learning model, while
achieving good results, is not medically interpretable
in the opinion of clinician
2.2 Research on Medical-Assisted
Diagnosis by Multimodal Deep
Learning
Multimodal thyroid cancer classification studies to
refer to the classification of thyroid cancer using
features of different modalities. The methods of
multimodal feature fusion (Ramachandram &
Taylor, 2017) can be generally classified into two
types: early fusion and late fusion.
Gong et al. propose a late fusion approach
(Gong, 2013) for benign and malignant classification
of thyroid tumors based on a composite weight
multi-classifier fusion method of a non-Bayesian
fusion framework, which outperformed a
single-modal classifier in terms of correctness,
sensitivity, and specificity.Song et al. propose an
image fusion method to assist in Alzheimer's disease
diagnosis (AD) (Song et al., 2021) by fusing gray
matter tissue regions of MRI with FDG-PET images
and then using 3DMulti-Scale CNN for evaluation.
This method has better overall performance and
outperforms state-of-the-art AD diagnostic methods..
Zheng et al. propose a multimodal fusion lung
adenocarcinoma pathology classification model
based on attentional mechanisms using deep
learning multimodal techniques combining clinical
information, CT images, and serum tumor markers
of patients (Zheng, 2021). The method outperformed
existing related studies in terms of accuracy as well
as AUC.Xu et al. proposed an early fusion approach
from cervical dysplasia diagnosis (Xu et al., 2016),
using AlexNet network to learn image features from
Cervigarm images, and then combining image
features with non-image features (clinical outcomes,
etc.), This method significantly outperforms using
only any single source of information and previous
multimodal frameworks.. Wang et al made
improvements to ShuffleNet and proposed a
3DShuffleNet-based model for Alzheimer's disease
assisted diagnosis (Wang et al., 2021), Their
proposed method obtains relatively good results
from a small computational cost.
these studies demonstrated the performance of
multimodal versus single-modal, respectively,
reflecting the key role of multimodal learning, and
Huang et al. also demonstrated the superiority over
multimodal learning (Huang et al., 2021), In contrast,
few studies in the field of thyroid cancer have
combined ultrasound image features with clinical
features and used them for classification, so we then
attempted to use multimodal data for feature fusion
and use it for thyroid cancer classification.
3 METHODS
In this paper, we propose a model combining
Densenet (Huang et al., 2017) , Multilayer
Perceptron (MLP) and Long Short Term Memory
(LSTM) (Greff et al., 2016) (Figure 1),which
incorporates ultrasound images, clinical information
and image geometric features to classify benign and
malignant
thyroid tumors of patients. DenseNet is
Thyroid Classification in Ultraound by Deep Multimodal Learning
217

Figure 1: Diagram of thyroid cancer classification model based on multimodal deep learning
used to extract features from ultrasound images, and
MLP is used to extract clinical information features
and image geometry features, and the extracted
feature vectors are stitched together to obtain a new
feature vector to be input into the LSTM before the
final classification by the classifier.
3.1 Datasets
Table 1:Baseline characteristics.
Patients Data set
sex Male 75
(25.8%)
Female 215
(74.2%)
age
(Years)
Age>4
5
175
(60.3%)
Age<=45 115
(39.6%)
The data for this experiment were obtained from
four hospitals, all of which were produced by
Samsung ultrasound instruments.Because some of
the patient data were missing some key information,
a total of 290 patients with 558 images was retained,
170 (30%) negative images and 388 (70%) positive
images, of which 25.8% were male and 74.2% were
female (Table 1).
3.2 Geometric and Clinical Features of
Images
Image geometric features (Zhihang, 2017): 10
features are selected from the many geometric
features, which are:
(1) Number of pixels in the focal area: The number
of white pixels in the focal area is calculated by
MASK.
(2) Total number of pixels in the image: the number
of pixels in the whole ultrasound image.
(3) Percentage of focal area: the number of pixels
in the focal area.
(4) Area size of the lesion: the area of the contour
formed by connecting the pixel centroids at the
border of the lesion area.
(5) Perimeter of the lesion: the sum of the pixel
points at the edge of the lesion area.
(6) Average gray values of the focal area:
superimpose the focal area with MASK and divide
the total gray value by the number of pixels.
(7) Focal area aspect ratio: the ratio of the width of
the height of the outer rectangle of the focal area.
𝐴𝑠𝑝𝑒𝑐𝑡 𝑅𝑎𝑡𝑖𝑜 =
(1)
(8) Lesion area firmness: ratio of lesion area to
convex bun area.
𝐸𝑥𝑡𝑒𝑛𝑡 =
(2)
(9) Equivalent diameter of the lesion area: the
diameter of a circle equalled to the area of the lesion
area.
𝐸𝑞𝑢𝑖𝑣𝑎𝑙𝑒𝑛𝑡𝐷𝑖𝑎𝑚𝑒𝑡𝑒𝑟 =
√
×
(3)
(10) Focal area orientation: the angle between the
short axis of the external rectangle of the focal area
and the horizontal axis.
Clinical features: Han et al (Yuren et al.,
2022)concluded that the benignity of thyroid cancer
is related to the patient's age as well as gender, so we
mainly counted and collated the patient's gender, age,
true length of the lesion, true width and true area of
the lesion as clinical features.
ISAIC 2022 - International Symposium on Automation, Information and Computing
218

3.3 Data Preprocessing
Medical Image Preprocessing:
For the original medical images, a series of
pre-processing processes are needed to remove the
interference information. The preprocessing process
is shown in Figure 2, where the original medical
image is Gaussian blurred with the kernel size set to
(9,9) and the standard deviation taken as 0. Then, it
is binarized with the kernel shape of rectangle and
the kernel size of
(5,5) for morphological opening
operation. Finally, contour detection is performed
and all the found contours are sorted by area size,
and the coordinates of the largest contour are
recorded and cropped. For the cropped image, a
mask of the lesion area
(Figure 3) is created
according to the annotation of the professional
doctor on the image.
Figure 2: Ultrasound image preprocessing flow chart,
original image (A), Gaussian blur (B), binarization (C),
morphological open operation (D), finding maximum
contour (E)
Figure 3: Original ultrasound image (A), physician labeled
image (B), and lesion area labeled image (C)
Clinical data and image geometric features data
preprocessing:
The gender features (male and female) in the clinical
data were integrated after converting them to 0
(female) and 1 (male), and then the clinical data
were normalized to 0 mean with the image geometric
feature data..
𝑥
∗
=
(4)
where is the original value of a feature, μ is the mean
of the feature in all samples, σ is the standard
deviation of the feature in all samples, and is the
standardized feature value.
3.4 Network Design
The core of deep learning based multimodal thyroid
tumor classification lies in the extraction of different
modal features, fusion of multimodal features, and
feature classification after fusion. In this paper, we
use an early fusion approach to perform fusion at the
feature layer. The proposed model in this paper has
three main parts: feature extraction, feature fusion,
and classification.The three parts are described in
detail in the following.
Feature extraction module:
The processed clinical data and image geometry data
are input to the MLP for feature extraction
respectively. The MLP has two hidden layers, the
first hidden layer has 32 neurons and the second
hidden layer has 128 neurons, both using Relu
activation function, and finally a feature vector of
length 1*128 is obtained. The ultrasound image
modality, on the other hand, uses Densenet121 for
feature extraction, gets the output of the last
convolutional layer, performs global average pooling
and then reshape to 1*128.
Feature fusion module:
The three feature vectors are stitched together to
obtain a 3*128 feature vector, and then the new
feature vector is input into the LSTM.
Classification module:
The output of the LSTM network is fed to the fully
connected layer for classification. The fully
connected layer consists of two hidden layers, the
first with 128 units and the second with 32 units, and
both with the Relu activation function. The final
output layer outputs the classification results using
the Sigmod activation function.
Thyroid Classification in Ultraound by Deep Multimodal Learning
219

Figure 4: Overall network structure
3.5 Initialization and Training
To demonstrate the superiority in the model proposed
of this paper, the model proposed to this paper is
compared with some classical single- models.
For the multimodal model, Batch is set to 8, the
optimizer is Adam, the learning rate is 0.0001, the
loss is binary_crossentropy, and 200 rounds are
trained, where the size of the ultrasonic modal input
image is set to (250,250) and initialized with the
pre-trained model weights.. The single-modal deep
learning models used for the result comparison are
ResNet50, EfficientNetB3, DenseNet121, and
InceptionResnetV2, the Settings are the same as for
multimodal models.
3.6 Evaluating Performance Metrics
In medical diagnosis, sensitivity and specificity are
important indicators, and we prepare several
indicators to evaluate the model performance.
Accuracy =
(6)
Specificity =
(7)
Precision =
(8)
Recall =
(9)
𝐹
=2∙
∙
(10)
TP: Positive samples predicted by the model as
positive class. TN: negative sample predicted by the
model as negative class. FP: negative samples
predicted by the model as positive class. FN: positive
samples predicted by the model to be negative.
ROC (Kootte et al., 2017)curve:Also known as
subject operating characteristic curves, the ROC plot
provides a quick visualization of the relationship
between sensitivity and specificity.
In order to make the classification results more
interpretable, Wu et al (Wu et al., 2022)proposed
to use the modal contribution index (MCI) to find
the contribution of each modality of the model.
Considering the different degree of contribution to
different modalities to the classification accuracy we
proposed the WMCI, adding the accuracy of each
modality as a weight.
MCI
=
∑
∑
,,
∑∑
,,
(11)
𝑊𝑀𝐶𝐼
=
∗
∑
∗
(12)
By calculating the output of the feature extraction
module, where denotes the Jth element of the mth
modal vector of the ith instance, FN is the number of
features, FM is the modality,N is the number of
instances, and W is the accuracy rate when each
modality is predicted individually.
4 RESULTS
With 588 images obtained from a total of 290
cases from four hospitals, 334 (60%) images were
used for the training set, 112 (20%) for the
validation set, and 112 (20%) for the test set.
Figure 5 shows the ROC curves on different
models. From the results in the figure, Resnet50 [28]
has an AUC of 0.693, EfficientnetB3 [29] has an
AUC of 0.649, Dencenet121 has an AUC of 0.654,
InceptionResnetV2 has an AUC of 0.612, MLP_Cli
has an AUC of 0.837, and MLP_Img has an AUC of
0.882, while the best results are achieved using our
proposed multimodal model, with an AUC of 0.933.
Table 2 shows the prediction results of each model
on the test set, and the thyroid cancer classification
model based on multimodal deep learning proposed
in this paper achieves an accuracy of 0.884,
precision of 0.865, recall of 0.859, and F1 of 0.862,
which is better than several other comparative
methods in all evaluation metrics. It indicates that
the introduction of data from different modalities is
beneficial for the classification of benign and
malignant thyroid cancer.
ISAIC 2022 - International Symposium on Automation, Information and Computing
220
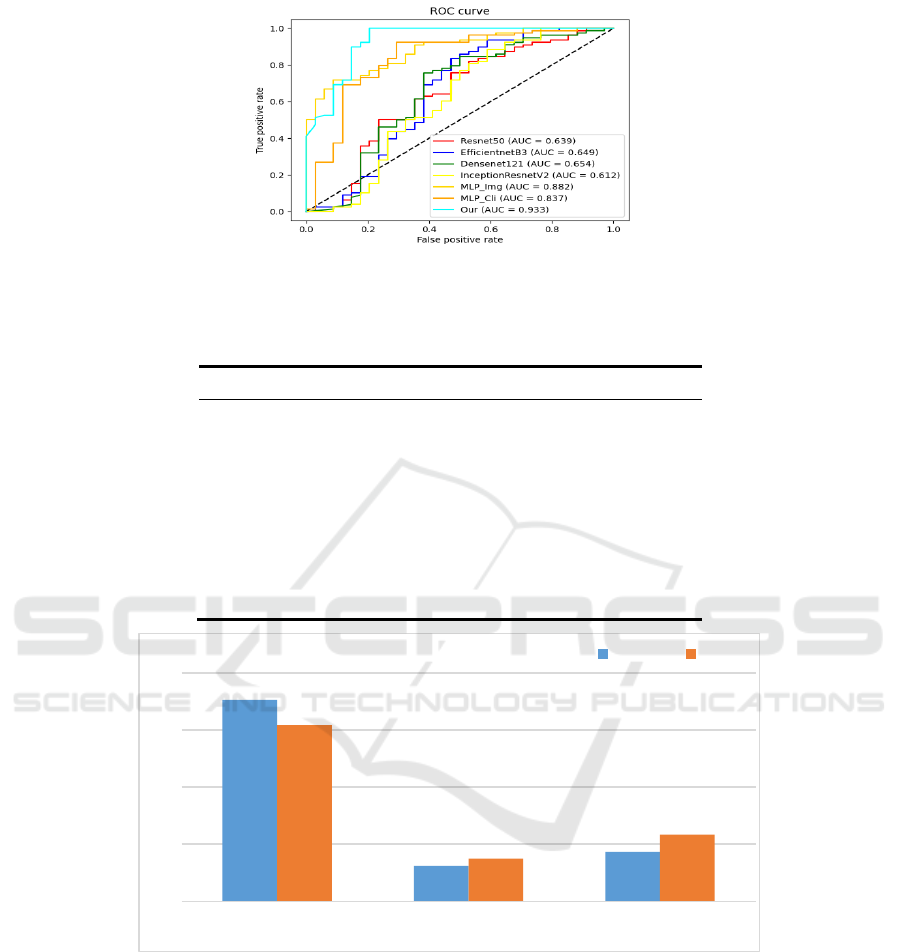
Figure 5: ROC plots for the multimodal model and each single-modal model, Mlp_cli is the Mlp model with clinical data as
input and Mlp_img is the Mlp model with image geometric features as input
Table 2:Diagnostic performance of Multimodal and Single-modal models.
Model ACC Precision Recall F1 AUC
ResNet50 0.705 0.644 0.631 0.636 0.639
InceptionResnetV2 0.732 0.680 0.625 0.634 0.612
Densenet121 0.705 0.639 0.615 0.620 0.654
EfficientNetB3 0.723 0.675 0.634 0.658 0.649
Mlp_cli 0.803 0.797 0.803 0.792 0.837
Mlp_img 0.821 0.816 0.821 0.816 0.882
Our 0.884 0.865 0.859 0.862 0.933
Figure 6:WMCI for Training Set and Test Set
Figure 6 shows the contribution of the model for
each modality in the training set and the test set. In
both datasets, the ultrasound image modality has the
largest contribution, with 0.706 for the ultrasound
image modality, 0.124 for the clinical information
modality, and 0.170 for the image geometry feature
modality in the training set, and 0.617 for the
ultrasound image modality, 0.149 for the clinical
This also indicates that in most cases, the
multimodal deep learning model relies more on the
ultrasound image modality for prediction.
5 DISCUSSION AND
CONCLUSION
This paper proposes a multimodal thyroid cancer
classification model based on deep learning method.
0
0,2
0,4
0,6
0,8
ultrasound images clinical information image geometric features
WMCI
Training Test
Thyroid Classification in Ultraound by Deep Multimodal Learning
221

According to our results, our model outperforms the
four single-modal networks used for comparison in
terms of accuracy, sensitivity,,and specificity.The
clinical information about patients and geometric
features of images play a role in improving the
classification of thyroid tumors and also validate
the superiority of the model.
Our study also has some limitations:
(1) The current collected and collated multimodal
dataset is relatively small, and the performance of
the model may be better if more samples are
available in the future.
(2) In the feature fusion part, we use early fusion,
which directly splices the three output feature
vectors, and can try other different feature fusion
methods.
(3) In this study, the objective is to classify the
benign and malignant thyroid tumors. The images
input to the model are the parts of the ultrasound
images that contain only the lesions, and it is still
necessary to segment the images according to the
doctor's labeled images when collecting and
organizing the data in the preliminary stage.
To the best of our knowledge, previous studies
have shown that deep learning algorithms
outperform medical professionals in certain clinical
outcomes, however, the use of deep learning
approaches alone is not applicable in clinical settings
(Ko et al., 2019), therefore, the main objective of
this study is to assist physicians in diagnosis and
reduce overdiagnosis and overtreatment. In future
studies the multimodal model will be further
improved by expanding the dataset used in the
experiment and adding more different clinical data
as features in the clinical information. In the feature
fusion part, different fusion strategies are used to
compare the effects of different fusion strategies on
the model performance so as to improve the
performance.
REFERENCES
Changfa, X., Xuesi, D., He, L., Maomao, C., Dianqin, S.,
Siyi, H., . . . Wanqing, C. (2022). Cancer statistics in
China and United States, 2022: profiles, trends, and
determinants. Chinese Medical Journal.
CHEN, J., & JIANG, L. (2017). Accurate pathological
diagnosis of thyroid cancer in the era of precision
medicine. Chinese Journal of Clinical Oncology, 44
(04), 181-185.
Durante, C., Grani, G., Lamartina, L., Filetti, S., Mandel, S.
J., & Cooper, D. S. (2018). The diagnosis and
management of thyroid nodules: a review. Jama, 319
(9), 914-924.
Fujita, H. (2020). AI-based computer-aided diagnosis
(AI-CAD): the latest review to read first. Radiological
physics and technology, 13 (1), 6-19.
Gong, R. (2013). THYROID TUMOR CLASSIFICATION
BASED ON MUTLI-MODE ULTRASOUND IMAGE
Harbin Institute of Technology].
Greff, K., Srivastava, R. K., Koutník, J., Steunebrink, B.
R., & Schmidhuber, J. (2016). LSTM: A search space
odyssey. IEEE transactions on neural networks and
learning systems, 28 (10), 2222-2232.
Guang-Yuan, Z., Xia-Bi, L., & Guang-Hui, H. (2018).
Survey on Medical Image Computer Aided Detection
and Diagnosis Systems. Journal of software, 29 (05),
1471-1514.
Huang, G., Liu, Z., Van Der Maaten, L., & Weinberger, K.
Q. (2017). Densely connected convolutional networks.
Proceedings of the IEEE conference on computer
vision and pattern recognition,
Huang, Y., Du, C., Xue, Z., Chen, X., Zhao, H., & Huang,
L. (2021). What makes multi-modal learning better
than single (provably). Advances in Neural
Information Processing Systems, 34, 10944-10956.
Jeong, E. Y., Kim, H. L., Ha, E. J., Park, S. Y., Cho, Y. J.,
& Han, M. (2019). Computer-aided diagnosis system
for thyroid nodules on ultrasonography: diagnostic
performance and reproducibility based on the
experience level of operators. European radiology, 29
(4), 1978-1985.
Juan-Xiu, T., Guo-Cai, L., Shan-Shan, G., Zhong-Jian, J.,
Jin-Guang, L., & Dong-Dong, G. (2018). Deep
Learning in Medical Image Analysis and Its
Challenges. Acta automatica Sinica, 44 (03), 401-424.
Ko, S. Y., Lee, J. H., Yoon, J. H., Na, H., Hong, E., Han,
K., . . . Park, V. Y. (2019). Deep convolutional neural
network for the diagnosis of thyroid nodules on
ultrasound. Head & neck, 41 (4), 885-891.
Kootte, R. S., Levin, E., Salojärvi, J., Smits, L. P., Hartstra,
A. V., Udayappan, S. D., . . . Holst, J. J. (2017).
Improvement of insulin sensitivity after lean donor
feces in metabolic syndrome is driven by baseline
intestinal microbiota composition. Cell metabolism,
26
(4), 611-619. e616.
Li, X., Zhang, S., Zhang, Q., Wei, X., Pan, Y., Zhao, J., . . .
Li, J. (2019). Diagnosis of thyroid cancer using deep
convolutional neural network models applied to
sonographic images: a retrospective, multicohort,
diagnostic study. The Lancet Oncology, 20 (2),
193-201.
Peng, S., Liu, Y., Lv, W., Liu, L., Zhou, Q., Yang, H., . . .
Zhang, X. (2021). Deep learning-based artificial
ISAIC 2022 - International Symposium on Automation, Information and Computing
222

intelligence model to assist thyroid nodule diagnosis
and management: A multicentre diagnostic study. The
Lancet Digital Health, 3 (4), e250-e259.
Qingwen, H., Yi, C., Huagui, L., & Bin, L. (2017).
Comparative analysis of CT and B ultrasound
diagnosis and pathological diagnosis of thyroid
carcinoma. China Foreign Medical Treatment, 36 (28),
179-180+183.
Ramachandram, D., & Taylor, G. W. (2017). Deep
multimodal learning: A survey on recent advances and
trends. IEEE signal processing magazine, 34 (6),
96-108.
Song, J., Zheng, J., Li, P., Lu, X., Zhu, G., & Shen, P.
(2021). An effective multimodal image fusion method
using MRI and PET for Alzheimer's disease diagnosis.
Frontiers in Digital Health, 3, 637386.
Sun, Z., Ke, Q., Rahmani, H., Bennamoun, M., Wang, G.,
& Liu, J. (2022). Human action recognition from
various data modalities: A review. IEEE transactions
on pattern analysis and machine intelligence.
Wang, Y., Liu, X., & Yu, C. (2021). Assisted diagnosis of
alzheimer’s disease based on deep learning and
multimodal feature fusion. Complexity, 2021.
Wu, X., Li, M., Cui, X.-w., & Xu, G. (2022). Deep
multimodal learning for lymph node metastasis
prediction of primary thyroid cancer. Physics in
Medicine & Biology, 67 (3), 035008.
Xu, T., Zhang, H., Huang, X., Zhang, S., & Metaxas, D. N.
(2016). Multimodal deep learning for cervical
dysplasia diagnosis. International conference on
medical image computing and computer-assisted
intervention,
YiCheng, Z., PengFei, J., Jie, B., Quan, J., & Ximing, W.
(2021). Thyroid ultrasound image classification using
a convolutional neural network. Annals of
translational medicine, 9 (20).
Yuren, H., Limei, L., & Rui, W. (2022). Clinical
Characteristics and Prognostic Factors of 'Thyroid
Cancer Patients. The Practical Journal of Cancer, 37
(06), 1000-1002.
Zhaohui, L., Haiqing, Z., Jingtao, D., Yukun, L., Qinglong,
K., Guoqing, Y., . . . Juring, L. (2010). Diagnostic
value of ultrasonographic features in thyroid nodule
properties. Chinese Medical Journal (46), 3272-3275.
Zheng, D. (2021). Research on Intelligent Lung Tumor
Analysis Technology Based on Multimodal Data
Fusion [Shanghai Institute of Technical Physics,
Chinese Academy of Science].
Zhihang, Z. (2017). Feature extraction and visualization of
ultrasonic image based on TI-RADS image based on
TI-RADS [ Southwest Jiaotong University].
Thyroid Classification in Ultraound by Deep Multimodal Learning
223
