
Utilization of Parafin and TiO
2
as Phase Change Materials (PCM) for
Processor Coolers
I Made Arsawan
1a
, I Putu Sastra Negara
1b
and I Gede Oka Pujihadi
2c
1
Politeknik Negeri Bali Badung Bali, Indonesia
2
Department of Mechanical Engineering, Politeknik Negeri Bali, Indonesia
Keywords: Processor Cooler, Heatsink, Phase Change Material.
Abstract: The development of new and renewable energy is an alternative that can be done to avoid an energy crisis.
One of the most prospective energy storage techniques is thermal energy storage. Thermal energy storage
technique is by utilizing the phase change of a material or known as Phase Change Material (PCM). Phase
change materials (PCM) are materials that change their physical characteristics when they absorb or release
heat energy. Many refrigeration technologies currently utilize PCM technology in their cooling process.
(Hikma et al. 2020) AC which was originally used massively and can damage the environment can be replaced
by EGACY on a regular basis. Utilization of PCM in the electronics field has begun to be developed, as
research conducted by(Kandasamy, Wang, and Mujumdar 2008) found that PCM in the heat sink cavity will
improve cooling performance compared to the case of a heat sink without PCM. In this study, we will try to
develop a PCM-based processor cooler, by making a test tool in the form of a PCM-based processor cooling
simulation. After the simulation is complete, the operational system is tested before taking data. Data
collection was carried out with a combination of PCM material in the form of paraffin and TiO
2
with several
variations of the mixture, namely with PCM material in the form of pure paraffin, paraffin + 2% TiO
2
, paraffin
+ 4% TiO
2
, paraffin + 6% TiO
2
, paraffin + 8% TiO
2
, and paraffin + 10 %TiO
2
. The results showed that the
addition of PCM into the heatsink can reduce the processor temperature and the addition of TiO
2
to paraffin
can stabilize the temperature that occurs in the processor. The addition of 4% and 6% TuO
2
provides the best
cooling effect on the processor compared to pure paraffin and TiO
2
concentrations of 2%, 8%, and 10%.
1 INTRODUCTION
The development of new and renewable energy is an
alternative that can be done to avoid an energy crisis.
Many things can be done to develop renewable
energy, such as developing solar energy, developing
micro hydro energy, developing wind energy and
others. One alternative in the effort to utilize
renewable energy is the development of energy
storage devices, which is as important as developing
renewable energy sources. Energy storage not only
reduces the mismatch between supply and demand, it
can also improve the performance and reliability of
energy systems and play an important role in energy
utilization more effectively. One of the most
a
https://orcid.org/0000-0002-2365-9401
b
https://orcid.org/0000-0002-1028-070X
c
https://orcid.org/0000-0003-0417-8538
prospective energy storage techniques is thermal
energy storage. Thermal energy storage technique is
by utilizing the phase change of a material or known
as Phase Change Material (PCM).
Phase change materials (PCM) are materials that
change their physical characteristics when they
absorb or release heat energy. Utilization of phase
changes of a material in the form of liquid and solid
phase changes. When a solid material is heated to a
temperature above its melting point, it absorbs heat
and melts. On the other hand, when a liquid is cooled
below its melting point, the liquid will solidify at a
constant temperature, for example water is put in a
freezer until it changes to a solid phase (Ice).
Furthermore, if you want to melt or condense a
material that can change phase it is necessary to
732
Arsawan, I., Negara, I. and Pujihadi, I.
Utilization of Parafin and TiO2 as Phase Change Materials (PCM) for Processor Coolers.
DOI: 10.5220/0011875800003575
In Proceedings of the 5th International Conference on Applied Science and Technology on Engineering Science (iCAST-ES 2022), pages 732-740
ISBN: 978-989-758-619-4; ISSN: 2975-8246
Copyright © 2023 by SCITEPRESS – Science and Technology Publications, Lda. Under CC license (CC BY-NC-ND 4.0)

absorb or release a certain amount of energy which is
called "latent heat" or "heat of fusion".
Many refrigeration technologies currently utilize
PCM technology in their cooling process. (Hikma et
al. 2020) Air conditioners that were originally used
massively and can damage the environment can be
replaced by EGACY on a regular basis, so that at
night, cold temperatures can be used as a source of
cooling energy that is stored first by the PCM and
then released during the day. Through pipes
connected to the building, air will flow into the room
and then spread to all parts of the room. When this
is implemented, the benefits of being an
environmentally friendly, economical and of course
energy efficient cooler will be obtained.(Risano, S,
and Pratama 2017) The difference in the efficiency
of using PCM CPO (Crude Palm Oil) in heat transfer
from the inner wall to the Building Intergated
Photovoltaics (BIPV) room can increase the
efficiency by 5.75%. (Putra et al. 2020) A study of
the performance of beeswax (PCM) phase change
materials and heat pipes as a passive battery cooling
system for electric vehicles resulted in the use of
heat pipes to reduce the battery temperature by 26.62
°C under a heat load of 60 W compared to casing
without passive cooling system. Furthermore, the
addition of RT 44 on the heat pipe resulted in a
maximum temperature decrease of 33.42 °C. Thus,
RT 44 HC is more effective than beeswax because
its melting temperature is within the battery's
recommended operating temperature range, and its
latent heat allows more heat to be absorbed than
beeswax.
The use of PCM in the electronics field has
begun to be developed, as research conducted by
(Kandasamy, Wang, and Mujumdar 2008) found
that PCM in the heat sink cavity will improve
cooling performance compared to the case of heat
sinks without PCM when the input power level is
relatively high. The use of liquid metal was
developed as PCM material in Heatsink by (Fan et
al. 2016) liquid metal PCM materials and organic
materials were compared as PCM materials in heat
zinc applications, where molten metal has the ability
to overcome oper heat better than organic materials
(Octadecanol) and The volumetric latent heat of
molten metal smelting is proportional to the latent
heat of organic PCM.
Research on PCM materials is growing,
currently, nano-PCM materials have been
developed. This nanotechnology has also been
investigated by (Bayat, Faridzadeh, and Toghraie
2018) regarding the investigation of the
performance of finned heat sinks with nano-level
phase change materials (NePCM) where the
addition of a small portion of nanoparticles (2%),
heat sink performance can be increased up to PCM.
melted completely. Increasing the percentage of
nanoparticles, can cause a decrease in the
performance of heat zinc. With the addition of 2%
aluminum oxide nanoparticles can produce better
heatsink performance compared to the case of
adding copper oxide with the same percentage.
The development of PCM on processor cooling
has not been widely developed, because the
microprocessor is susceptible to liquid fluid which
results in the failure of the processor performance.
Previous research has been done on processor
cooling by using liquid fluid that flows through heat
zinc, where the results of the multi-channel flow
model provide a better cooling effect than the pool-
shaped flow model. In this study, it will be tried to
develop the use of local materials, namely lard as
PCM material combined with faraffin wax in
processor cooling.
2 PHASE CHANGE MATERIAL
(PCM)
PCM can be classified into two, namely organic and
anorganic. This grouping is based on the melting
point and latent heat of fusion. There is no single
material that can fulfill all the desired properties, so
PCM is also developed which is a combination of 2
groups of materials (Sharma et al. 2009).
2.1 Organic PCM
Usually organic PCM has a low temperature range, is
expensive and has a low average latent heat per unit
volume and density. Most organic PCMs are
flammable in nature. Organic PCM can be
distinguished as paraffin and non-paraffin.
2.1.1 Paraffins
Paraffins consist of a mixture of mostly straight chain
n-alkanes CH3-(CH2)-CH3. Crystallization of the
chain (CH3)- releases some latent heat. The melting
point and latent heat of fusion increase with the length
of the chain. The quality of paraffin as a smelting heat
storage material is caused by its wide temperature
range. Some of the melting points and latent heat of
smelting of paraffin can be seen in Table 1.
Utilization of Parafin and TiO2 as Phase Change Materials (PCM) for Processor Coolers
733

Table 1: Melting Point and Latent Heat of Melting Some
Types of Paraffins.
Number of
Atom C
Melting Poin
(
o
C)
Latent Heat of
Melting (kJ/kg)
14 5.5 228
15 10 205
16 16.7 237.1
17 21.7 213
18 28.0 244
19 32.0 222
20 36.7 246
21 40.2 200
22 44.0 249
23 47.5 232
24 50.6 255
25 49.4 238
26 56.3 256
27 58.8 236
28 61.6 253
Source: Sharma et al.2009
2.1.2 Nonparaffin
PCM from non-paraffin materials is PCM that is
commonly encountered with quite a lot of variation in
properties. Each of these materials has special
characteristics / properties unlike paraffin which has
almost the same properties. This type is the most
common category of PCM. Among the non-paraffinic
materials, the most common types are esters, fatty
acids, alcohols and glycol types (Abhat et al. 1981).
This group is often further subdivided into groups of
fatty acids and other nonparaffinic organics. These
materials are generally flammable and should not be
exposed to high temperatures, near flames and
oxidizing agents. The description of non-paraffin
PCM can be seen in Table 2, while the fatty acid PCM
can be seen in Table 3.
Table 2: Melting Point and Latent Heat of Melting Some
Non Paraffin.
Materials Melting Point
(
o
C)
Latent Heat of
Melting (kJ/kg)
Formic acid 7.8 247
Caprilic acid 16.3 149
Glycerine 17.9 198.7
α-Lactic acid 26 184
Methyl palmitat 29 205
Phenol 41 120
Bee wax 61.8 177
Gyolic acid 63 109
Azobenzene 67.1 121
Acrylic acid 68.0 115
Materials Melting Point
(
o
C)
Latent Heat of
Melting (kJ/kg)
Glutaric acid 97.5 156
Catechol 104.3 207
Quenon 115 171
Benzoic acid 124 167
Benzamide 127.2 169.4
Oxalate 54.3 178
Alpha naphol 96 163
Source: Sharma et al.2009
Table 3: Melting Point Heat of Latent Melting Some Fatty
Acids.
Materials Melting
Point (
o
C)
Latent Heat
of Melting
(kJ/kg)
Acetic acid 16.7 184
Poly ethylene glycol 20.25 146
Capric acid 36 152
Eladic acid 47 218
Lauric acid 49 178
Pentadecanoic acid 52.5 178
Tristearin 56 190
Mirystic acid 58 199
Palmatic acid 55 163
Stearic acid 69.4 199
Acetamide 81 141
Source: Sharma et al.2009
2.2 Anorganic
PCM anorganic PCM is classified as salt hydrate and
metallic. This type of PCM is not very cold and the
heat of fusion will not decrease during rotation.
2.2.1 Hydrates of Salts
Hydrates of salt can be seen as mixtures of inorganic
salts with water to form certain crystalline solids of
the general formula AB.nH2O. The solid-liquid
change of the salt hydrate is a dehydration process of
the salt hydration. Salt hydrates usually melt into a
salt hydrate with very small moles of water.
AB.nH2O → AB.mH2O + (n-m)H2O
or into anhydrous form,
AB.nH2O → AB + nH2O
At their melting point, the hydrate crystals split into
anhydrous salt and water or into a lower hydrate and
water. Salt hydrates are the most important and
widely studied type of PCM in energy storage
systems. The most prominent properties of this type
of PCM are high latent heat of fusion per unit volume,
relatively high thermal conductivity (almost twice
that of paraffin) and small volume change during
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
734
melting. This type of PCM is also less corrosive,
compatible with plastics and only a few types are
toxic. Many types of salt hydrates are inexpensive to
use as heat stores. In Table 4 it can be seen several
types of PCM from salt hydrates.
Table 4: Melting Point and Latent Heat of Melting Some
Salt Hydrates.
Materials Melting
Point (
o
C)
Latent Heat
of Melting
(kJ/kg)
K
2
HPO
4
.6H
2
O 14,0 109
FeBr3.6H
2
O 21,0 105
Mn(NO
3
)
2
.6H
2
O 25,5 148
FeBr
3
.6H
2
O 27,0 105
CaCl
2
.12H
2
O 29,8 174
LiNO
3
.2H
2
O 30,0 296
LiNO
3
.3H
2
O 30 267
Na
2
O
3
.10H
2
O 32,0 241
Na
2
SO
4
.10H
2
O 32,4 173
KFe(SO4)2.12H20 33 138
CaBr
2
.6H
2
O 34 124
LiBr
2
.2H
2
O 34 134
Zn(NO
3
)
2
.6H
2
O 36,1 223
Source: Sharma et al.2009
2.2.2 Metals
This type of metal also includes metals with low
melting points and metal alloys. This type of PCM
has not received much attention because it is very
heavy. If volume is a concern, this type is the choice
because it has a high latent heat of fusion per unit
volume. This metal has a high thermal conductivity
so that no additional heavy filler is needed. A list of
some metallic materials can be seen in Table 5.
Table 5: Melting Point and Latent Melting Heat of Some
Metallics.
Materials Melting
Point (
o
C)
Latent Heat of
Melting
(kJ/kg)
Gallium-gallium
Antimony eutectic
29,8 -
Gallium 30,0 80,3
Cerrolow eutectic 58 90,9
Bi-Cd-In eutectic 61 25
Cerrobend
eutectic
70 32,6
Bi-Pb-In eutectic 70 29
Bi-In eutectic 72 25
Bi-Pb-tin eutectic 96 -
Bi-Pb eutectic 125 -
Source: Sharma et al.2009
2.3 PCM Combination
PCM combination is a composition with the lowest
melting point of two or more components, each of
which melts and solidifies to form a mixture of
crystalline components during the crystallization
process (George 1989). This type of PCM almost
always melts and solidifies without separation
because they solidify into a crystalline mixture,
giving the components little chance to separate. At the
time of melting the two components melt sequentially
with unwanted separation.
Table 6: List of PCM Combinations of Organic-Anorganic.
Materials Melting
Point
(
o
C)
Latent
Heat of
Melting
(kJ/kg)
CaCl
2
.6H
2
O+CaBr
2
.6H
2
O 14,4 140
Triethylethane+water+urea 13,4 160
CaCl
2
+MgCl
2
+6H
2
O 25 95
NH
3
CONH
2
+ NH
3
CONH
2
27 163
Naphtalene+benzoic acid 67 123,4
Freezer salt -50 325
Freezer salt -23 330
Freezer salt -16 330
Source: Sharma et al.2009
2.4 State of the Art
(Hosseinizadeh, Tan, and Moosania 2011) This study
compared heatsinks with different numbers of fins to
cool electronic components, where the results obtained
in heat absorption from a heat zing source with 7 fins
gave an average temperature at low heat zing. Thicker
heat zing results in better heat zing performance and 4
mm and 6 mm thickness have the best effectiveness in
absorbing heat from the source. The higher the fin, the
lower the heat zing temperature. (Markandeyulu,
Krishna Devanuri, and Kiran Kumar 2016) For thermal
management of electronic components, PCM can be a
promising option because it does not need external
assistance and because of its compatibility in size.
PCM can remove heat from electronic components at
a constant temperature where the temperature rise can
be effectively limited. By using PCM, product failures
and assembly failures can be reduced which can further
lead to an increase in productivity. The main problem
with PCM is its low thermal conductivity. This
problem can be overcome by adding high thermal
conductivity materials (eg conductivity enhancers),
nanoparticles, matrix metal foam and metal fins.
(Rehman et al. 2018) The results show that the heat
zing performance with PCM is better than the heat zing
Utilization of Parafin and TiO2 as Phase Change Materials (PCM) for Processor Coolers
735

performance without PCM. The higher volume
fraction of PCM results in more drop in heat zing
temperature at the same input power. Different PCM
materials exhibit different behavior. (Fan et al. 2016)
The results showed that PCM material with molten
metal had a greater impact on temperature reduction on
heat zing than 1-octadecanol organic PCM material.
Judging from the protection time, the liquid metal has
a protection twice as long as 1-octadecanol. (Wang et
al. 2021) Higher alcohol/graphite foam PCM materials
have the ability to lower the heat zing temperature
better than PCM materials using higher alcohol with a
better percentage of 24%. The heat capacity of PCM
higher alcohol/graphite foam material reaches 102.32
J/K with a thermal conductivity of 54.22 W/cm2.
3 EXPERIMENT SETUP AND
PROCEDURE
3.1 Research Design
This study was designed using simulation, where the
processor simulated as a source of heat energy, so a
heater with a power of 120 W is made with a heat
capacity of 40 - 70
o
C where this temperature is the
operating temperature of the processor. The heatsink
is made with dimensions of 73 x 73 x 55 mm, as
shown in Figure 1.
PCM material uses paraffin base material with
several variations of TiO
2
concentration and the test
device will be made as shown in Figure 2.
3.2 Determination of Data Sources
The data used in this study is primary data in the form
of temperature data obtained from temperature
measurements placed on the heatsink using a
thermocouple and other primary data in the form of a
mixture of PCM material, namely paraffin and TiO
2
.
In addition to primary data, secondary data is also
obtained from related research data sources in the
form of PCM material characteristics.
3.3 Research Variables
The variables of this study are independent
variables, dependent variables and control variables.
The independent variables in this study are PCM
material and the shape of the heatsink that is made
to vary. The dependent variable is the temperature
generated by the system due to changes in the
variation of the PCM material and the shape of the
Heatsink. As a control variable, namely the input
power given to the system.
Figure 1: Heatsink Model.
(Fan et al. 2016)
Figure 2: Model Design Test Equipment.
3.4 Research Materials
The materials used in this research are PCM materials
in the form of paraffin and TiO2, and the selected
heatsink material is aluminum because it has good
heat conductivity.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
736
3.5 Research Instruments
Several research instruments used in this study are
temperature measuring instruments, namely
thermocouples, AC power meters which are used to
supply power to the system, AC Adapter which
functions to convert alternating current (AC current)
into direct current (DC current), one for the heating
unit that functions as a heat source as a simulation of
the processor heat, Digital PID Temperature
Controller to read the temperature, Solid State Relay
SSR 25A to disconnect and connect current to the
heating system which is controlled via digital PID.
3.6 Research Procedure
This research will begin by making a test instrument
in the form of a PCM-based processor cooling
simulation. After the simulation is complete, the
operational system is tested before taking data. Data
collection was carried out with a combination of
parafin and TiO
2
with a mixture as shown in table 7.
Table 7: Sample Mixture of Paraffin and TiO
2
as PCM.
No
Mixed Percentage (%)
Parafin TiO
2
1 100 0
2 98 2
3 96 4
4 94 6
5 92 8
6 90 10
3.7 Data Analysis Methods
In this study, the data analysis method used is a
descriptive statistical method. Temperature data
obtained from measurements are presented in tabular
form or in graphical form as a basis for making
decisions. The data will be further processed so that it
is known the effect of using PCM and the shape of the
Heatsink on the cooling effectiveness of the
processor. In addition to temperature data, photo data
obtained from digital cameras that are used to observe
phenomena that occur in PCM materials during
testing will be analyzed qualitatively.
4 RESULTS AND DISCUSSION
Simulated processor temperature by heating with
heatsink cooling without PCM, heatsink with PCM
using pure paraffin and heatsink with PCM material
with some mixture of paraffin and TiO2 can be seen
in Figure 3.
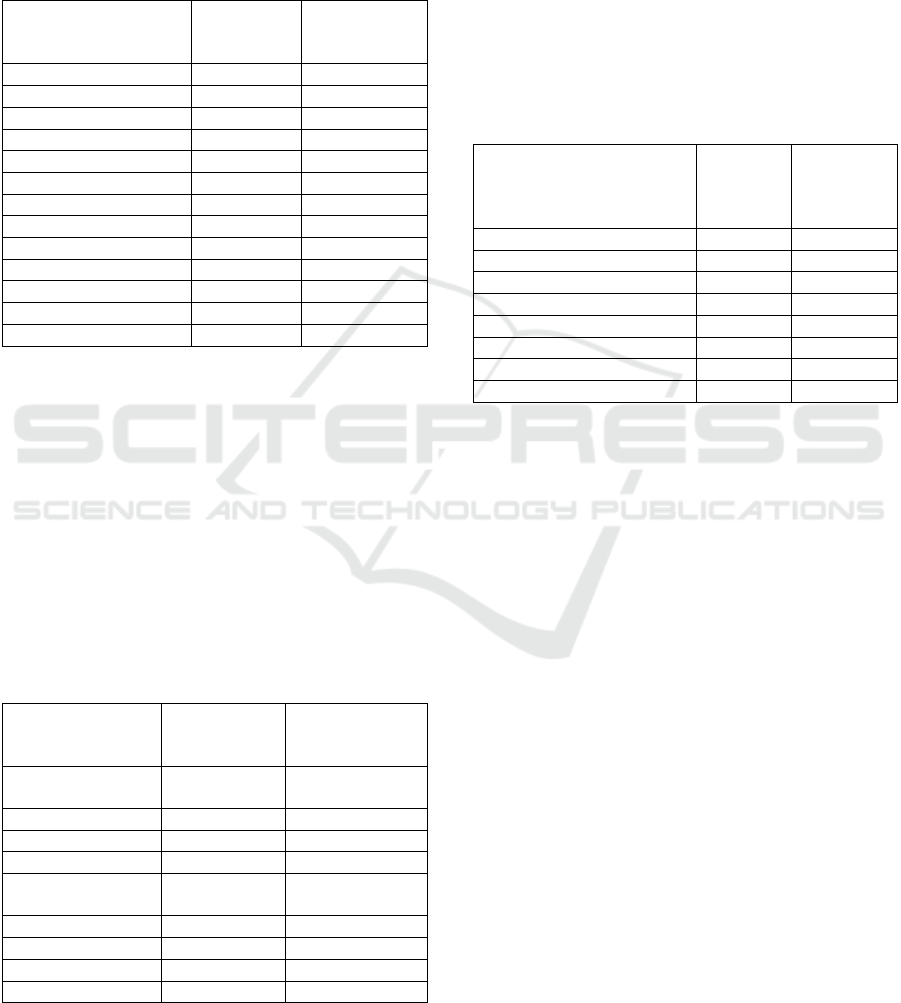
Figure 3: Chart of the effect of adding PCM to the heatsink
on the cooling effect of the processor.
From Figure 3, it can be seen that the cooling of the
processor with a heatsink without a PCM shows that
the temperature continues to increase from 0 seconds
to 150 seconds, and the processor temperature
reaches 93
o
C in 150 seconds. Processor temperature
that continues to increase due to cooling by
conduction method from the processor to the heatsink
and free convection alone is not able to eliminate the
presence of heat in the processor. The addition of
paraffin to the heatsink is able to stabilize the
temperature on the processor below 50
o
C. The use of
TiO2 mixed with paraffin as PCM material is able to
increase the thermal conductivity of PCM as
evidenced by the faster cooling effect that occurs in
the processor, where the greater the TiO2 content
given to paraffin, the faster the cooling effect.
4.1 Effect of Using Heatsink without
PCM on Processor Cooling
Cooling with heatsink without PCM shows a
significant increase in processor temperature starting
from 0 minutes to 15 minutes continues to increase.
The processor temperature at 47
o
C only lasted until
the first 3 minutes, then the processor temperature
continued to increase, meaning that cooling the
processor only by relying on the heatsink was not
enough, maybe because the area of conduction heat
transfer from the processor to the heatsink was not
enough. Figure 4 shows the power input On at minute
1 to minute 3 then at minute 4 the power input is Off
and the 5th minute is On again and so on. The input
power is set at a temperature of 47
o
C if the processor
temperature reaches 47
o
C then the input power is Off
and if the processor temperature is below 46
o
C then
the input power is On.
0
10
20
30
40
50
60
70
80
90
100
0123456789101112131415
Temperature (oC)
Time (menit)
Haeatsink
Without PCM
Heatsink with
pure paraffin
PCM
Heatsink with
PCM Parafin +
2%TiO2
Heatsink with
PCM Parafin +
4%TiO2
Heatsink with
PCM Parafin +
6%TiO2
Heatsink with
PCM Parafin +
8%TiO2
Utilization of Parafin and TiO2 as Phase Change Materials (PCM) for Processor Coolers
737

Figure 4: Input Power On/Off On Processor Cooling with
Heatsink without PCM.
4.2 Effect of Using Heatsink with PCM
Made of Pure Paraffin
The addition of pure paraffin to the heatsink affects
the cooling effect on the processor, where the
processor reaches the 7th minute the temperature is
still below 47
o
C which is indicated by the supply the
power is in the On position and at the 8th minute the
power supply is Off, meaning that the processor
temperature has reached 47oC or more and at the 9th
minute the power supply is On, it means that the
processor temperature is below 47
o
C, this is because
at a temperature of 47
o
C the paraffin has started to
melt so it absorbs heat that occurs in the processor so
that the processor temperature drops again. In the
13th to 15th minute the power supply is turned off
again because the temperature that has occurred has
reached 47
o
C or more and at the 15th minute the
power supply is back on. An overview of On/Off
Supply power based on heating time can be seen in
Figure 5.
Figure 5: Input Power On/Off On Processor Cooling with
Heatsink Using Pure Paraffin As PCM.
4.3 Effect of Using Heatsink with PCM
Made of Paraffin + 2% TiO
2
The addition of a mixture of paraffin + 2% TiO2 on
the heatsink has an impact on the temperature that
occurs in the processor. With the addition of 2% TiO
2
to paraffin, it provides a fairly stable cooling effect,
as evidenced by the persistence of the processor
temperature below 47
o
C until the 8th minute and at
the 9th - 11th minute the temperature is still in the
range of 47
o
C - 48
o
C, then at the 12th minute up to
15 minutes the processor temperature is below 47
o
C
Again, due to the PCM material on the heatsink has
melted and the process of absorbing heat from the
processor to the heatsing occurs. An overview of
On/Off Supply of power based on heating time can be
seen in Figure 6.
Figure 6: Input Power On/Off in Processor Cooling with
Heatsink Using Paraffin + 2% TiO
2
as PCM.
4.4 Effect of Using Heatsink with PCM
Made of Paraffin + 4% TiO
2
Addition of a mixture of paraffin + 4% TiO
2
to the
heatsink has an impact on the temperature that occurs
on the processor. With the addition of 4% TiO
2
in
paraffin, it provides a fairly stable cooling effect
where the processor temperature is only at 10 minutes
which exceeds 47
o
C, as evidenced by the power
supply being On at all times except Off at 10 minutes.
This shows that the addition of 4% TiO
2
to paraffin
has a significant impact on the cooling process of the
processor. An overview of On/Off Supply of power
based on heating time can be seen in Figure 7.
Figure 7: Input Power On/Off In Processor Cooling with
Heatsink Using Paraffin + 4% TiO
2
As PCM.
1111
0
1
0
1
0
1
0
1
0
1
0
1
0
1
0123456789101112131415
Power Supply
On/Off
Time (minute)
1111111
0
1111
000
1
0
1
12345678910111213141516
Power Supply On/Off
Time (minute)
11111111
000
11111
0
1
12345678910111213141516
Power Supply On/Off
Time (minute)
1111111111
0
11111
0
1
0123456789101112131415
Power Supply On/Off
Time (minute)
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
738

4.5 Effect of Heatsink Utilization with
PCM Made of Paraffin +6% TiO
2
The addition of a mixture of paraffin + 6% TiO
2
on
the heatsink has an impact on the temperature that
occurs on the processor. With the addition of 6% TiO
2
to paraffin, it provides a fairly stable cooling effect
where the processor temperature is only at 10 minutes
which exceeds 47
o
C, as evidenced by the power
supply being On at all times except Off at 10 minutes.
This shows that the addition of 6% TiO
2
to paraffin
has a significant impact on the cooling process of the
processor. The difference with 4% TiO
2
content is in
the temperature difference at each time, which is the
overall processor temperature at each time is lower
than PCM with 4% TiO
2
content. An overview of
On/Off Supply of power based on heating time can be
seen in Figure 8.
Figure 8: Input Power On/Off on Processor Cooling with
Heatsink Using Paraffin + 6% TiO
2
As PCM.
4.6 Effect of Using Heatsink with PCM
Made of Paraffin +8% TiO
2
The addition of a mixture of paraffin + 8% TiO
2
on
the heatsink has an impact on the temperature that
occurs on the processor. With the addition of 8% TiO
2
to paraffin, the temperature effect in the early minutes
is lower than the percentage of TiO
2
below 8%, but
the achievement of temperatures above 47
o
C is also
faster. In this mixture of paraffin + 8% TiO
2
, the
processor temperature is also less stable, as shown in
Figure 9. On/Off fluctuations occur in the power
supply due to temperature fluctuations that occur in
the processor due to an increase in the conductivity of
the PCM material which causes the PCM material to
melt earlier.
Figure 9: Input Power On/Off on Processor Cooling with
Heatsink Using Paraffin + 8% TiO
2
As PCM.
4.7 Effect of Using Heatsink with PCM
Made of Paraffin + 10% TiO
2
The addition of a mixture of paraffin + 10% TiO
2
on
the heatsink has an impact on the unstable
temperature that occurs in the processor. Processor
temperature characteristics that occur in a mixture of
paraffin + 10% TiO
2
are almost similar to a mixture
of paraffin + PCM 8% TiO
2
. An overview of On/Off
Supply of power based on heating time can be seen in
Figure 10.
Figure 10: Input Power On/Off on Processor Cooling with
Heatsink Using Paraffin + 8% TiO
2
As PCM.
5 CONCLUSION
From the research that has been done, it can be
concluded as follows:
1. The addition of PCM to the heatsink has a
significant effect on the temperature that occurs
in the processor, where the addition of PCM to
heatzing can reduce the temperature that occurs
in the processor compared to using a heatsink
without PCM.
1111111111
0
11111
0
1
0123456789101112131415
Power Supply On/Off
Time (minute)
1111111
00
111
0
111
0
1
12345678910111213141516
Power Supply On/Off
Time (minute)
1111111
00
111
0
111
0
1
12345678910111213141516
Power Supply On/Off
Time (minute)
Utilization of Parafin and TiO2 as Phase Change Materials (PCM) for Processor Coolers
739

2. The addition of TiO2 concentration in paraffin
as PCM material can stabilize the temperature
that occurs in the processor.
3. The addition of 4% and 6% TiO2 concentrations
in paraffin gave the best impact on processor
cooling.
ACKNOWLEDGMENT
Thank you to the Bali State Polytechnic for the
financial assistance that has been given so that this
research can be carried out. Researchers really hope
that this research can be continued with several
heatsink models and other PCM composite material
models, so that an effective and efficient processor
cooling system is found.
REFERENCES
Abhat, A, D Heine, M Heinisch, N A Malatidis, and G
Neuer. 1981. “Development of a Modular Heat
Exchanger with an Integrated Latent Heat Storage.”
Report No. BMFT FBT.
Bayat, Milad, Mohammad Reza Faridzadeh, and Davood
Toghraie. 2018. “Investigation Of Finned Heat Sink
Performance With Nano Enhanced Phase Change
Material (NePCM).” Thermal Science and Engineering
Progress. https://doi.org/10.1016/j.tsep.2017.10.021.
Fan, Li Wu, Yu Yue Wu, Yu Qi Xiao, Yi Zeng, Yi Ling
Zhang, and Zi Tao Yu. 2016. “Transient Performance
of a Thermal Energy Storage-Based Heat Sink Using a
Liquid Metal as the Phase Change Material.” Applied
Thermal Engineering 109. https://doi.org/10.1016/
j.applthermaleng.2016.08.137.
Hikma, Nurfi, Saktioto Jurusan, Fisika Fakultas,
Matematika Dan Ilmu, Pengetahuan Alam, and
Universitas Riau. 2020. Air Conditioning Di Kota
Pekanbaru.
Hosseinizadeh, S. F., F. L. Tan, and S. M. Moosania. 2011.
“Experimental and Numerical Studies on Performance
of PCM-Based Heat Sink with Different Configurations
of Internal Fins.” In Applied Thermal Engineering. Vol.
31. https://doi.org/10.1016/j.applthermaleng.2011.07.0
31.
Kandasamy, Ravi, Xiang Qi Wang, and Arun S. Mujumdar.
2008. “Transient Cooling of Electronics Using Phase
Change Material (PCM)-Based Heat Sinks.” Applied
Thermal Engineering 28 (8–9): 1047–57.
https://doi.org/10.1016/j.applthermaleng.2007.06.010.
Markandeyulu, Thota, Jaya Krishna Devanuri, and K. Kiran
Kumar. 2016. “On the Suitability of Phase Change
Material (PCM) for Thermal Management of Electronic
Components.” Indian Journal of Science and
Technology 9 (S1). https://doi.org/10.17485/ijst/2016/
v9is1/107939.
Rehman, Tauseef ur, Hafiz Muhammad Ali, Ahmed Saieed,
William Pao, and Muzaffar Ali. 2018. “Copper
Foam/PCMs Based Heat Sinks: An Experimental Study
for Electronic Cooling Systems.” International Journal
of Heat and Mass Transfer 127. https://doi.org/
10.1016/j.ijheatmasstransfer.2018.07.120.
Risano, A Yudi Eka, M Dyan Susila E S, and Yoga
Pratama. 2017. “Aplikasi Minyak Kelapa Sawit Pada
Photovoltaic Yang Terintegrasi Pada Dinding
Bangunan Sebagai Pendingin Pasif Untuk
Meningkatkan Efisiensi Dan Menurunkan Beban
Pendingin Ruangan.” Turbo : Jurnal Program Studi
Teknik Mesin 6 (1). https://doi.org/10.24127/trb.v6i1.4
60.
Sharma, Atul, V. V. Tyagi, C. R. Chen, and D. Buddhi.
2009. “Review on Thermal Energy Storage with Phase
Change Materials and Applications.” Renewable and
Sustainable Energy Reviews. https://doi.org/10.1016/
j.rser.2007.10.005.
Wang, Shisong, Yuming Xing, Zhaolong Hao, Jianbao Yin,
Xu Hou, and Zixian Wang. 2021. “Experimental Study
on the Thermal Performance of PCMs Based Heat Sink
Using Higher Alcohol/Graphite Foam.” Applied
Thermal Engineering 198. https://doi.org/10.1016/
j.applthermaleng.2021.117452.
Putra, P., Liriwati, F. Y., Tahrim, T., Syafrudin, S., &
Aslan, A. (2020). The students learning from home
experience during covid-19 school closures policy in
indonesia. Jurnal Iqra, 5(2).
George, J. M. (1989). Mood and absence. Journal of
applied psychology, 74(2), 317.
iCAST-ES 2022 - International Conference on Applied Science and Technology on Engineering Science
740
