
Antibacterial Activities of Ethanol Extract of Karamunting
(Melastoma malabathricum L.) Leaf and Flowers on Salmonella typhi,
Escherichia coli, Staphylococcus aureus
Isnaini
1
*, Lia Y. Budiarti
2
, Noor Muthmainah
2
, Dimas S. Baringgo
3
, Ririn Frisilia
3
,
Nanda Sulistyaningrum
3
, Irawati F. Batubara
3
, Wuri Sofiratmi
3
, Wiresa D. Renalta
3
1
Departement of Pharmacology and Therapy, Faculty of Medicine, University of Lambung Mangkurat Banjarmasin, South
Borneo, Indonesia
2
Departemen Mikrobiology, Faculty of Medicine, University of Lambung Mangkurat Banjarmasin, South Borneo,
Indonesia
3
Student of Faculty of Medicine, University of Lambung Mangkurat Banjarmasin, South Borneo, Indonesia
Keywords: Melastoma malabathricum L, Karamunting, Salmonella typhi, Escherichia coli, Staphylococcus aureus
Abtract: Karamunting (Melastoma malabathricum Linn) is native spesies from Borneo. This plant is easily found in
Borneo as shrub. M. malabathricum L has not been used optimally, only considered a pest. M.
malabathricum L contains flavonoid, saponin, tanin and alkoloid, which serve as antibacterial agents. In this
study we tested the antibacterial activity of M. malabathricum L leaves and flowers against bacteria
Salmonella typhi ATCC 14028, Escherichia coli ATCC 25922 and Staphylococcus aureus ATCC 25923.
Testing of antibacterial activity using diffusion method by measuring the inhibition zone formed around the
paper disk. The results of phytochemical scheming of ethanol extract of the leaves indicates M.
malabathricum L to contain flavonoid, saponin, tannin, and alkaloid, and flowers to emit flavonoid, saponin
and tannin. Profoundly, its ethanolic exttract of the leaves have the antibacterial agents respectively lead to
the most effective inhibitory effect 28.2 mm in diameter on Salmonella typhi, and that of its flowers of 27.2
mm on Escherichia coli.
1 INTRODUCTION
Melastoma malabathricum L (karamunting) is a
native species from South Borneo. This plant is
easily found in Borneo as shrub. M. malabathricum
L has not been used optimally, only considered a
pest. Based on the study, M. malabathricum L
flowers contain flavonoid, saponin, and tannin
components (Isnaini et al., 2010). Flavonoid have
activity as antioxidant (Unoufin et al., 2017),
anticancer (Raffa et al., 2017), antibacterial
(Unoufin et al., 2017).
Flavonoid contained in the flower of M.
malabathricum L, namely quercetin, kaempferol,
and antosianin (Janna et al., 2006; Isnaini et al.,
2017). Quercetin, kaempferol and anthocyanin are
antibacterial (Borrás-Linares et al., 2015; Valle et
al., 2016; Yang et al., 2017).
Each part of the plant has a different activity
because of the different content. Antibacterial
activity of melaboma malabathricum L. leaves and
flowers is unknown in bacterial Salmonella typhi
ATCC 14028, Escherichia coli ATCC 25922 and
Staphylococcus aureus ATCC 25923
2 MATERIAL AND METHOD
2.1 Materials
The research materials used were flower and leaves
M. malabathricum, isolate Salmonella typhi ATCC
14028, Escherichia coli ATCC 25922 and
Staphylococcus aureus ATCC 25923, which was
cultured in Microbiology Laboratory of Medical
Faculty UNLAM, ethanol 70%, so that Mc conkey,
Mueller Hinton (MH), CMC-Na, sterile aquades,
blank disc paper, Brain Heart Infusion (BHI),
standard solution of Mc farland I of 3.108 cfu / ml,
standard ampicillin disk, standard chloramphenicol
disk
2.2 Extraction
Leaves and flowers of M. malabathricum L obtained
in Kelurahan Guntung Manggis, Banjarbaru, South
Kalimantan. Identification of plant species to be
studied was done by Basic Laboratory of Faculty of
Biology MIPA UNLAM with sample no. 095/TS-
02/011. Extraction was done by maceration method
using 70% ethanol solvent with a ratio of 1: 5 and
soaked for 24 hours with 3 repetitions. The
Isnaini, ., Budiarti, L., Muthmainah, N., Baringgo, D., Frisilia, R., Sulistyaningrum, N., Batubara, I., Sofiratmi, W. and Renalta, W.
Antibacterial Activities of Ethanol Extract of Karamunting (Melastoma malabathricum L.) Leaf and Flowers on Salmonella typhi, Escherichia coli, Staphylococcus aureus.
DOI: 10.5220/0009846600002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1
ethanolic extract will be evaporated using a rotary
evaporator at a low pressure with a temperature of
60 ° C until a thick ethanolic extract is obtained.
2.3 Phytochemical Screening
The phytochemical screening used ethanol extract.
The identification of alkaloids using Dragendorff
reagent. The flavonoids were identified using
magnesium. The identification of tannins were
used 1% FeCl and identification of saponin used
olive oil, if the extract have saponin will have stable
emulsion (Moreira, 1979; Buveniswari et al., 2011)
2.4 Preparation of Bacteria
The bacteria used in this study was grown on a
medium for Mc conkey. After that it was incubated
in the incubator for 24 hours at 370C. The colonies
have good colonies are selected, which form round
colonies rather convex, clear and slippery then
grown on BHI seedling for 6 hours at 37
0
C. Further
dilute the suspension with sterile aquades until the
turbidity is proportional to the standard of Mc
Farland I (3.108 cfu / ml).
2.5 Antibacterial Test
Microorganism standardized with Mc Farland I,
smeared with sterile cotton swabs on MH agar
medium, then blank paper disks immersed in leaves
and flowered ethanol extract of M. malabathricum L
for 3 hours were placed on MH medium then
incubated at 37° C for 24 hours . How to measure
antibacterial power by measuring the diameter of a
radical zone using a ruler in millimeters.
Replication is done 3 times.
3 RESULT AND DISCUSSION
Results of phytochemical screening of ethanolic
extract from leaves and flowers of M.
malabathricum L showed in table 1. In the extract
ethanolic from leaves of M. malabathricum L
content flavonoid, alkaloid, saponin and tannin, but
in the flowers there is not have alkaloid.
Table 1: The content of phytochemical compounds on M.
malabathricum L
Compds
Leaves
Flowers
Flavonoid
+
+
Alkaloid
+
-
Saponin
+
+
Tannin
+
+
Results of activities antibacterial from ethanolic
extract from leaves and flowers of M.
malabathricum L showed in figure 1 and figure 2.
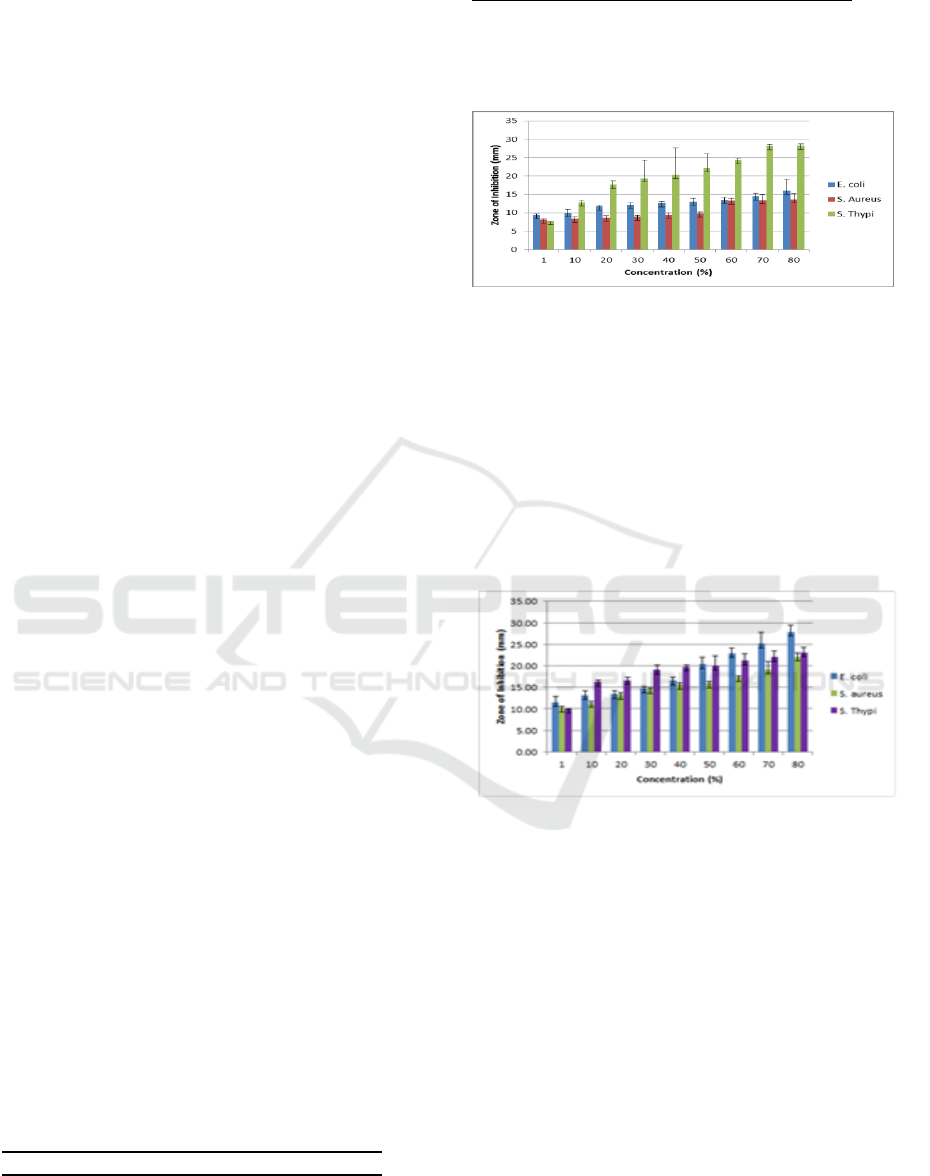
Figure 1: Zone of Growth Inhibition caused ethanolic
extract of M. malabathricum L leaves
Extract ethanolic from leaves M. malabathricum
L has the greatest activity in bacterial S. Thypii,but
extract ethanolic from flowers M. malabathricum L
has the greatest activity in bacterial E. coli.
Differences in phytochemical content of extract
ethanolic from leaves and floers cause different
activities. The extract ethanolic from flower does
not contain alkaloid compounds, so the activity
antibacterial is more effective for E. coli.
Figure 2. Zone of Growth Inhibition caused ethanolic
extract of M. malabathricum L flowers
Flavonoids are polyphenolic compounds. The
flavonoid derivative in the hydroxyl group in the β
ring is more active against microorganisms than in
the 2-OH group (Maftuch et al., 2016). This
suggests that the target of this component is a
lipophilic compound through a bacterial membrane.
Flavonoids have the ability to form complexes with
soluble extracellular proteins and bacterial cells
(Bilal et al., 2017). There are three mechanisms of
flavonoids, which inhibit nucleic acid synthesis,
inhibit cytoplasmic membrane function and inhibit
energy metabolism (Maftuch et al., 2016).
Alkaloids form intercellate with double helix
DNA and uncouple respiration (Bilal et al., 2017).
Tanin is a phenolic polymeric compound. The
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2

antimicrobial metabolism of tannins is associated
with microbial adhesion inactivation, enzyme cell
envelope transport protein, causing toxicity in
bacterial filaments, and tannins also bind to protein
walls to inhibit bacterial growth (Pandey and
Kumar, 2013).
4 CONCLUSION
The differences in phytochemical content of leaves
and flowers of M. malabathricum L cause
differences in antibacterial activity
ACKNOWLEGMENTS
Thanks to the Medical Faculty of the University
Lambung Mangkurat Banjarmasin in South Borneo,
which has provided grants to this research
REFERENCES
Bilal, M., T. Rasheed, H.M.N. Iqbal, H. Hu, X. Zhang,
W. Wang. 20.17. Macromolecular agents with
antimicrobial potentialities : A drive to combat
antimicrobial resistance. International Journal of
Biological Macromolecules 103 : 554 - 574
Borrás-Linares, I., S.Fernández-Arroyo, D.Arráez-Roman,
P.A.Palmeros-Suárez, R.Del Val-Díaz, I.Andrade-
Gonzáles, A.Fernández-Gutiérrez, J.F.Gómez-Leyva,
A.Segura-Carretero. 2015. Characterization of
phenolic compounds, anthocyanidin, antioxidant and
antimicrobial activity of 25 varieties of Mexican
Roselle (Hibiscus sabdariffa). Industrial Crops and
Products 69 : 385-394
Buvaneswari K, Ramamoorthy D, Velanganni J.
Preliminary phytochemical and antimicrobial activity
studies on the leaves of the Indian plant Thevetia
neriifolia Juss. World J Agric Sci. 2011;7(6):659–666.
Isnaini, M. Bakhriansyah, Hendra W. 2010. Potential
antidiiarrhea of M. malabathricum L fruits in male-
induced by Oleum ricini in Mus musculus. Research
report. Not published
Isnaini, Nur Permatasari, Karyono Mintaroem, M. Aris
Widodo. 2017. Analysis of quercetin and kaempferol
levels in various phase of flowers Melastoma
malabathricum L. International Journal of Plant
Biology 8 : 69 – 72
Janna, OA., Khairul A, Maziah M., Mohd Y. 2006. Flower
pigment analysis of Melastoma malabathricum.
African Journal of Biotechnology (5) 2 : 170-174
Maftuch, I. Kurniawati, A. Adam, I’ah Zamzami. 2016.
Antibacterial Effect Of Gracilaria verucosa bioactive
on Fish Pathogenic Bacteria. Egyptian Journal Of
Aquatic Research 42 : 405 – 410
MoreiraEA.Contribuic¸ãoparaoestudofitoquímicode
Lobelia hassleri A Zahlb e Lobelia stellfeldii R Braga
Companulaceae. Tribuna Farm. 1979;47:13–39.
A.K. Pandey and S. Kumar. 2013. Perspective on Plant
Products as Antimicrobials Agents : A Review.
Pharmacologia 4 (7) : 469 – 480
Raffa, D., B.M. Maria, V. Raimondi, F. Plescia, G.
Daidone. 2017. Recent discoveries of anticancer
flavonoids. European Journal of Medicinal Chemistry
142 : 213-228
Unuofin, J.O., G.A.Otunola, A.J. Afolayan. 2017.
Phytochemical screening and in vitro evaluation of
antioxidant and antimicrobial activities of Kedrostis
africana (L.) Cogn. Asian Pacific Journal of Tropical
Biomedicine 7 (10) : 901-908
Valle, P., M.R. García-Armesto, D. Arriaga, C. González-
Donquiles, P. Rodríguez-Fernández, J. Rúa. 2016.
Antimicrobial activity of kaempferol and resveratrol in
binary combinations with parabens or propyl gallate
against Enterococcus faecalis. Food Control 61 : 213-
220
Yang, X., W. Zhang, Zh. Zhao, N. Li, Zh. Mou, D. Sun,
Y. Cai. W. Wang, Y. Lin. 2017. Quercetin loading
CdSe/ZnS nanoparticles as efficient antibacterial and
anticancer materials. Journal of Inorganic
Biochemistry 167 : 36-48
Antibacterial Activities of Ethanol Extract of Karamunting (Melastoma malabathricum L.) Leaf and Flowers on Salmonella typhi,
Escherichia coli, Staphylococcus aureus
3
