
Cell Cycle Inhibition and Apoptosis Induction Activities of N-hexane
Fraction of Cyperus Rotundus L. Rhizome
Masfria
1
, Urip Harahap
2
, Denny Satria
3
1
Department of Pharmaceutical Chemistry,
2
Department of Pharmacology,
3
Department of Pharmaceutical Biology
Faculty of Pharmacy, University of Sumatera Utara
Keywords: Cell cycle, Apoptosis, Cyperus rotundus L., rhizome, n-hexane.
Abstract: Breast cancer is one of the leading cause of death and the most common cancer type amongst women in the
world after cervic cancer. To evaluate the cell cycle inhibition and apoptosis induction activities on T47D
cell lines of n-hexane fraction (nHF) of Cyperus rotundus L. rhizomes. Ethanol extract was obtained by
maceration method and was fractionated with n-hexane. Cytotoxic activity was examined with MTT assay,
and cell cycle inhibition, apoptosis induction and cyclin D1 expression were assessed with flow cytometry
method. Cytotoxic activity of nHF was found to have IC
50
of 71.69 ± 0.34 µg/mL, nHF at concentration 35
µg/mL caused accumulation in G
0
-G
1
and S phase accumulation (56.89% and 19.36%), increased early
apoptosis (26.30%) and decreased expression of cyclin D1 (26.30%). The results reveal that nHF of
Cyperus rotundus L. rhizomes has cell cycle inhibition and apoptosis induction activities. Our further study
is to isolation compounds which responsible for these activities.
1 INTRODUCTION
Breast cancer is one of the leading cause of death
and the most common cancer type amongst women
worldwide in 2012 (WHO, 2015). Breast cancer is
the second cause of cancer death in developed
countries after lung cancer. A recent study has
reported that breast cancer is the first in the
predicted new cancer cases, and the second most
incidence death cause of women suffering from
cancer in the United States (Siegel, et. al., 2015).
Cyperus rotundus L. (Cyperaceae) have been
used as the drug of stomachache, disorders bowel,
menstrual irregularities (Peerzada, et al., 2015).
Bioassay investigations indicated which the extract
of Cyperus rotundus L. exerts antioxidant,
antibacterial, insecticidal activity and its essential oil
have activity as antiradical, antimutagenic and
cytotoxic. Essential oil and steroids/ triterpenoids
could extracted with non polar solvent such as n-
hexane and chloroform (Hemanth, et al., 2014; Hadi,
et al., 2007; Vitaglione, et al., 2004; Lanciotti, et al.,
2004; Kilani, et al., 2008; Tenore, et al., 2011; Nam,
et al., 2016; Sonwa and Konig, 2001; Liu, et al.,
2016; Kilani, et al., 2007; Jirovetz, et al., 2004;
Memariani, et al., 2016). Cyclin D1 has an important
role in cell cycle process in G
0
-G
1
phase (Żurynń, et
al., 2016). This study aimed to determine cell cycle
inhibition and apoptosis induction activities of n-
hexane fraction of Cyperus rotundus L. rhizomes.
2 MATERIALS AND METHODS
2.1 Plant and Chemicals Material
Fresh rhizomes of Cyperus rotundus L. was
collected from Paya Tumpi, Aceh Tengah regency,
Nangroe Aceh Darussalam province, Indonesia.
Cyperus rotundus L. was identified in Herbarium
Medanense, Faculty of Mathematics and Natural
Sciences, University of Sumatera Utara. Chemicals
used were annexin-V (BioLegend), cyclin D1
antibody (Santa Cruz), distilled water, DMSO
(Sigma), [3-(4,5-dimethylthiazole-2-yl)-2,5diphenyl
tetrazolium bromide] (MTT) (Sigma), propidium
iodide reagent (BioLegend).
Masfria, ., Harahap, U. and Satria, D.
Cell Cycle Inhibition and Apoptosis Induction Activities of n-Hexane Fraction of Cyperus rotundus L. Rhizome.
DOI: 10.5220/0009846200002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

2.2 Preparation of Extract
Ethanol extract of Cyperus rotundus L. rhizomes
(10g) was repeatedly fractionated with n-hexane
(3x100 mL) at separating funnel. The supernatant
was collected, and then evaporated under reduced
pressure to give a viscous fraction and then dried on
water bath to dry (Satria, et al., 2015; Anggraeni, et
al., 2015; Hasibuan, et al., 2015).
2.3 Cytotoxicity Assay
The cells were treated with nHF. In this test, the
T47D cell line (cancer cells which isolated from a 54
years old woman which mutation on p53) was
grown in RPMI 1640 medium, medium containing
10% Fetal Bovine Serum (Gibco), 1% penicillin-
streptomycin (Gibco), and fungizone 0.5% (Gibco)
in a flask in a humidified atmosphere (5% CO
2
) at
37
o
C. The inoculums seeded at 1x10
4
cells/mL at an
optimal volume of 0.1 mL per well. After 24 h
incubation, the medium was discharged and treated
by EE. After incubation 24 h, the cells were
incubated with 0.5 mg/mL MTT for 4 h in 37
o
C.
Viable cells reacted with MTT to produce purple
formazan crystals. After 4 h, SDS 10% as stopper
(Sigma) in 0.01N HCl (Merck) was added to
dissolve the formazan crystals. The cells were
incubated for 24 h in room temperature and
protected from light. After incubation, the cells were
shaken, and absorbance was measured using
microplate reader at λ 595 nm. The data which were
absorbed from each well were converted to
percentage of viable cells
(Harahap, et al., 2018;
Dalimunthe, et al., 2018; Satria, et al., 2017).
2.4 Preparation of Cells for Flow
Cytometry Analysis
T47D cells (5x10
5
cells/well) were seeded into 6-
well plate and incubated for 24 h. After that, the
cells were treated with nHF and then incubated for
24 h. Both floating and adherent cells were collected
in conical tube using tripsin 0.025%. The cells were
washed thrice with cold PBS and centrifuged 2500
rpm for 5 min. The supernatant was separated, while
the sediment was collected (Satria, et al., 2015;
Anggraeni, et al., 2015).
2.5 Cell Cycle Analysis
Cells were fixed in cold 70% ethanol in PBS at -
20
o
C for 2 h. The cells were washed thrice with cold
PBS resuspended and incubated in ethanol 70% for
60 min then centrifuged at 3000 rpm for 3 min,
and PI reagent (containing PI 40 µg/mL and
RNAse 100 µg/mL) added to sediment and
resuspended and incubated at 37
o
C for 30 min. The
samples were analysed using FACScan flow
cytometer (Dalimunthe, et al., 2017; Nugroho, et al.,
2014).
2.6 Apoptosis Analysis
Annexin V reagent was added to sediment and
suspended and incubated at 37
o
C for 30 min. The
samples were analyzed using FACScan flow
cytometer (Satria, et al., 2017; Handayani, et al.,
2017).
2.7 Cyclin D1 Expression
Sediment cells were fixed with ethanol 70% stand
for 2 h in -20
0
C and cyclin D1 antibody was added
and incubated at 37
o
C for 10 min. The samples were
analyzed using FACScan flow cytometer (Żurynń, et
al., 2016)
2.8 Statistical Analysis
Data was expressed as mean ± SD. All statistics
were analyzed using the SPSS 21 software.
3 RESULTS AND DISCUSSION
3.1 Inhibitory Concentration 50%
(IC
50
)
MTT method was used to determine percentage of
cell viability after incubation for 24 h. In every
treatment nHF was shown to inhibit cells growth.
The IC
50
value of nHF was 71.69 ± 0.34 µg/mL. The
cytotoxicity estimate of natural product is related to
content of active compound in these plants including
Cyperus rotundus L. This plant contain
monoterpenes, sesquiterpenes, and steroids
estimated as active compounds (Nidugala, et al.,
2016, Yadav, et al., 2012; Nidugala, et al., 2017).
3.2 Effect on Cell Cycle and Apoptosis
To evaluate the effect of nHF to increase cell death
by modulating cell cycle, we concentrated on it for
further studies using flow cytometry method. The
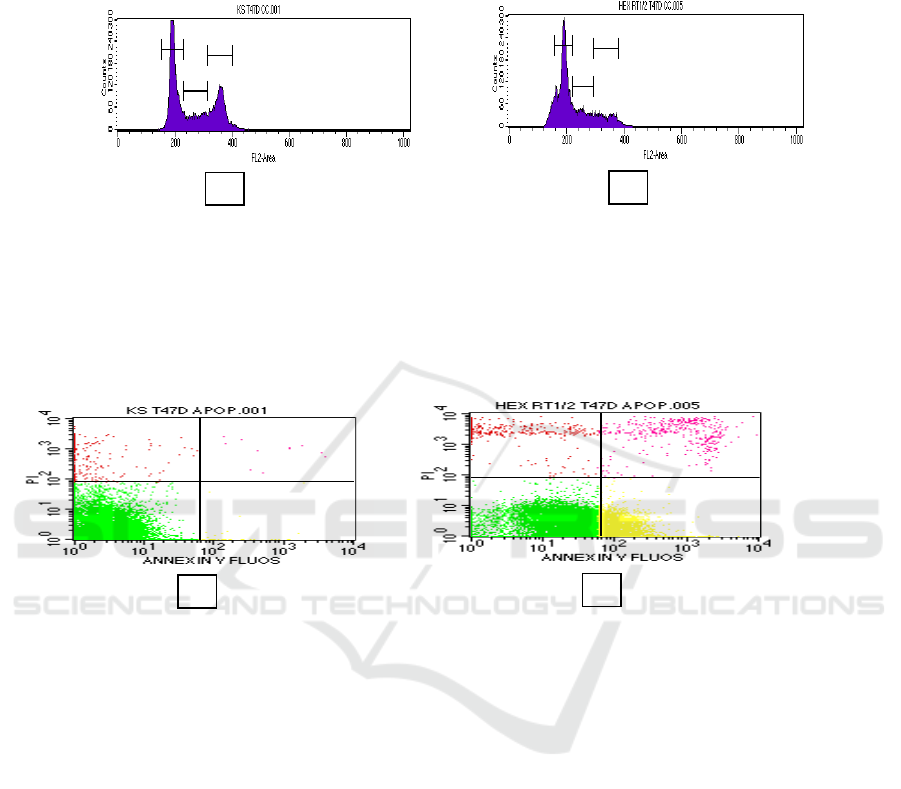
effect of nHF is given in Figure 1. Whereas
treatment of nHF in 35 µg/mL caused cell
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
accumulation at G
0
-G
1
and S phase accumulation
(56.89% and 19.36%) and for control cell (52.28%
and 16.80%). This fact was to indicate that nHF can
inhibit cell grow at G
0
/G
1
phase. Recent study have
reported which monoterpenes exert anticancer
activities and as chemopreventive agents (Elson and
Yu, 1994; Kellof, et al., 1996; Crowell, et al., 1999).
Figure 1. Cell cycle analysis using flow cytometry. T47D cells were treated by nHF for 24h and stained using propidium
iodide. (a) control cells; (b) nHF 35 µg/mL.
As shown in Figure 2, the cells in the upper and
lower right quadrants represent late apoptotic/
necrotic and early apoptotic cells, respectively. The
percentage of nHF at 35 µg/mL and control in early
apoptotic (26.11% and 3.65%), in late
apoptotic/early necrotic (4.26% and 2.96%), and in
late necrotic (4.88% and 2.41).
Figure 2. Apoptosis analysis using flow cytometry. T47D cells were treated by nHF for 24h and stained using Annexin-V.
(a) control cells; (b) nHF 35 µg/mL.
nHF has increased the cells to apoptosis in early
apoptosis if compared to control cell. Apoptosis is
processed in cells which cause programmed cell
death with alters on morphology, membrane
blebbing and chromatine (Ruddin,et al., 1997).
3. 3 Analysis of Cyclin D1 Expression
To evaluate the effect of nHF to decrease cyclin
D1 expression, we concentrated on it for further
studies using the flow cytometry method. The effect
of nHF is given in Figure 3. Whereas treatment of
nHF in 35 µg/mL caused cell accumulation in M1
area (26.30%) and for control cell (11.19%).
Evaluation of cyclin D1 expression was performed
using flow cytometry method with cyclin D1
antibody as shown in Figure 3.
M1
GO- G1
S-phase
G2- M
M5
GO- G1
S-phase
G2- M
R1
M1
GO- G1
S-phase
G2- M
M5
GO- G1
S-phase
G2- M
R1
R2
R3
R4
R1
R2
R3
R4
R1
a
b
a
b
Cell Cycle Inhibition and Apoptosis Induction Activities of n-Hexane Fraction of Cyperus rotundus L. Rhizome
3
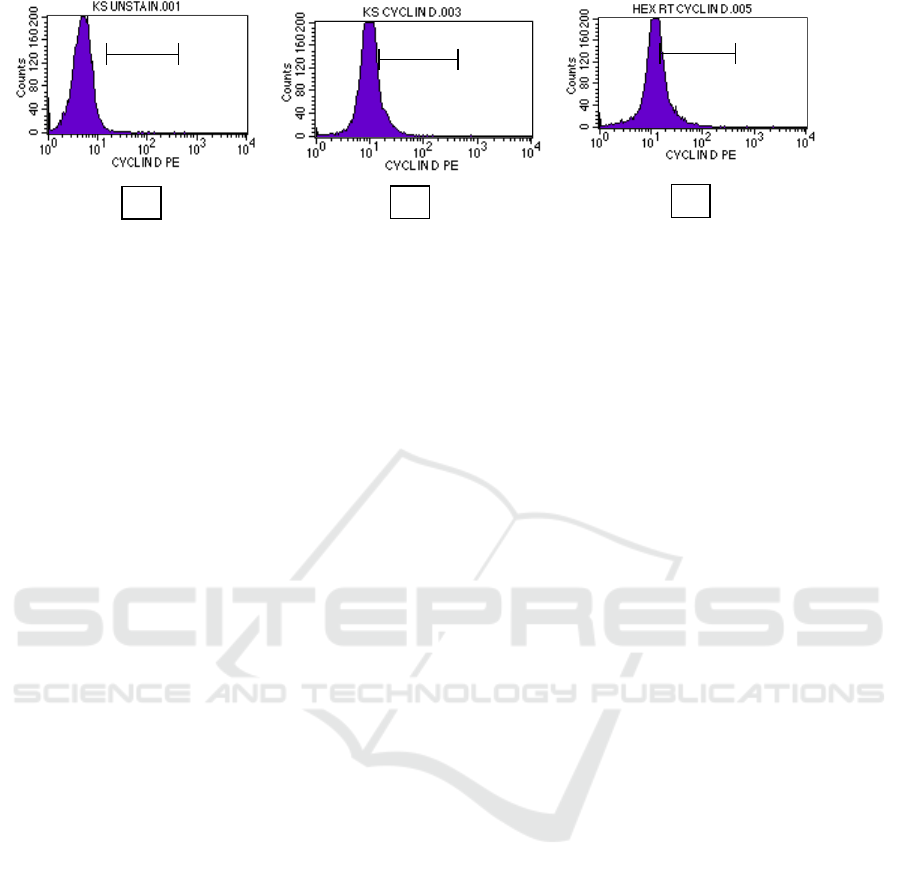
Figure 3. Cyclin D1 analysis using flow cytometry. T47D cells were treated by nHF for 24h and stained using cyclin D1
antibody. (a) control cells unstaining; (b) control cells; (c) nHF 35 µg/mL.
Geraniol is one of monoterpene which has
suppressed the MCF-7 growth through induction cell
cycle arrest in G
1
phase, reduce the level of cyclin
D1, cyclin dependent kinase 4 (CDK4), cyclin E and
cyclin A (Duncan, et al., 2004).
Based on the results above, we conclude that n-
hexane fraction of Cyperus rotundus L. rhizome has
cytotoxic activity towards T47D breast cancer cells
which have some mechanism such as induction of
apoptosis and inhibition of cell cycle especially in
inhibit cyclin D1 expression.
ACKNOWLEDGEMENT
We gratefully thank to Research Center University
of Sumatera Utara through “Hibah Penelitian Guru
Besar” Research Grant 2018 “No:2590/
UN5.1.R/PPM/2018” for financial support in the
study.
REFERENCES
Anggraeni R, Hadisahputra S, Silalahi J, Satria D (2014).
Combinational effects of ethylacetate extract of
Zanthoxylum acanthopodium DC. with doxorubicin on
T47D breast cancer cells. Int J PharmTech Res, 6,
2032-5.
Crowell PL (1999). Prevention and therapy of cancer by
dietary monoterpenes. J Nutr 129:775S–778S.
Dalimunthe A, Hasibuan PAZ, Satria D. (2017). Cell cycle
arrest activity of Litsea cubeba Lout. heartwood and
fruit extracts against T47D breast cancer cells. Asian J
Pharm Clin Resc, 10(11), 404-6.
Dalimunthe A, Hasibuan PAZ, Silalahi J, Sinaga SF,
Satria D. (2018). Antioxidant activity of alkaloid
compounds from Litsea cubeba Lour. Orient J Chem,
34(2), 1149-52.
Duncan RE, Lau D, El-Sohemy A and Archer MC. (2004).
Geraniol and beta-ionone inhibit proliferation, cell
cycle progression, and cyclin-dependent kinase 2
activity in MCF-7 breast cancer cells independent of
effects on HMG-CoA reductase activity. Biochem
Pharmacol 68: 1739-1747.
Elson CE and Yu SG (1994) The chemoprevention of
cancer by mevalonate-derived constituents of fruits
and vegetables. J Nutr 124:607–614.
Hadi SM, Bhat SH, Azmi AS, Hanif S, ShamimU, Ullah
MF. (2007). Oxidative breakage of cellular DNA by
plant polyphenols: a putative mechanism for
anticancer properties. Semin Cancer Biol. 17, 370–6.
Handayani S, Susidarti RA, Jenie RI, Meiyanto E. (2017).
Two active compounds from Caesalpinia sappan L. in
combination with cisplatin synergistically induce
apoptosis and cell cycle arrest on WiDr cells. Adv
Pharm Bull, 7(3), 375-80.
Harahap U, Hasibuan PAZ, Sitorus P, Arfian N, Satria D.
(2018). Antimigration activity of an ethylacetate
fraction of Zanthoxylum acanthopodium DC. fruits in
4T1 breast cancer cells. Asian Pac J Cancer Prev,
19(2), 565-9.
Hasibuan PAZ, Jessy C, Denny S (2015). Combination
effect of ethylacetate extracts of Plectranthus
ambonicius (Lour.) Spreng. with doxorubicin againts
T47D breast cancer cells. Int J Pharm Pharm Sci, 7,
155-9.
Hemanth K, Razack S, Nallamuthu I, Khanum F. (2014)
Phytochemical analysis and biological properties of
Cyperus rotundus L. Ind. Crop Prod. 52, 815–26.
Jirovetz L, Wobus A, Buchbauer G, Shafi MP, Thampi
PT. (2004). Comparative analysis of the essential oil
and SPME-headspace aroma compounds of Cyperus
rotundus L. roots/tubers from South-India using GC,
GC-MS and olfactometry. J. Essent. Oil-Bearing
Plants. 7, 100-6.
Kelloff GJ, Boone CW, Crowell JA, Steele VE, Lubet RA,
Doody LA, Malone WF, Hawk ET and Sigman CC
(1996) New agents for cancer chemoprevention. J Cell
Biochem 26:1–28.
Kilani, S. (2007). Chemical investigation of different
extracts and essential oil from the tubers of (Tunisian)
Cyperus rotundus. Correlation with their antiradical
and antimutagenic properties. Ann. Microbiol. 57,
657–64.
M1
M1
M1
a
b
c
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

Kilani, S. (2008). Comparative study of Cyperus rotundus
essential oil by a modified GC/MS analysis method.
Evaluation of its antioxidant, cytotoxic, and apoptotic
effects. Chem. Biodivers. 5, 729–42.
Lanciotti R, Gianotti A, Patrignani F, Belleti N, Guerzoni
ME, Gradini F. (2004). Use of natural aroma
compounds to improve shelf life and safety of
minimally processed fruits. Trends Food Sci. Tech. 15,
201–8.
Liu XC, Lu XN, Liu QZ, Liu ZL. (2016). Chemical
Composition and Insecticidal Activity of the Essential
Oil of Cyperus rotundus Rhizomes Against Liposcelis
bostrychophila (Psocoptera: Liposcelididae). J. Essent.
Oil Bear. Pl. 19(3), 640–47.
Memariani T, Toktam H, Hossein K, Amaneh M, Maryam
G. (2015). Evaluation of the cytotoxic effects of
Cyperus longus extract, fractions and its essential oil
on the PC3 and MCF7 cancer cell lines. Oncology
Report, 11, 1353-60.
Nam JH, Nam DY, Lee DU. (2016). Valencene from the
rhizomes of Cyperus rotundus inhibits skin
photoaging-related ion channels and UV-induced
melanogenesis in B16F10 melanoma Cells. J. Nat.
Prod. 79, 1091–6.
Nidulaga H, Ramakrishna A, Ashwini P, Ravishankar B.
(2016). In vitro cytotoxic activity of rhizome extracts
of Cyperus rotundus L. against colon carcinoma and
Ehrlich ascites carcinoma. J App Pharm Sci, 6(11),
172-5.
Nidulaga H, Ramakrishna A, Ashwini P, Ravishankar B.
(2017). Cyperus rotundus extract exert anticancer
activity on Ehrlich ascites carcinoma. Eur J Pharm
Med Resc, 4(8), 297-304.
Nugroho AE, Hermawan A, Nastiti K, Suven, Elisa P,
Hadibarata T, Meiyanto E (2014). Immunomodulatory
effects of hexane insoluble fraction of Ficus septica
Burm. F. in doxorubicin-treated rats. Asian Pacific J
Cancer Prev, 13(11), 5785-90.
Peerzada AM, Ali HH, Naeem M, Latif M, Bukhari AH,
Tanveer A. (2015). Cyperus rotundus L.: Traditional
uses, phytochemistry, and pharmacological activities.
J. Ethnopharmacol. 174, 540–60.
Rudin CM, Thompson CB. (1997) Regulation and clinical
relevance of programmed cell death. Annu Rev Med,
48, 267-81.
Satria D, Furqan M, Hadisahputra S, Rosidah (2015).
Combinational effects of ethylacetate extract of Picria
fel-terrae Lour. and doxorubicin on T47D breast
cancer cells. Int J Pharm Pharm Sci, 7, 73-6.
Satria D, Silalahi J, Haro G, Ilyas S, Hasibuan PAZ.
(2017). Antioxidant and antiproliferative activities of
an ethylacetate fraction of Picria fel-terrae Lour.
herbs. Asian Pac J Cancer Prev, 18(2), 399-403.
Siegel RL, Miller KD, Jemal A (2015). Cancer statistics.
CA Cancer J Clin. 65, 5-29.
Sonwa MM and König WA. (2001). Chemical study of
the essential oil of Cyperus rotundus. Phytochemistry.
58, 799–810.
Tenore GC. (2011). Antimicrobial and antioxidant
properties of the essential oil of Salvia lanigera from
Cyprus. Food Chem. Toxicol. 49, 238–43.
Vitaglione P, Morisco F, Caporaso N, Fogliano V. (2004).
Dietary antioxidant compounds and liver health. Crit.
Rev. Food. Sci. Nutr. 44, 575–86.
WHO (2015). World Cancer Report 2014.
Yadav VR, Sahdeo P, Bokyung S, Ramaswamy K, Bharat
BA (2010). Targetting inflammatory pathways by
triterpenoids for prevention and treatment of cancer.
Toxins, 2, 2428-66.
Żurynń A, Anna L, Barbara SM, Anna KW, Maciej G.
(2016). The effect of sulforaphane on the cell cycle,
apoptosis and expression of cyclin D1 and p21 in the
A549 non-small cell lung cancer cell line. Int J Onco
48: 2521-33.
Cell Cycle Inhibition and Apoptosis Induction Activities of n-Hexane Fraction of Cyperus rotundus L. Rhizome
5
