
Evaluation of Antibacterial Activity of Different Extract of Ipomoea
aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
Mohamed Rasny Mohamed Razik
1*
, S. Angielina
1
, Reyadh Al- Rashidi
1
, Samer Al-Dhalli
1
,
Jiyauddin Khan
1
, Kiran Chanabasappa Nilugal
1
, Santosh Fattepur 1, Kaleemullah, M
1
, Shariq
Baber
1
, Chen Jie
1
, Fadli Asmani
1
, Eddy Yusuf
2
1
School of Pharmacy, Management and Science University, 40100 Shah Alam, Selangor, Malaysia;
2
International Center for Halal Studies, Management and Science University, 40100 Shah Alam, Selangor, Malaysia
Keywords: Ipomoea aquatica, Staphylococcus aureus, Streptococcus pyogenes.
Abstract: The skin is the largest organ in the body and can be vulnerable to various microbial infection. Although
antibiotics are clinically proven to be useful in the treatment of bacterial skin infections, they are largely
subjected to antibiotic resistance and adverse effects. This has led to the screening of several medicinal
plants for their potential antimicrobial activity since they are less expensive, has reduced occurrence of
adverse effects and widespread availability. The aim of this research will focus on evaluating the
antibacterial activity of different extracts of Ipomoea aquatica leaves against Staphylococcus aureus and
Streptococcus pyogenes that causes skin infections. Leaves were extracted separately with 95% methanol
and 95% ethanol using maceration process. Phytochemical screening was done for each extract and the
minimum inhibitory concentration (MIC) was determined for each extract against both bacteria using 10
different concentrations ranging from 10mg/ml up to 100mg/ml via disc diffusion method in triplicates.
Two concentrations above the MIC from each extract were selected and antibacterial assay of the different
extracts against the two bacteria respectively was performed using disc diffusion method in triplicates. MIC
for methanolic extract against both bacteria was 10mg/ml, while MIC for ethanolic extract was 10mg/ml
against Staphylococcus aureus and 30mg/ml against Streptococcus pyogenes. Methanolic extract of the
plant at a concentration of 90mg/ml and 100mg/ml was statistically significant against Streptococcus
pyogenes with a significance value of 0.00 (p<0.05), with 100mg/ml having larger mean inhibition zone of
17.00mm ± 0.00mm than 90mg/ml (14.33mm ± 0.58mm). Statistical analysis was performed using one-way
ANOVA (Tukey’s Test). Both methanolic and ethanolic extract of the leaves has positive antibacterial
activity against both Staphylococcus aureus and Streptococcus pyogenes at different concentrations.
1 INTRODUCTION
The skin is the largest organ in the body and acts as
a static, stationary or inert wrapping for the body.
Large numbers of microorganisms are present in the
various components of the skin. For example, the
number of bacteria on the skin surface can range
from 1000 organisms per square centimetre to more
than 10 million. The principal members of the
normal skin flora are Diphtheroids, Staphylococci
and Fungi (Hall, 2001). Skin infections are clinical
entities comprising of many etiology, manifestations
and severity that involves microbial invasion of the
layers of the skin (Ki and Rotstein, 2008). Many
types of bacteria can cause infection to the skin, in
which the most common ones are Staphylococcus
and Streptococcus.
According to CDC, Staphylococcus aureus (S.
aureus) is a type of bacteria that about 30% of
people carry in their noses and often found on the
surface of the skin. It is usually harmless, but
invasive staphylococcus infections can lead to life
threatening medical complications in as little as 12
hours (Dr. . Tom Frieden, MD, 2013). On the other
hand, Streptococcus pyogenes (S. pyogenes) can
cause a variety of diseases in immunocompetent
individuals, from pharyngo-tonsillitis to life-
threatening invasive diseases, such as streptococcal
Razik, M., Angielina, S., Al-Rashidi, R., Al-Dhalli, S., Khan, J., Nilugal, K., Fattepur, S., Kaleemullah, M., Baber, S., Jie, C., Asmani, F. and Yusuf, E.
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus pyogenes.
DOI: 10.5220/0009845100002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

toxic shock syndrome, and rapidly progressing deep-
tissue infections, such as necrotizing fasciitis.
(Johansson et al., 2010).
Although antibiotics have been clinically proven
to be useful in the treatment of bacterial skin
infections, they are largely subjected to limitations
such as antibiotic resistance and adverse effects. The
progressing failure of chemotherapeutics and
resistance to antibiotics has led to the screening of
several medicinal plants for their potential
antimicrobial activity (Oyewole and Kalejaiye,
2012). Unlike conventional medicines or treatments,
herbal treatments have several advantages in that
they are less expensive, more effective in certain
chronic conditions, has reduced occurrence of
adverse effects as well as widespread availability.
In this regard, one of the plant which is being
evaluated for its therapeutic efficacies is Ipomoea
aquatica (I. aquatica). In the ancient science of
Indian medicine and homeopathy, extracts of I.
aquatica leaves are administered orally to alleviate
antioxidant related disorders.
The plant is also used effectively against
nosebleed and high blood pressure. Furthermore, its
leaf extract can be used to reduce blood sugar levels
and as an antibiotic against Escherichia coli,
Pseudomonas aeruginosa and Bacillus subtilis. The
floral buds are used as an anthelmintic (Prasad et al.,
2005).
Water spinach is a perennial herb found
throughout India, Sri Lanka, Tropical Asian
countries, Africa and Australia. It is grown as weed
in India and USA, while in Malaysia, China,
Singapore and Hong Kong, it is grown commercially
(Mbatchou and Dawda, 2012). It is also known with
its common name which is swamp morning glory or
‘kangkung’ in Malaysia. I. aquatica, a green leafy
vegetable which is a rich source of amino acids and
vitamins, has been explored for the isolation and
identification of its bioactive compounds that
provides many health benefits. The leaves of I.
aquatica contains 90% moisture, 4.3%
carbohydrates, 3% protein, 2% mineral matter, 0.9%
fibre, 0.4% fat, 0.6mg/100g of nicotinic acid,
120mg/100g of riboflavin, 137mg/100g of Vitamin
C and 11mg/100g of Vitamin E (Mbatchou and
Dawda, 2012).
Plants are potential sources of natural bioactive
compounds such as primary and secondary
metabolites. Flavonoids are one of the secondary
metabolites produced by plants and are present in
most plant tissues and often in vacuoles. The basic
structures of flavonoid molecules are composed of
three rings with various substitutions, including
glycosylation, hydrogenation, hydroxylation,
methylation and sulfation. Flavonoids are
hydroxylated phenolic substances known to be
synthetized by plants in response to microbial
infection and they have been found to act as
antimicrobial agents against a wide array of
microorganisms in vitro. Their activity is probably
due to their ability to complex with extracellular and
soluble protein and to complex with bacterial cell
wall (Yadav and Agarwala, 2011). The type of
flavonoids present in I. aquatica leaves are quercetin
3-methyl ether and quercetin 4-methyl ether.
Therefore, the aim of this research will focus on
evaluating the antibacterial activity of different
extracts of I. aquatica leaves against S. aureus and
S. pyogenes that causes skin infections.
2 METHODOLOGY
2.1 Preparation of Plant Extract using
Maceration Process
2kg of fresh leaves of I. aquatica obtained was first
weighed and washed thoroughly using running tap
water to remove all adhering foreign materials and
soil particles. The leaves were then dried under
shade and sun for seven days. The amount of dried
leaves was weighed again in order to calculate the
percentage of moisture content in the plant. The
weight obtained was 101.27g. After that, the dried
leaves were coarsely powdered using a mechanical
blender. The amount obtained was weighed and
equally separated into two portions. One part (50g)
was macerated with 95% ethanol and the other part
(50g) with 95% methanol.
Both was allowed to stand at room temperature
for 7 days with occasional shaking. The final
extracts obtained was clarified by filtration using
filter papers. The filtrates were then concentrated
under vacuum in a rotary evaporator in order to
remove the solvent and obtain a solid mass. The
solid mass of both methanolic and ethanolic extracts
was weighed and the percentage yield of the plant
obtained after extraction was calculated.
2.2 Phytochemical Screening of Leaf
Extracts
Both methanolic and ethanolic extracts of I.
aquatica leaves were evaluated for qualitative
determination of primary and secondary metabolites
by preliminary phytochemical screening respectively
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2

(Yadav and Agarwala, 2011). The tests done for the
presence primary metabolites were Molisch’s Test
for the presence of carbohydrates, Millon’s Test for
the presence of proteins, Ninhydrin Test for the
presence of amino acids and Filter Paper Test for the
presence of fats and oil. The tests done to detect
secondary metabolites were Alkaline Reagent Test
for the presence of flavonoids, Liebermann’s Test
for the presence of glycosides, Mayer’s Test for the
presence of alkaloids, Foam Test for the presence of
saponins, Salkowski’s Test for the presence of
steroids as well as Ferric Chloride Test for the
presence of phenols and tannins.
2.3 Determination of Minimum
Inhibitory Concentration (MIC)
using Disc Diffusion Method
The minimum inhibitory concentration was
determined for both methanolic and ethanolic
extracts of I. aquatica leaves against both S. aureus
and S. pyogenes respectively. This was done by
preparing different concentrations of each extract in
w/v (100mg/ml, 90mg/ml, 80mg/ml, 70mg/ml,
60mg/ml, 50m/ml, 40mg/ml, 30mg/ml, 20mg/ml and
10mg/ml). Empty sterile discs were impregnated in
each concentration of the extracts for a sufficient
time and then they were placed on agar plates that
has been inoculated with the selected bacteria
strains. The control group for this assay was empty
sterile discs that has been impregnated in distilled
water. The plates were then incubated at 37
0
C for 24
hours in an incubator. After 24 hours, each plate was
observed and the zone of inhibition of each sample
was measured and recorded. MIC was determined
by observing the lowest concentration of plant
extract that was able to inhibit the bacteria growth.
From this, a suitable concentration of the plant
extract was used for Antibacterial Assay using Disc
Diffusion method for both methanolic and ethanolic
extracts of I. aquatica leaves.
2.4 Antibacterial Assay using Disc
Diffusion Method
Strains: Strains of S. aureus and S. pyogenes
Medium: Mueller Hinton Agar (MHA) for S.
aureus
: Nutrient Agar (NA) for S. pyogenes
Samples: Concentrations of 30% and 40% of
methanolic extract of I. aquatica leaves against S.
aureus and concentrations of 90% and 100% against
S. pyogenes.
Concentrations of 70% and 90% of ethanolic extract
of I. aquatica leaves against S. aureus and
concentrations of 80% and 90% against S. pyogenes.
Positive control (Antibiotic): Vancomycin disc
(30mcg/disc)
Negative control (Solvent): Distilled water
The antibacterial activity of I. aquatica leaves were
tested using Disc Diffusion method. A suitable
concentration of both methanolic and ethanolic
extracts of I. aquatica leaves as stated above were
prepared respectively, in which sterile paper discs
were impregnated for a sufficient time. The positive
control, Vancomycin discs (30mcg/disc) kept in
refrigerator was taken out and left to cool to room
temperature before use.
The negative control, distilled water was
prepared and empty sterile discs were impregnated
in them respectively. S. aureus and S. pyogenes was
inoculated on the agar plates that were prepared and
stored earlier, respectively.
The discs impregnated in methanolic extract,
ethanolic extract, distilled water as well as
Vancomycin discs was then placed on each agar
plates with appropriate distance between each disc.
All the plates were incubated in an incubator at 37
0
C
for 24 hours. After incubation, the agar plates were
observed and the diameter of zone of inhibition of
each and every agent and disc used was measured.
These procedures were performed in triplicates in
order to obtain the mean and standard deviation
(n=3, mean ± standard deviation) zone of inhibition
for each agent used.
2.5 Statistical Analysis
The results obtained were statistically analysed
using One-Way Analysis of Variance (ANOVA) and
Tukey’s Test via Statistical Package for the Social
Science (SPSS) software. ANOVA was followed by
Tukey’s Test for control, standard and test group
comparisons for statistical evaluation. p value less
than 0.05 was considered statistically significant.
3 RESULTS AND DISCUSSION
The moisture content of I. aquatica leaves used in
this research was 94.95%, while its percentage yield
obtained after extraction with methanol and ethanol
was 89.16% and 96.80% respectively. Maceration of
the leaves with methanol produced more amount of
extract compared to ethanol.
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
3
Percentage of Moisture Content (MC) in I. aquatica
leaves
% Moisture Content = Initial Weight (IW) – Dried Weight
(DW) X 100%
Initial Weight
(IW)
= 2000g – 100.91g X 100%
2000g
= 94.95%
Percentage of Yield obtained after Extraction of I.
aquatica leaves
Methanolic Extract of I. aquatica leaves
% Yield after Extraction = Initial Weight (IW) – Final
Weight (FW) X 100%
Initial
Weight (IW)
= 50.0g – 5.42g
X 100%
50.0g
= 89.16%
Ethanolic Extract of I. aquatica leaves
% Yield after Extraction = Initial Weight (IW) – Final
Weight (FW) X 100%
Initial
Weight (IW)
= 50.0g – 1.60g
X 100%
50.0g
= 96.80%
Both methanolic and ethanolic extracts of I.
aquatica leaves were tested for the presence of
primary and secondary metabolites respectively.
According to the results, methanolic extract of the
leaves contained carbohydrates, amino acids,
flavonoids, glycosides, alkaloids, saponins, steroids,
phenols as well as tannins. Meanwhile, the ethanolic
extract of the leaves showed positive results for the
presence of carbohydrates, proteins, amino acids,
flavonoids, glycosides, alkaloids, saponins, steroids,
phenols and tannins. According to a study conducted
in 2011 by Yadav & Agarwala, it was found that
flavonoids were responsible for the antimicrobial
activity of I. aquatica leaves. Flavonoids are
hydroxylated phenolic substances known to be
synthetized by plants in response to microbial
infection and they have been found to act as
antimicrobial agents against a wide array of
microorganisms in vitro. Their activity is probably
due to their ability to complex with extracellular and
soluble protein and to complex with bacterial cell
wall (Yadav and Agarwala, 2011). Therefore, the
presence of flavonoids in both methanolic and
ethanolic extracts of I. aquatica leaves in this study
can be said to be accountable for its antibacterial
activity.
Table 1: Results of phytochemical screening for both methanolic & ethanolic extracts of I. aquatica leaves.
METABOLITES
TEST
OBSERVATIONS
INTERPRETATION
(METHANOLIC
EXTRACT)
INTEPRETATION
(ETHANOLIC
EXTRACT)
Carbohydrates
Molisch’s Test
Appearance of a
violet ring at the
interphase.
Present
Present
Proteins
Millon’s Test
Turning of white
precipitate to red
upon gentle heating.
Absent
Present
Amino Acids
Ninhydrin Test
Appearance of violet
colour.
Present
Present
Fats & Oil
Filter Paper Test
No permanent
staining of the filter
paper.
Absent
Absent
Flavonoids
Alkaline
Reagent Test
Intense yellow
changed to
colourless.
Present
Present
Glycosides
Liebermann’s
Test
Colour changed from
violet to blue / green.
Present
Present
Alkaloids
Mayer’s Test
Formation of yellow
coloured precipitate.
Present
Present
Saponins
Foam Test
Formation of stable
foam.
Present
Present
Phenols &
Ferric Chloride
Appearance of blue-
Present
Present
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

Tannins
Test
black coloration.
Steroids
Salkowski’s Test
Red colour produced
in the lower
chloroform layer.
Present
Present
According to Shamli et al 2015, phytochemical
analysis of acetone and petroleum ether extract of
I.aquatica showed that proteins, carbohydrates,
tannins, phenols and terpenoids were present in both
exctarct and glycoside and flavonoids were absent in
petroleum ether extract. Whereas, Steroids, alkaloids
and saponin were absent in both extract (Shamli,
Chandra and Nadu, 2015).
Methanolic Extract of I. aquatica Leaves
Ethanolic Extract of I. aquatica Leaves
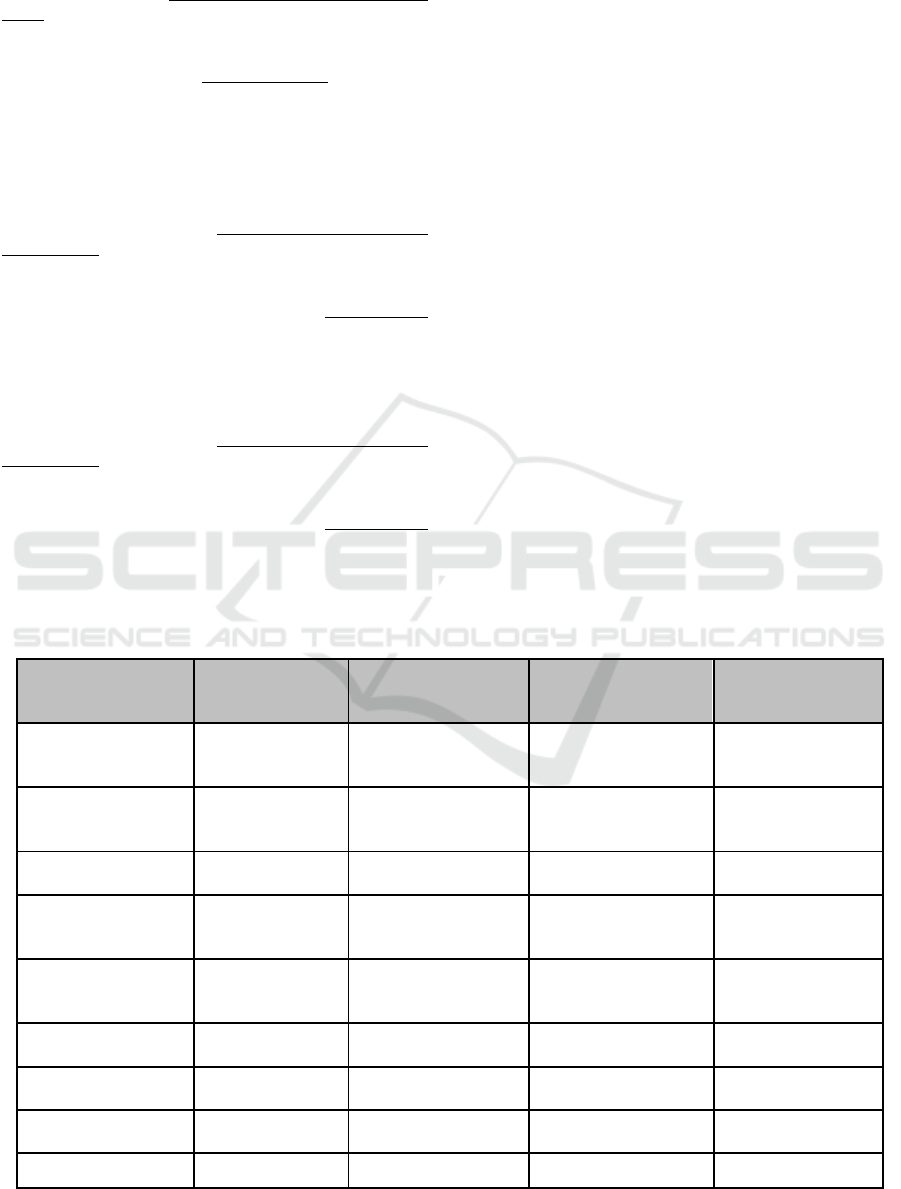
Molisch’s Test for Carbohydrate
Molisch’s Test for Carbohydrate
Millon’s Test for Protein
Millon’s Test for Protein
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
5
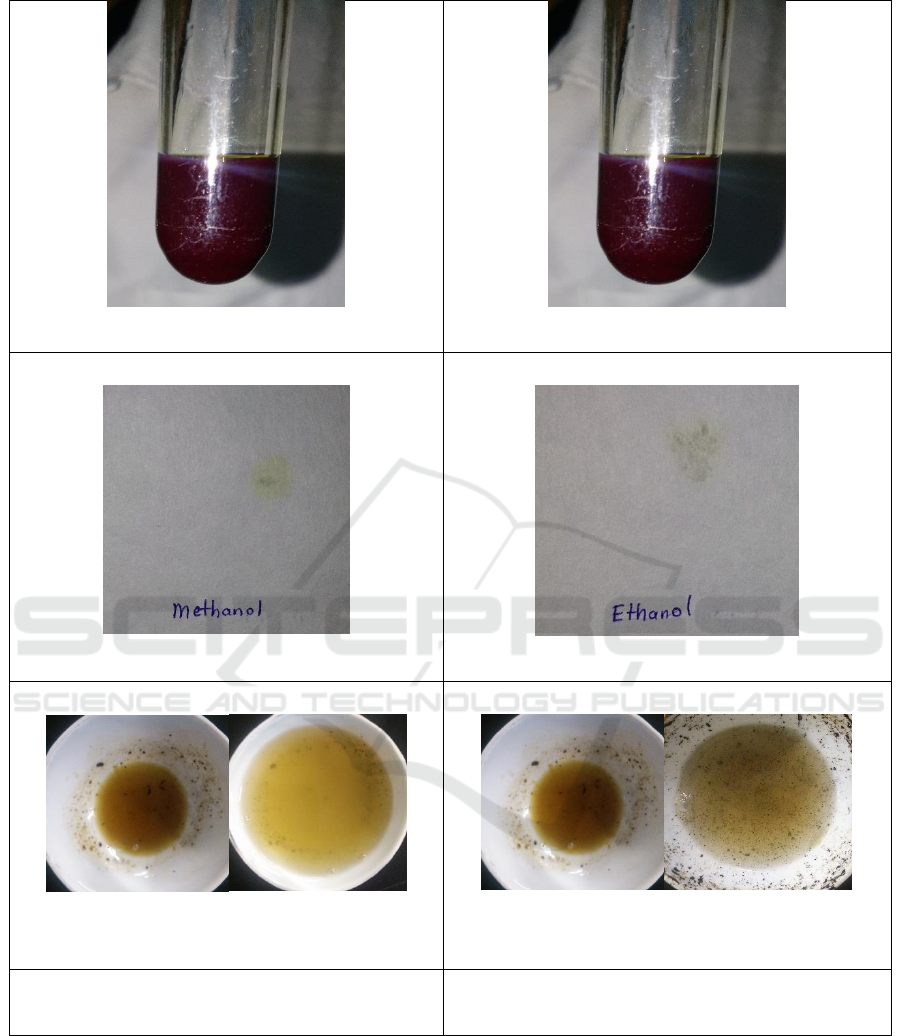
Ninhydrin Test for Amino Acids
Ninhydrin Test for Amino Acids
Filter Paper Test for Fats & Oil
Filter Paper Test for Fats & Oil
Alkaline Reagent Test for Flavonoids
Alkaline Reagent Test for Flavonoids
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
6

Liebermann’s Test for Glycosides
Liebermann’s Test for Glycosides
Mayer’s Test for Alkaloids
Mayer’s Test for Alkaloids
Foam Test for Saponins
Foam Test for Saponins
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
7
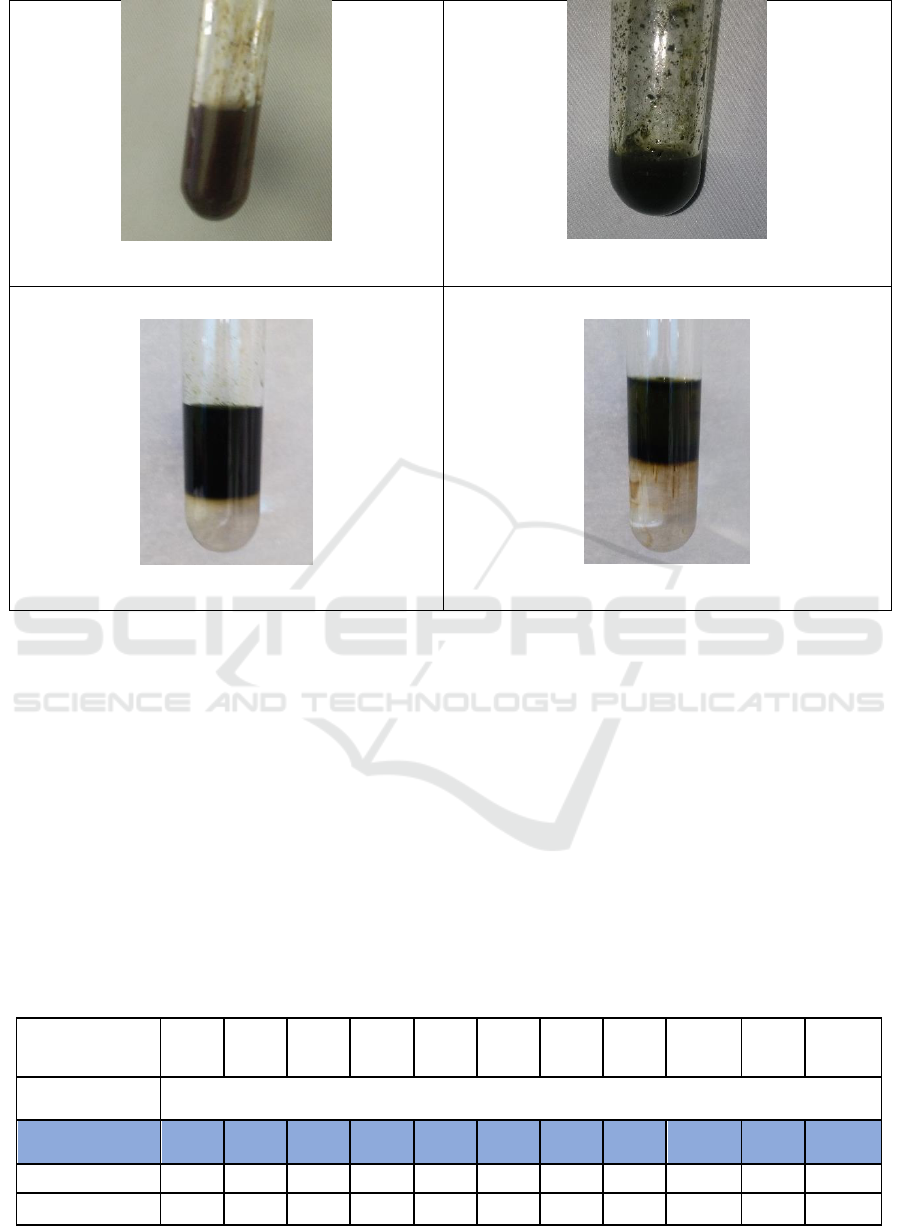
Ferric Chloride Test for Phenols &Tannins
Ferric Chloride Test for Phenols &Tannins
Salkowski’s Test for Steroids
Salkowski’s Test for Steroids
Figure 1: Results of Phytochemical Screening
The determination of MIC for both methanolic
and ethanolic extracts of I. aquatica leaves were
determined using ten different concentrations of
both extracts, ranging from 10mg/ml up to
100mg/ml via Disc Diffusion method, which was
done in triplicates (Table 2).
As for methanolic extract of the leaves, the
lowest concentration that showed and were able to
inhibit the growth of S. aureus was 10mg/ml with a
mean zone of inhibition of 6.00mm (n=3, mean),
while for S. pyogenes, the MIC was also 10mg/ml,
but with a mean zone of inhibition of 4.00mm (n=3,
mean).
On the other hand, as for ethanolic extract of the
leaves, the lowest concentration that showed and
were able to inhibit the growth of S. aureus was
10mg/ml with a mean zone of inhibition of 3.33mm
(n=3, mean), while for S. pyogenes, the MIC was
30mg/ml, with a mean zone of inhibition of 4.00mm
(n=3, mean). After the determination of MIC for
both methanolic and ethanolic extracts of the leaves,
two concentrations above the MIC with the highest
mean value were chosen to be tested for antibacterial
activity against S. aureus and S. pyogenes
respectively.
Table 2: Minimum Inhibitory Concentration (MIC) for methanolic and ethanolic extracts of I. aquatica leaves after tested
against S. aureus and S. pyogenes respectively.
Concentration
(mg/ml)
10
20
30
40
50
60
70
80
90
100
(-)
Control
Extract /
Bacteria
Mean Zone of Inhibition (mm)
Methanolic
Extract
S. aureus
6.00
4.67
6.33
6.33
5.67
5.67
0.00
3.33
0.00
0.00
0.00
S. pyogenes
4.00
5.33
6.00
6.00
6.67
5.33
6.33
3.00
9.33
9.00
0.00
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
8
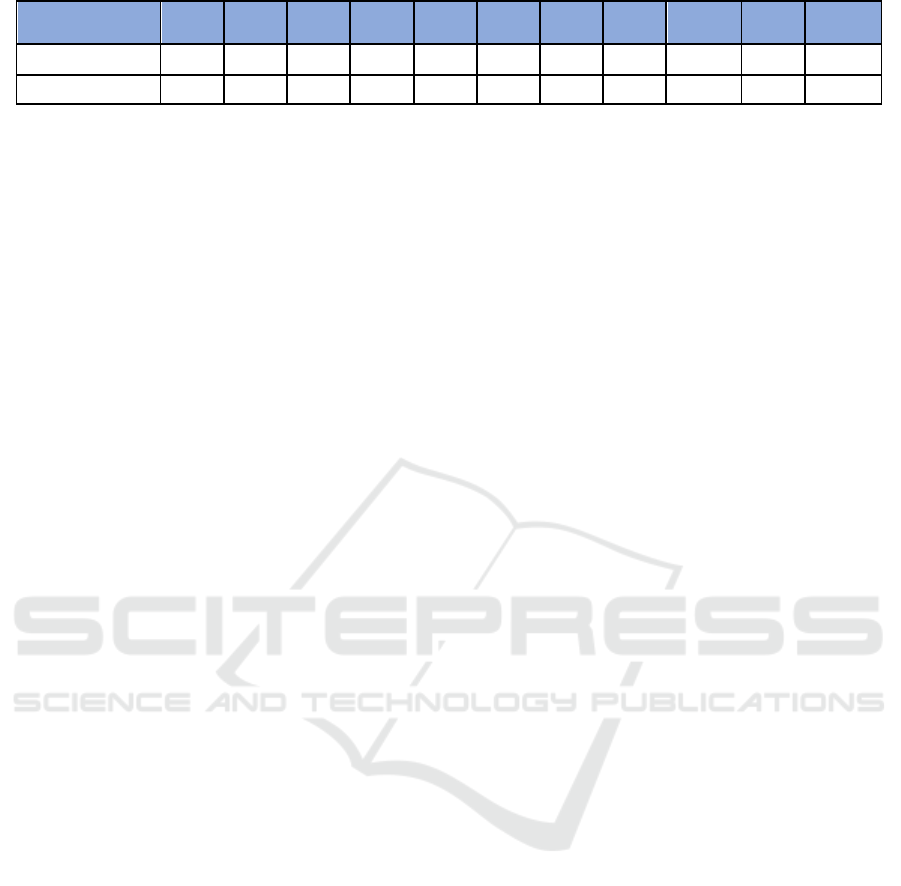
Ethanolic
Extract
S. aureus
3.33
2.00
6.33
6.67
6.00
6.00
7.67
5.67
6.67
5.33
0.00
S. pyogenes
0.00
0.00
4.00
7.33
5.00
7.00
5.67
9.67
10.67
9.33
0.00
From the determination of MIC for methanolic
extract of I. aquatica leaves, concentrations of
30mg/ml and 40mg/ml of the extract were observed
to have the highest mean values for the zone of
inhibition of the growth of S. aureus, and therefore
they were chosen to perform antibacterial testing
against S. aureus using disc diffusion method. The
results obtained showed that both concentrations had
antibacterial activity against S. aureus with a mean
and standard deviation of 7.33mm ± 0.58mm (n=3,
mean ± standard deviation). According to one-way
ANOVA test, both the concentrations did not show
significant effects against S. aureus since the p value
was 1.00 (p > 0.05) when compared to the positive
control, Vancomycin (30mcg/disc) which had a
mean and standard deviation of 23.67mm ± 1.15mm
(n=3, mean ± standard deviation), with a p value of
0.00 (p < 0.05) (Table 4).
One-way ANOVA using Post-hoc Tukey’s test
was performed in order to evaluate the significance
of antibacterial activity of the concentrations
obtained from both methanolic and ethanolic
extracts of I. aquatica leaves against the test bacteria
(S. aureus and S. pyogenes). Based on the
interpretation from ANOVA test, p values lower
than 0.005 (p<0.05) were considered significant for
antibacterial activity and vice versa.
On the other hand, concentrations of 90mg/ml
and 100mg/ml of methanolic extract of I. aquatica
leaves were prepared to test its antibacterial activity
against S. pyogenes. The results obtained showed
that the extract of 100mg/ml had larger zone of
inhibition, with a mean and standard deviation of
17.00mm ± 0.00mm (n=3, mean ± SD) than
90mg/ml of extract.
At the same time, comparison between each
group using ANOVA test (Table 5) showed that
each group had significant effects against S.
pyogenes, since the significance value was 0.00 (p <
0.05), in which it can be said that 100mg/ml of the
extract has higher significant antibacterial effect
than 90mg/ml of the extract since 100mg/ml has
higher mean zone of inhibition against S. pyogenes.
For ethanolic extract of I. aquatica leaves, the
concentrations of the extract used were 70mg/ml and
90mg/ml in order to test the susceptibility of S.
aureus towards the extract. The higher concentration
(90mg/ml) of the extract had larger zone of
inhibition compared to 70mg/ml of the extract.
However, there were no significant difference
between the two concentrations against S. aureus
according to ANOVA test (Table 6). This is because
the p value for each concentration when compared to
one another was 0.754 (p > 0.05).
Meanwhile, the concentrations of 80mg/ml and
90mg/ml of ethanolic extract was used to test its
antibacterial activity against S. pyogenes. Clearly,
the higher concentration of the extract had bigger
zone of inhibition with a mean and standard
deviation of 7.67mm ± 0.58mm (n=3, mean ±
standard deviation) compared to 80mg/ml of the
extract. Nonetheless, ANOVA test showed that there
was no significant difference in the antibacterial
activity between the two concentrations against S.
pyogenes since the p value was 0.754 (p > 0.05)
when both concentrations were compared to each
other (Table 7).
When we compare the antibacterial activity
between methanolic and ethanolic extract of I.
aquatica leaves against S. aureus and S. pyogenes, it
can be said that both extract require different
concentrations in order to inhibit the bacteria’s
growth (Figure 1 and 2). However, when we
compare the antibacterial activity of methanolic and
ethanolic extract of I. auatica to the standard
positive control (Vancomycin); both methanolic and
ethanolic extract of I. aquatica got low antibacterial
activity than vancomycin.
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
9
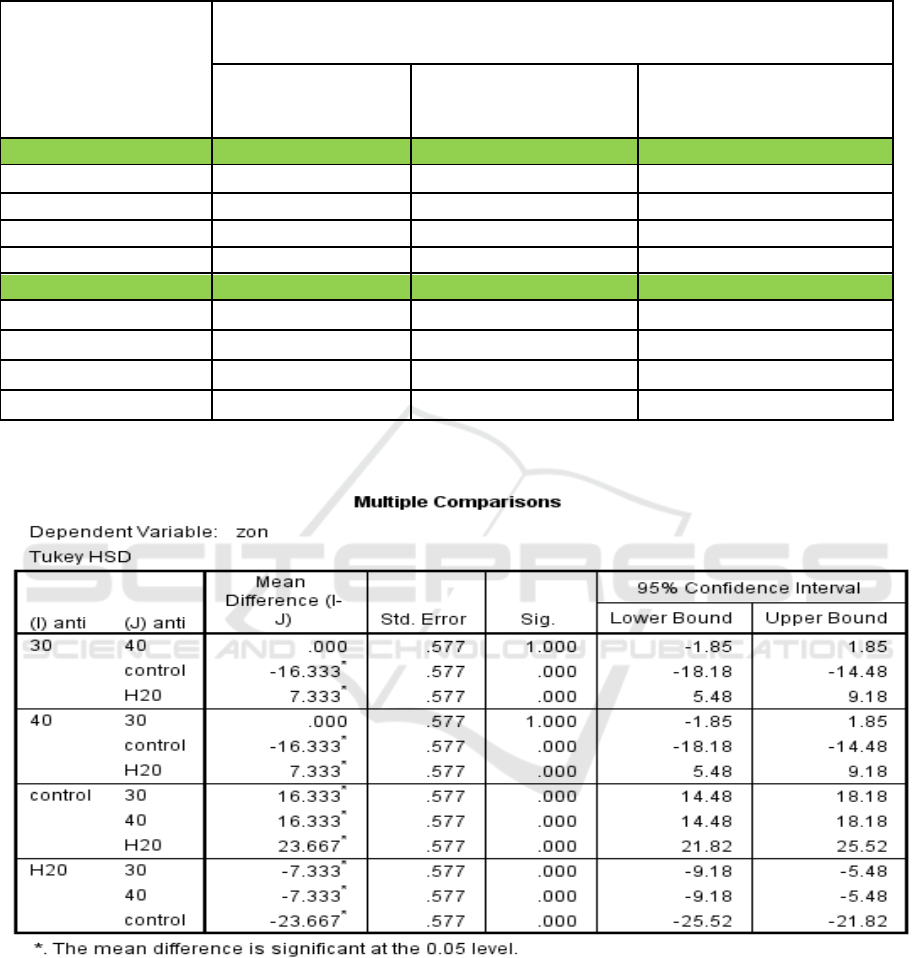
Table 3: Mean and SD of zone of inhibition for methanolic and ethanolic extracts of I. aquatica leaves and controls after
tested against S. aureus and S. pyogenes respectively.
Bacteria / Concentration
of Extract (mg/ml)
Mean Zone of Inhibition (mm) ± Standard Deviation (SD)(mm)
Sample Extract
(-) Control
(+) Control (Vancomycin
30mcg/disc)
Methanolic Extract
S. aureus / 30
7.3 ± 0.6
0.0 ± 0.0
23.7 ± 1.2
S. aureus / 40
7.3 ± 0.6
0.0 ± 0.0
23.7 ± 1.2
S. pyogenes / 90
14.3 ± 0.6
0.0 ± 0.0
35.0 ± 0.0
S. pyogenes / 100
17.0 ± 0.0
0.0 ± 0.0
35.0 ± 0.0
Ethanolic Extract
S. aureus / 70
5.0 ± 0.0
0.0 ± 0.0
24.3 ± 1.2
S. aureus / 90
5.7 ± 1.2
0.0 ± 0.0
24.3 ± 1.2
S. pyogenes / 80
7.3 ± 0.6
0.0 ± 0.0
30.0 ± 0.0
S. pyogenes / 90
7.7 ± 0.6
0.0 ± 0.0
30.0 ± 0.0
Table 4: Significance of each methanolic extract of I. aquatica Leaves compared to one another against S. aureus according
to their zone of inhibition.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
10
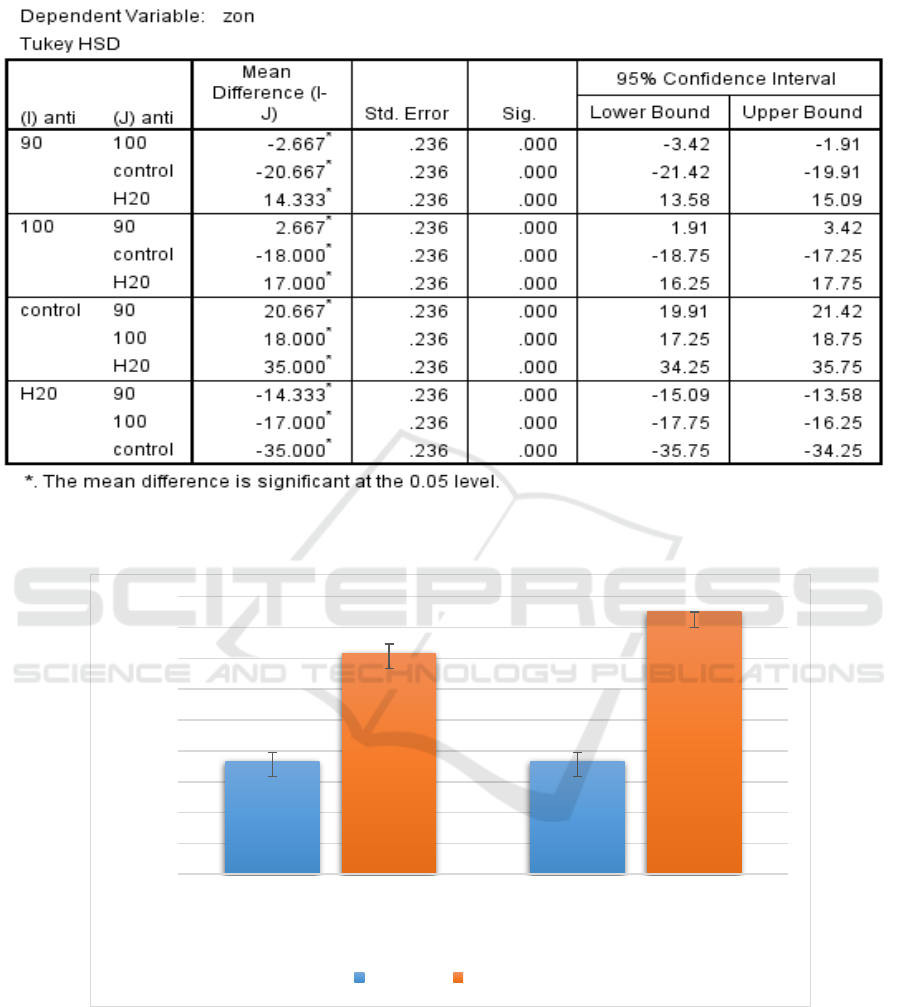
Table 5: Significance of each methanolic extract of I. aquatica Leaves compared to one another against S. pyogenes
according to their zone of inhibition.
Figure 2: Evaluation of the mean zone of inhibition exhibited by different concentrations of methanolic extract of I.
aquatica leaves against both S. aureus and S. pyogenes.
0
2
4
6
8
10
12
14
16
18
30 90 40 100
Mean Zone of Inhibition (mm)
Concentration of Methanolic Extract (mg/ml)
S. aureus S. pyogenes
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
11
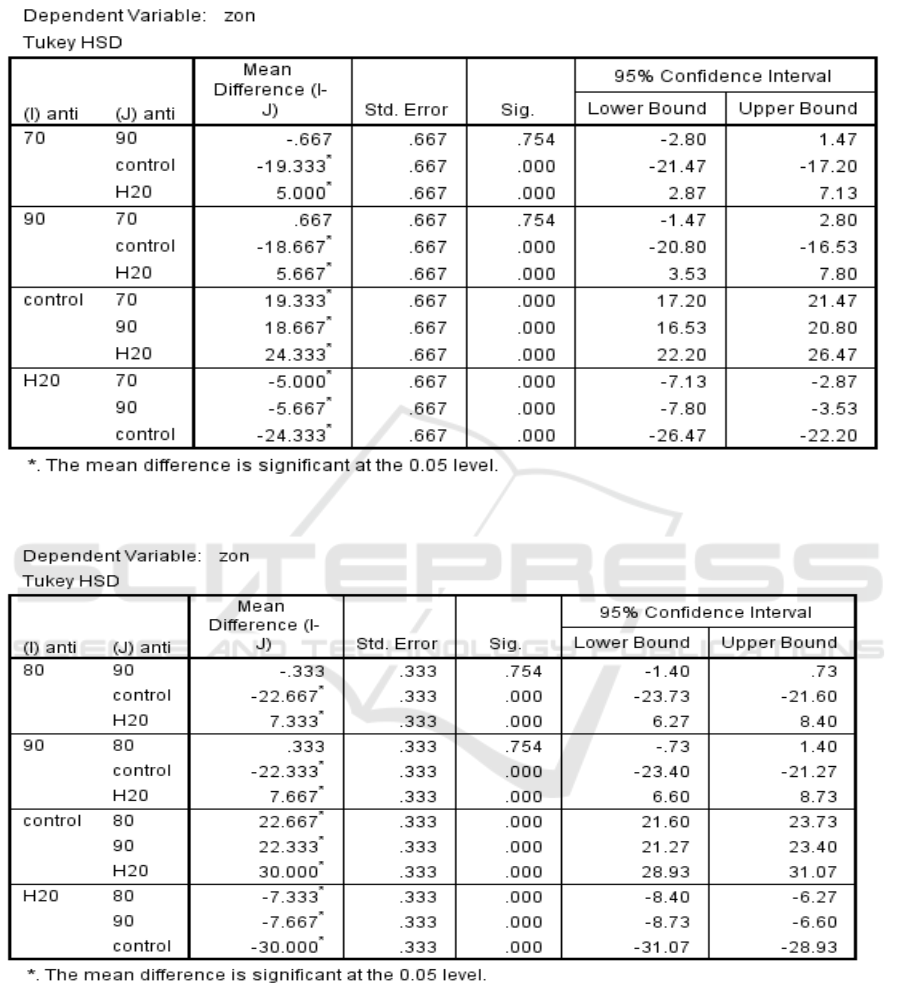
Table 6: Significance of each Ethanolic extract of I. aquatica Leaves compared to one another against S. aureus according
to their zone of inhibition.
Table 7: Significance of each Ethanolic extract of I. aquatica Leaves compared to one another against S. pyogenes
according to their zone of inhibition.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
12
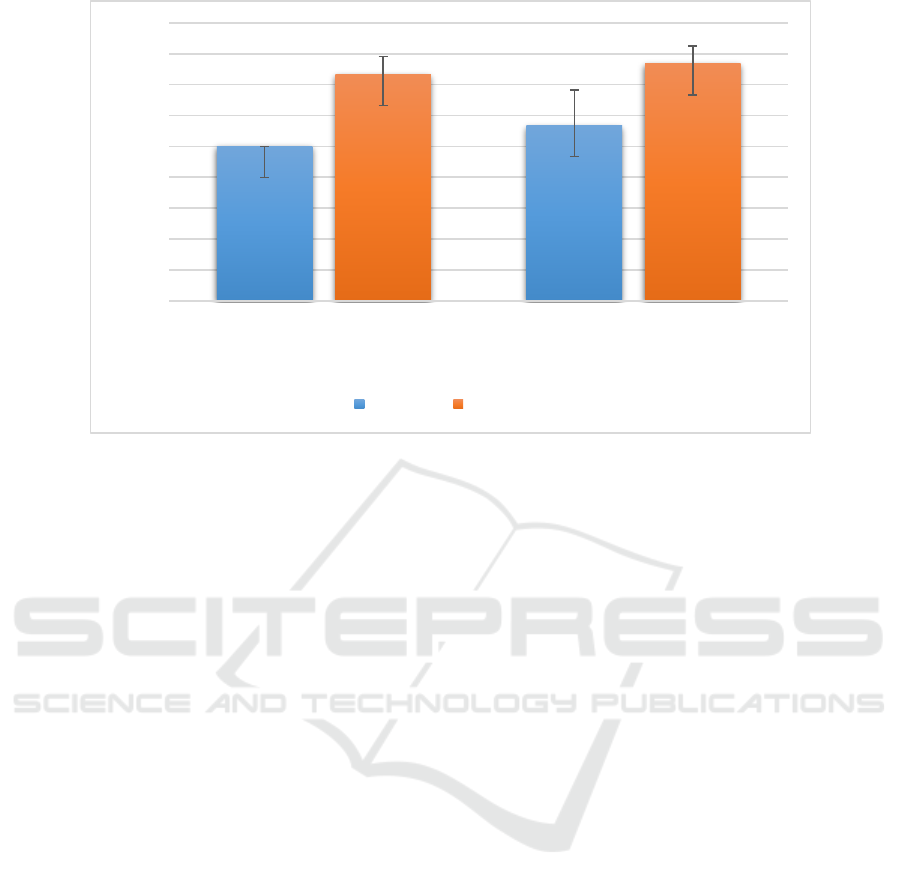
Figure 3: Evaluation of the mean zone of inhibition exhibited by different concentrations of ethanolic extract of I. aquatica
leaves against both S. aureus and S. pyogenes.
4 CONCLUSION
Based on the conducted study, it can be concluded
that both methanolic and ethanolic extracts of I.
aquatica leaves contain flavonoids that is thought to
be responsible for its antimicrobial properties. At the
same time, both type of the extracts showed positive
antibacterial activity and were effective against both
notorious S. aureus and S. pyogenes that are known
to cause skin infections. However, each extract
require different concentrations in order to inhibit
the bacteria’s growth. Further studies such as
determination of the total flavonoid content of I.
aquatica leaves and fractionation of the extract of I.
aquatica leaves can be carried out in order to isolate
the leaves’ constituents as well as improving its
antibacterial properties. Moreover, future research
can be done on developing a formulation using
ethanolic extract of I. aquatica leaves in order to
heal and fight infection on the skin caused by S.
pyogenes, since in this research, ethanolic extract of
the leaves showed a significant effect against the
mentioned bacteria.
ACKNOWLEDGEMENT
The author is thankful to all the research committees
and lecturers of School of Pharmacy, Management
and Science University (MSU), Malaysia for their
endless support, teaching and guidance as well as in
providing all the required materials, equipment and
laboratory facilities throughout the completion of
this research.
CONFLICT OF INTEREST
The author confirms that there is no conflict of
interests.
REFERENCES
Dr. . Tom Frieden, MD, M. (2013) ‘Antibiotic Resistance
Threats’, Cdc, pp. 22–50. doi: CS239559-B.
Hall, B. J. (2001) ‘Skin Infections’, pp. 533–560. doi:
10.1017/CBO9780511576829.
Johansson, L. et al. (2010) ‘Getting under the skin: the
immunopathogenesis of Streptococcus pyogenes deep
tissue infections.’, Clinical infectious diseases : an
official publication of the Infectious Diseases Society
of America, 51(1), pp. 58–65. doi: 10.1086/653116.
Ki, V. and Rotstein, C. (2008) ‘Bacterial skin and soft
tissue infections in adults: A review of their
epidemiology, pathogenesis, diagnosis, treatment and
site of care’, Can.J.Infect.Dis.Med.Microbiol.,
19(1712–9532 (Print)), pp. 173–184.
Mbatchou, V. C. and Dawda, S. (2012) ‘Phytochemical
and pharmacological profile of genus Icacina’,
Phytopharmacology, 2(2), pp. 135–143. doi:
10.4103/0019-5359.121115.
Oyewole, O. A. and Kalejaiye, O. A. (2012) ‘Original
article The antimicrobial activities of Ethanolic
extracts of Basella alba on selected microorganisms’,
0
1
2
3
4
5
6
7
8
9
70 80 90 90
Mean Zone of Inhibition (mm)
Concentration of Ethanolic Extract (mg/ml)
S. aureus S. pyogenes
Evaluation of Antibacterial Activity of Different Extract of Ipomoea aquatic Leaves against Staphylococcus aureus and Streptococcus
pyogenes
13

1, pp. 113–118.
Prasad, K. N. et al. (2005) ‘Isolation of a free radical-
scavenging antioxidant from water spinach (Ipomoea
aquatica Forsk)’, Journal of the Science of Food and
Agriculture, 85(9), pp. 1461–1468. doi:
10.1002/jsfa.2125.
Shamli, M., Chandra, J. H. and Nadu, T. (2015)
‘EVALUATION OF ANTIBACTERIAL ACTIVITY
OF DIFFERENT SOLVENT EXTRACTS OF
MEDICINAL PLANT’, Journal of Chemical and
Pharmaceutical Sciences, 8(1), pp. 52–54.
Yadav, R. N. S. and Agarwala, M. (2011) ‘Phytochemical
analysis of some medicinal plants’, Journal of
Phytology, 3(12), pp. 10–14. doi: 10.1021/np800144q.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
14
