
The Antibacterial Activity of Isolated Flavonoid Fractions from
Ethanol Ethanolic Peel of Citrus Sinensis (Valencia Orange) with
Citrus Limon (Lemon) against Staphylococcus Aureus and
Pseudomonas Aeruginosa
Nik Nur Shamiha N. D
1,2*
, Ghayatery Nagatamby
1
, Fadli Asmani
1
, Eddy Yusof
2
1
School of Pharmacy, Management and Science University, Selangor Darul Ehsan, Malaysia
2
ICHLAS, Management & Science University, Selangor, Malaysia
Keywords: Citrus sinensis; Citrus limon; Combination antibacterial activity; Flavonoid; Skin and soft tissue infections
(SSTIs)
Abstract: Background: Skin and soft tissue infections (SSTIs) are most common infections encountered by all
physicians. Eventhough pharmacological industries have produced a number of new antibiotics, resistance to
these drugs by microorganisms has increased. Objective: The aim of this study is to evaluate the antibacterial
activity of isolated flavonoid fractions from ethanolic peel extract of C.sinensis (Orange), C.limon (Lemon)
and its combination against S.aureus and P.aeruginosa. Methods: The ethanolic peel extract of both plant were
screened for phytochemical identification of flavonoid by lead acetate test and shinoda test. The extract of
both plants were evaluated for preliminary antibacterial activity using disk diffusion method. Thin-layer
chromatography and column chromatography was performed to isolate flavonoids. Isolated flavonoids were
subjected to determination of minimum inhibitory concentration and antibacterial assay by disk diffusion
method. Results: Isolated flavonoids of C.sinensis (17mm, 8mm), C.limon (20mm, 9mm) and its combination
(24mm, 14mm) produced antibacterial activity that is comparable to the Ciprofloxacin disc (30mm, 9mm)
against S.aureus and P.aeruginosa respectively. Thus, these results suggested that C.limon produced a better
antibacterial activity against both bacteria compared to C.sinensis. However, the combination of both plants
isolated flavonoid fractions produced much better antibacterial activity against S.aureus and P.aeruginosa in
comparison with individual flavonoid fractions of both plants. Conclusion: Therefore, Citrus fruits peels that
is being as primary waste in juicing industries can be further developed as marketable natural source of
antibiotic as a treatment of SSTIs.
INTRODUCTION
Skin and soft tissue infections (SSTIs) are reflect
inflammatory microbial invasion of the epidermis,
dermis and subcutaneous tissues (Matthew
S.Dryden, 2010). Some skin colonizing
microorganisms, in particular Staphylococcus
aureus (S.aureus) and β-haemolytic group A
streptococci but also Gram-negative bacteria such
as Pseudomonas aeruginosa (P.aeruginosa), viruses
and fungi, have the potential to cause infection,
particularly when the skin barrier is breached
(Lacey et al., 2015). SSTIs are treated by using
antibiotics. The problem arises as these bacteria are
developing resistance strains against antimicrobial
agents as a result, there is an urgent need to find an
alternative way to cure the bacterial infections.
Plant would be a better alternative because of their
antimicrobial traits, which are due to compounds
synthesized in the secondary metabolism and it has
lesser side effects compared with synthetic
antibiotics (Kailash D. Sonawane et al., 2011).
Citrus fruits have been of interest for extraction
of antimicrobial metabolites by large numbers of
researchers but the peels are less studied (Akhilesh
et al., 2012). Moreover, the peels part of citrus fruits
is abundance with secondary metabolites which
serves as plant defense mechanisms against
predation by microorganisms, insects, and
herbivores (Xinmiao Lv et al., 2015). Among the
secondary metabolites, flavonoids are known for its
antimicrobial properties. Since, they are known to
Shamiha N. D., N., Nagatamby, G., Khan, J., Asmani, F. and Yusuf, E.
The Antibacterial Activity of Isolated Flavonoid Fractions from Ethanol Ethanolic Peel of Citrus Sinensis (Valencia Orange) with Citrus Limon (Lemon) against Staphylococcus Aureus and
Pseudomonas Aeruginosa.
DOI: 10.5220/0009844400002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All r ights reserved
1

be synthesized by plants in response to microbial
infection, it should not be surprising that they have
been found in vitro to be effective antimicrobial
substances against a wide array of microorganisms
(Xinmiao Lv et al., 2015).
There are previous studies on antimicrobial
activity of essential oil from peels of orange and
lemon against several microorganisms (Shalu
Hasija et al., 2015). However, there are lack of
studies on antibacterial activity of flavonoid
isolated from peels of orange and lemon. Moreover,
there are no studies on combined antibacterial
activity of flavonoid isolated from orange peel and
lemon peel. Hence, this study is mainly focused on
investigating the antibacterial activity of combined
flavonoid fractions from ethanolic peel extract of
C.sinensis with C.limon against a gram-positive
organism (S.aureus) and a gram-negative organism
(P.aeruginosa) that is linked with SSTIs.
2 METHODOLOGY
2.1 Collection and Preparation of
Plant Materials
The peels of lemon and oranges part was separated
from the fruits. The peels were air-dried at room
temperature for almost a week until constant
weight. Then, it was grinded into coarse powder by
using a mechanical blender (Sapna B.Shetty et al.,
2015).
Both peels’ powder was macerated for a week
with 96% ethanol. Filtered and evaporated the
solvent by using rotary evaporator to obtain a
concentrate extract of the peels. The extract were
stored in cold room until further use (Augustine
Ahmadu & Ufuoma Omonigho, 2013).
2.2 Preliminary Screening for
Flavonoid
Both of the plants extract were subjected for two
tests: Lead acetate and Shinoda test, for
identification of flavonoids (Sangha R. Bijeka et al.,
2015).
2.3 Preliminary Antibacterial Assay
Both of the plants extract was tested on its
antibacterial activity against S.aureus and
P.aeruginosa by disk diffusion method with three
different concentration (1mg/mL, 10mg/mL and
100mg/mL).
2.4 Thin-Layer Chromatography
(TLC)
TLC was conducted on both plants extract by using
five different solvent mixture (Oyvind M. Andersen
& Kenneth R. Markham, 2006) as described in
Table 1.
Table 1: Different solvent mixture for TLC.
No
Solvent mixture
1
Chloroform-Acetic acid 100:4
2
Chloroform-Methanol-Acetic acid 90:5:5
3
Chloroform-Methanol-Water 40:10:1
4
Chloroform-Methanol-Water 65:45:12
5
Ethyl acetate-Methanol-Water 50:3:10.
TLC was conducted to determine the number of
flavonoid components in both plants extract and
also to identify solvent mixture that provide best
fractionation or separation between those
components (Abe Rita Temidayo, 2013).
2.5 Column-Chromatography
(Fractionation/Isolation of
Flavonoid)
Best solvent mixture identified from TLC was used
for isolating flavonoid fractions from both plants
extract (Abe Rita Temidayo, 2013). Chloroform-
Acetic acid 100:4 solvent system was used for
C.Limon while Chloroform-Methanol-Water
40:10:1 was selected for C. Sinensis. Silica gel was
used as adsorbent. Fractions were collected in
several test tubes and tested on TLC to ensure all the
flavonoid components are collected. Moreover, the
collected fractions were also tested on lead acetate
test again to ensure it is flavonoid fractions.
2.6 Determination of Minimum
Inhibitory Concentration (MIC)
Stock solution of flavonoid fraction was
prepared for both plants and its combination:
100mg/ml. Then, from this stock solution
transferred 1ml into test tube that contains 1ml
ethanol (50mg/ml), these procedure were repeated
until several dilution from 25 mg/ml - 0.2mg/ml.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2

Each concentration was tested on selected bacteria
by disk diffusion method. The first lowest
concentration that produced zone of inhibition
represent the MIC.
2.7 Antibacterial Assay
The in- vitro antibacterial activities was carried out
using the disk diffusion method. Mueller Hinton
Agar (MHA) plate was inoculated with selected
bacteria; S.aureus and P.aeruginosa. The MHA was
prepared and stored at 4°C. Three different
concentration of flavonoid fraction were placed on
the plate (lemon, orange and its combination).
Positive control was Ciprofloxacin and negative
control was Ethanol. Plates were incubated at 37ºC
for 24 hours in the incubator. Zone of inhibition was
measured and repeated for three times.
3 RESULTS AND DISCUSSION
Both plants peel extracts showed positive result for
flavonoid screening by producing yellow
precipitate (Lead acetate test) and orange (Shinoda
test). The result of preliminary antibacterial assay
was, both plants peel extracts showed antibacterial
effect against S.aureus and P.aeruginosa except
C.limon extract does not show any antibacterial
effect against P.aeruginosa at lowest concentration
(1mg/mL). Upon TLC for both plants extract on
different solvent mixture (Table 1), solvent mixture
number 3 was selected for orange while solvent
mixture 1 was selected for lemon to isolate its
flavonoid components. Solvent mixture was chosen
based on two factors which are good retention factor
(0.25 – 0.35) and spot on TLC (considerable
distance between one spot to another). Table 2 and
Table 3 shows the result of isolating flavonoid
fractions from orange and lemon peel extract
respectively.
Table 2: Flavonoid fractions of C.sinensis peel extract
(orange)
Fractions (C.sinensis)
Flavonoid test
(Lead acetate test)
1-5 (Presence spot)
Yellow precipitate
(Component A)
11-15 (Presence spot)
Yellow precipitate
(Component B)
Table 3: Flavonoid fractions of C.limon peel extract
(Lemon).
Fractions (C.limon)
Flavonoid test
(Lead acetate test)
1-5 (Presence spot)
Yellow precipitate
(Component A + B)
12-14 (Presence spot)
Yellow precipitate
(Component C)
17-19 (Presence spot)
Yellow precipitate
(Component D)
21-23 (Presence spot)
Yellow precipitate
(Component E)
Table 4 shows the MIC of flavonoid fractions
from the peel extract of orange, lemon and its
combination respectively against the selected
bacteria. The result is suggestive that when the
flavonoid fractions are combined (lemon with
orange), a lesser concentration of flavonoid is
needed to inhibit the growth of both S.aureus and
P.aeruginosa than individual flavonoid fraction of
orange and lemon. Furthermore, it is noted that a
higher concentration of flavonoid is needed to
inhibit growth of P.aeruginosa compared to
S.aureus.
Table 4: MIC of flavonoid from orange, lemon and its
combination.
Figure 1 shows graph represent comparison of
flavonoid fractions antibacterial activity against
S.aureus and P.aeruginosa. All three flavonoid
fractions produced antibacterial effect against
S.aureus and P.aeruginosa. It is noted that
antibacterial effect of flavonoid against S.aureus is
more compared to P.aeruginosa. It is associated
with impermeability of gram-negative bacteria
(P.aeruginosa) towards antimicrobial agents, in this
case, it is flavonoids. The wall structure of Gram-
negative bacteria, and specifically the presence of
an outer envelope, is often responsible for the
impermeability of these micro-organisms to
antimicrobial agents (S.P. Denyer & J.Y. Maillard,
2002). When the antibacterial effect of C.sinensis
and C.limon is compared, C.limon produced a better
antibacterial effect against both bacteria. This can
be due to presence of more flavonoid components
in C.limon (Component A-E) than C.sinensis
(Component A & B). Moreover, the combination of
both plants flavonoid produced a further increase in
Microorganis
m
MIC (mg/mL)
C.sinensi
s
C.limo
n
Combinatio
n
S.aureus
12.5
0.78
0.2
P.aeruginosa
50
50
25
The Antibacterial Activity of Isolated Flavonoid Fractions from Ethanol Ethanolic Peel of Citrus Sinensis (Valencia Orange) with Citrus
Limon (Lemon) against Staphylococcus Aureus and Pseudomonas Aeruginosa
3
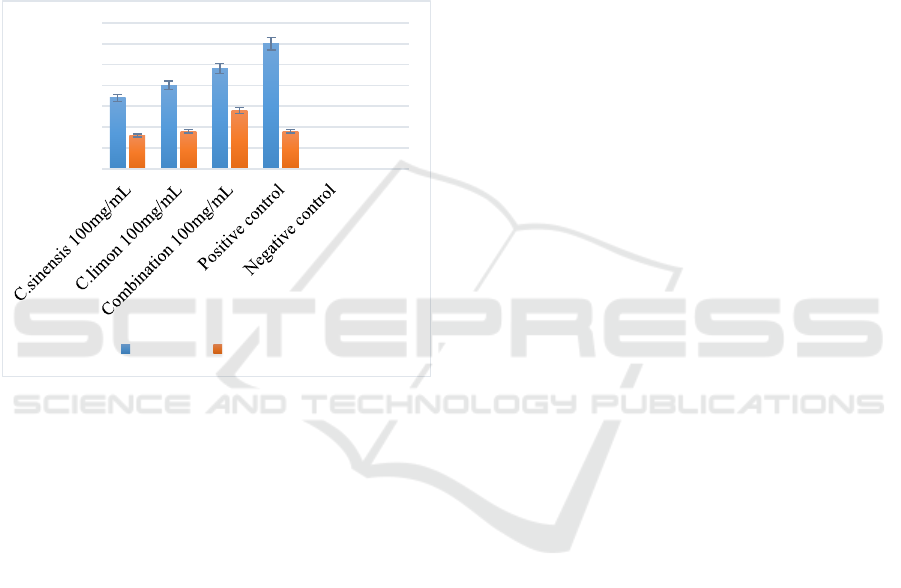
antibacterial effect against S.aureus and
P.aeruginosa. Combined flavonoid fractions
produced an antibacterial effect against S.aureus
(24mm) which was nearly equal to positive control,
Ciprofloxacin disc (30mm). Both flavonoid fraction
of C.sinensis (8mm) and C.limon (9mm) produced
effective antibacterial effect against P.aeruginosa
comparable to positive control (9mm). Also it was
found that combination of C.sinensis and C.limon
flavonoid fractions produced an antibacterial effect
against P.aeruginosa (14mm) better than the
positive control (9mm).
Figure 1: Graph that compare the antibacterial activity of
flavonoid fractions from orange, lemon and its
combination against S.aureus and P.aeruginosa.
4 CONCLUSIONS
This research has found that flavonoid fractions of
all three (C.sinensis, C.limon and its combination)
significantly (p<0.05) produced inhibitory effect
against S.aureus and P.aeruginosa.
Thus,C.sinensis, C.limon and its combination holds
promise as a potential to be developed into solely
herbal based antimicrobial agent to cure SSTIs.
ACKNOWLEDGEMENTS
The author is very thankful and grateful towards the
research committee and lecturers of Management
and Science University, Malaysia for providing all
the needed materials, equipment as well continuous
guidance and support throughout completing this
research project.
REFERENCES
Akhilesh Khushwaha, Raghvendra Pratap Singh,
Vikas Gupta, and Madhulika Singh. (2012).
Antimicrobial properties of peels of Citrus
fruits. International Journal of Universal
Pharmacy and Life Sciences 2(2).
Augustine A Ahmadu and Ufuoma Omonigho
(2013). Flavonoids from the leaves of
Physalis angulata Linn. African Journal of
Pharmaceutical Research & Development, 5,
40-43.
Kailash D. Sonawane. Maruti J. Dhanavade,
Chidamber B. Jalkute, and Jai S. Ghosh
(2011). Study Antimicrobial Activity of
Lemon (Citrus lemon L.) Peel Extract.
British Journal of Pharmacology and
Toxicology 2(3): 119-122, 2011.
Lacey, K. A., Geoghegan, J. A., & McLoughlin, R.
M. (2016). The Role of Staphylococcus
aureus Virulence Factors in Skin Infection
and Their Potential as Vaccine Antigens.
Pathogens (Basel, Switzerland), 5(1).
Matthew S.Dryden. (2010). Complicated skin and
soft tissue infection. J Antimicrob
Chemother 2010; 65 Suppl 3: iii35–44.
Oyvind M. Andersen & Kenneth R. Markham.
(2006). Flavonoids: Chemistry,
Biochemistry and applications.
S.P. Denyer and J.-Y. Maillard. (2002). Cellular
impermeability and uptake of biocides and
antibiotics in Gram-negative bacteria.
Journal of Applied Microbiology
Symposium Supplement 2002, 92, 35S–45S.
Sangha R Bijekar, M.C Gayatri, L Rajanna. (2015),
Antimicrobial Activity of Isolated Flavonoid
Fractions from Drypetes Roxburghii (Wall.)
Huresawa and Its Phytochemical
Fingerprinting. International Journal of
Innovative Research in Science, Engineering
and Technology, 4, 6214-6224.
Sapna B. Shetty, Prabu Mahin-Syed-Ismail, Shaji
Varghese, Bibin Thomas-George,
Pathinettam KandathilThajuraj, Deepak
Baby, Shaista Haleem, Sreeja Sreedhar,
Darshan Devang-Divakar. (2016).
Antimicrobial effects of Citrus sinensis peel
extracts against dental caries bacteria: An in
vitro study. Journal of clinical and
experimental denstistry.
Shalu Hasija, Geeta Ibrahim,and Ashok Wadia.
(2015). Antimicrobial Activity of Citrus
Sinensis (Orange), Citrus Limetta (Sweet
Lime) and Citrus Limon (Lemon) Peel Oil
17
20
24
30
0
8
9
14
9
0
0
5
10
15
20
25
30
35
S.aureus P.aeruginosa
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

on Selected Food Borne Pathogens.
International Journal of Life Sciences
Research, 3, 35-39.
Xinmiao Lv, Siyu Zhao, Zhangchi Ning, Honglian
Zeng, Yisong Shu, Ou Tao, Cheng Xiao,
Cheng Lu, and Yuanyan Liu (2015). Citrus
fruits as a treasure trove of active
natural metabolites that potentially provide
benefits for human health. Chemistry
Central Journal, 9, 68.
The Antibacterial Activity of Isolated Flavonoid Fractions from Ethanol Ethanolic Peel of Citrus Sinensis (Valencia Orange) with Citrus
Limon (Lemon) against Staphylococcus Aureus and Pseudomonas Aeruginosa
5

BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
6

The Antibacterial Activity of Isolated Flavonoid Fractions from Ethanol Ethanolic Peel of Citrus Sinensis (Valencia Orange) with Citrus
Limon (Lemon) against Staphylococcus Aureus and Pseudomonas Aeruginosa
7
