Composition Ratio of Lactose and Corn Starch in Granule Capsule
Formulation of 70% Ethanol Extract Justicia Gendarussa Leaves as
Male Contraceptive
Bambang Prajogo EW
1
, Esti Hendradi
2
,and Pramudita Riwanti
1
1
Pharmacognosy Department, Faculty of Pharmacy, Airlangga University, Surabaya
2
Chemical Pharmacy Department, Faculty of Pharmacy, Airlangga University, Surabaya
Keywords: Justicia gendarussa, lactose, corn starch.
Abstract: The objective of this study were to make a good physical properties of Justicia gendarussa granules with
lactose and corn starch as filler. Optimizations were made into 3 formulas. The difference of each formula
was in the ratio of corn starch and lactose. Formula 1 used ratio 3:7 for corn starch and lactose, Formula 2
used ratio 1:1 for lactose and corn starch, Formula 3 used ratio 7:3 for corn starch and lactose. Physical
evaluation was held to evaluate and choose the best granule like flowability, fines content, angle of repose,
moisture content, compressibility. The result for granules optimization, flowability formula 1 was 3,29 ±
1,08 g/s, formula 2 was 6,04 ± 1,80 g/s, formula 3 was 6,48 ± 1,32 g/s. Angle of repose for F1,2 and 3 were
30,54 ± 1,14o, 29,98 ± 0,34oand 26,98 ± 0,00o. Compressibilty index were 12,00% , 10,00% and 11,99%.
Moisture content 1,82%, 2,08% and 2,75%. Fines content were above 20%. From the evaluation, F2 was
selected as the best formula.
1 INTRODUCTION
Justicia gendarussa is a tropical plant which grow
in tropic land including Indonesia. This plant have
been used by society of Papua as male
contraceptive
30
. Major components from genus
Justicia are alkaloid, lignan, flavonoid and
terpenoid. Gendarussa leaves also contain tannin,
kalium, volatile oil.calcium oxalate and also
alkaloid (justicina) which is toxic.
8
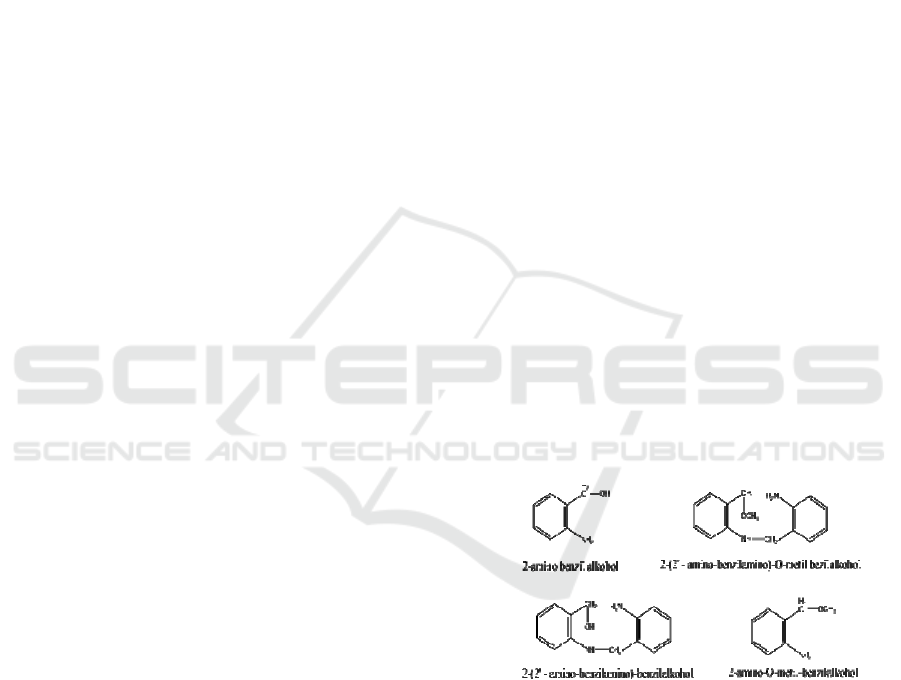
Alkaloid have been isolated from J.gendarussa
leaves are 2-amino benzyl alcohol; 2-amino-o-
metyl benzyl alcohol; 2-(2’-amino-benzilamino)
benzil alcohol; 2-(2’amino-benzil)-o-metil-benzil
ackohol (Figure.1).
7
Figure.1 : Chemical structure aromatic amin substituted
isolated from J.gendarussa leaves.
7
Flavonoids from Justicia gendarussa Burm. f.
are 6,8-di-C-α-L-arabinosil-4', 5,7 trihydroxy-
flavon or 6,8-di-C-α-Larabinosilapigenin and this
compound called gendarusin A, C-α-L-
arabinopiranosil-4 ', 5,7 - trihydroxy-8-C-β-D-
silopiranosilflavone or 6-C-α-L-arabinosil- 8-C-β-
D-silosilapigenin and this compound called
gandarusin B. Other flavonoids are gandarusin C,
D and E. Gandarusin A is major component,
steroid, volatile oil, alkaloids and other flavonoids
Composition Ratio of Lactose and Corn Starch in Granule Capsule Formulation of 70 .
DOI: 10.5220/0009844200002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

(gandarusin B,C,D and E) are minor components
in 70% ethanol extract Justicia gendarussa Burm.f.
31
J.gendarussa extract have an antifertility effect
that can inhibit spermatozoa penetration in-vitro
with inhibit of hyaluronidase enzyme.
29
Therefore,
J.gendarussa was developed into phytopharmaca
drug. Phytopharmaca drug must be produced based
on required standar to ensure the quality of
product. The production process must conform the
standards of GMP. GMP’s requirement including
raw material, equipment, sanitation and hygiene.
Further development of 70% ethanol extract
J.gendarussa leaves into phytopharmaca drug,
need formulation which have been conducted by
several researchers. Granules formulation from
water extract J.gendarussa leaves with avicel and
lactose as filler made by wet granulation gave poor
results because of the tablet hardness and slow
disintegration time (almost 20 minutes). Based on
that results, the filler change into lactose and corn
starch and gave good results
39
, the hardness of
tablets and disintegration time were decreased. The
addition of non-ionic surfactant,tween 80 is also
made to improve the physical requirements and the
dissolution rate of gendarusin A in the granules
formula.
2
Then developed further by replacing
Tween 80 with Poloxamer 188 as surfactant. In
this study, the surfactant used is sodium lauryl
sulfate. Replacement poloxamer 188 (non-ionic)
with sodium lauryl sulphate which are anionic
surfactants is based on research conducted by
Alkhamis et al. (2003) who found that non-ionic
surfactant demonstrated the ability solubilization
smaller than the anionic and cationic surfactants on
solubilization glikazid. Additionally, poloxamer
188 has a relatively expensive price so that less
effective if will be developed later. This formula
then regarded as the chosen formula by
considering the parameters that can produce good
product as an infertility drug.
Table 1 : Relationship between % compressibility and
flowability
40
%
Compres
s
ibilit
y
Flowability
5-12 Perfect
12-16 Good
18-21 Moderate
23-28 Poor
28-35 Poor
35-38 Very poor
> 40 Ver
y
-ver
y
poo
r
(cohesive)
2 MATERIALS AND METHOD
2.1 Materials
Lactose (Lactose Monohydrate, Leprino USA),
Corn starch (Amylum Maydis, Cargill Bio-
Chemical China, Cab-o sil (Pluronic F-68, Sigma
Life Scine USA), Sodium Lauryl Sulfate (SLS),
Methanol p.a (Merck), Ethanol 70% Nylon
membrane 0,2 µm (Whatman), Filter holder
(Millipore), Aquadest.
2.1.1 Standarized Simplicia of J.Gendarussa
Nine months of J.gendarussa leaves have been
harvested. Fresh leaves then made into simplicia
by sortation (remove mechanical parts except leaf
that are not needed like bark, stem, etc) and then
washed and dried in drying cabinet at tempertaure
below50°C. Dried simplicia then milled to a
powder with a certain size
12
2.1.2 Extraction
Gandarussa leaves powder extracted with
macseration using ethanol 70% (1:10) for 24 hours.
Re-extraction until three times. After extraction,
the solvents were allowed to evaporate using rotary
evaporator. Thus the highly concentrated ethanol
extract were obtained. The extract then stored in
refrigerator at 4
o
C for further use for formulation
process.
2.1.3 Formulation
Optimization were held with 3 formulas.
J.gendarussa leaves granules were prepared by wet
granulation method. Corn starch, lactose were used
2

as filler, sodium lauryl sulfate 1% was used as
surfactant and cab o sil was used as glidant. The
difference for each formula was on the ratio
between lactose and corn starch. Formula 2 used
ratio 1:1 for lactose and corn starch, Formula 1
used ratio 3:7 for lactose and corn starch, Formula
3 used ratio 7:3 for corn starch and lactose.
Physical evaluation is held to evaluate and choose
the best granule.
2.2 Evaluation of Granules
2.2.1 Flow Rate and Angle of Repose
The angle of repose was determined by allowing
granules to flow through a funnel and fall freely
onto a graph paper on a horizontal surface. The
time taken for the weighed granules to flow out
completely was recorded
5
. This was performed in
triplicate.
Flow rate was obtained by the equation below:
Flow rate = weight of granules / time
The height and diameter of the resulting cone were
measured and the angle of repose is calculated
from this equation:
tan Ø =h/r
Where :
h is the height of the powder cone and r is the
radius of the powder cone
2.2.2 Bulk Density
The bulk density (ρ
b
) of granules was determined
by filling the material into a tarred graduated
cylinder to the 100 ml mark. The graduated
cylinder was weighted and the bulk density
calculated as the ratio of the sample weight to
sample volume
ρbulk= W / V
Where :
ρbulk = Apparent bulk density,
W = Weight of the sample,
V = Apparent volume of powder
2.2.3 Tapped Density
A suitable amount of granules was placed in a 100
ml measuring cylinder. After absorbing its initial
volume, the sample was tapped 500 times initially
followed by an additional taps of 750 times until
the difference between succeeding measurement is
less than 2% and then tapped volume, was
measured, to the nearest graduated unit. Tapped
density was calculated using
equation
ρtab = W / Vf
Where :
ρtab = Tapped Density,
W = Weight of the sample,
Vf = Tapped volume of powde
2.2.4 Moisture Content
Moisture content determination using Ohauss
electronic Moisture balance 45 with place about
0,5-1g sample in sample pan. The sample pan must
lie flat in the pan handler. Then, press the start
button to start analyze. After ten minutes, %
moisture content can read.
2.2.5 Fines Content
The determination of fines is done by inserting 100
grams of granules into a sieve with a hole diameter
of 140 mesh (the equivalent of 100 micrometers).
Then sieve vibrated for 20 minutes at a speed of 10
rpm. Weigh the amount of powder that escaped
sieve (Wade and Weller, 1994). The amount of
fines should not more than 20%. Particles that are
larger than 250 μm is relatively free flowing,
whereas particles have a size below 100 μm (fines)
cause problems in the flow properties due to the
occurrence of a large cohesive force.
3
2.2.6 Statistical Analysis
Data analysis was performed on the physical
parameters of the granules and then performed
statistical analysis by one-way ANOVA (One-Way
ANOVA). To determine whether there were
significant differences between the formula, then
followed by the Tukey-HSD test to determine any
formula that provides a meaningful difference.
Statistical analysis includes flow rate, angle of
repose, moisture content and% compressibility
with 95% confidence level (α = 0.05). When
Asymp. Sig. α < (0.05), then Ho is rejected and Ha
accepted.
Composition Ratio of Lactose and Corn Starch in Granule Capsule Formulation of 70
3

3 RESULTS AND DISCUSSION
J.gendarussa leaves extract have a viscous
consistency therefore need filler composition like
corn starch and lactose that can improve the
physical properties of granules. With a viscous
consistency and large water content, granulation
process carried out by wet granulation to improve
the flowability and compactibilty of granule mass.
Materials used as filler are lactose and corn
starch. Lactose in the tablet formulation excipients
serves as good as it can condense the mass of
granules in the wet granulation or direct
compression and can improve the flow properties
because the lactose has a large specific gravity.
24
It
is also the most widely used filler because it does
not react with almost all of the ingredients.
Generally, formulation with lactose showed a good
rate of drug release, quick dry granules,
disintegration time is not very sensitive to changes
in the tablet hardness.
25
However, lactose may
increase the hardness of the tablet therefore need a
disintegrant to overcome. Corn starch has a lower
specific gravity than the lactose that can help the
bonds between the extract particles is not too
strong so the combination of this two excipients
lactose and corn starch can improve the physical
quality of granules extract (J. gendarussa) as
phytopharmaca drug.
The solubility of J.gendarussa extract that
partially soluble caused a slow release of the active
ingredients therefore the absorbtion and effect will
be slow. Materials with low water solubility caused
bad wetting because of their interfacial tension
between the water phase, vapor phase and solid
phase. As a result, drug will be difficult
dissolved.
25
This requires the addition of a
surfactant to improve the solubility.
In this study, the surfactant used was sodium
lauryl sulphate (SLS). With the addition of this
SLS, can reduce the surface tension between the
particles, which occurs damping effect that makes
the contact between the granules with media is
large so the active substance is easier to get out of
the granule and dissolve into the media. SLS as a
wetting agent can also improve the dissolution rate
of the drug due to its mechanism.
3
The result of granules can be seen in figure.2,
and evaluation of each formulas can be seen in
table 2.
Figure.2: Granules formula 1,2,3 resulted from
optimization
From the results in table 2.2, the flow rate of
the granules, formula 1 was 3.28 ± 1.08 g / s,
formula 2 was 6.04 ± 1.80 g / s, formula 3 was
6.48 ± 1.32 g / s. Based on the statistic results
using ANOVA, there was no significant difference
in flow rate between formula 1,2 and 3. formula
granule flow velocity is considered good if it is in
the range of 4-10 g/s. Formula 2 and 3 meet these
requirements, while the formula 2 has a flow rate
of <4 g/s so it can be considered to have difficult
flow properties.
3
The flowability can also be viewed from the
angle of repose. Angle of repose resulting from
formula 1 was 30.54 ± 1.14
o
, formula 2 was 29.98
± 0.34
o
and formula 3 was 26.98 ± 0,00
o
. From
the results of statistical tests on granules extract (J.
gendarussa) , there was a significant difference
between the angle of repose formula 1 and 3,
Formula 2 and 3 on the confidence level of 0.95%
(α = 0.05). Angle of repose illustrate the magnitude
of the frictional forces between the particles, so
4

can demonstrate the flow properties of a
granular indirectly.
6
Based on the angle of repose,
all of
granules formula were meets the criteria of the
granules with good flow properties, which have the
angle of repose between 20-30
o
.
44
Good flow
properties will make the die filling fulfilled evenly
so the weight of the capsule is not distorted.
25
Granule flow properties also have a
relationship with compressibility. Granules have a
perfect flow properties (granule flow freely) if it
has a range of 5-12% compressibility.
40
In formula
1,2 and 3 the compressibility respectively were
12.07%, 9.68% and 13.11%. All formula meets the
compressibility range with perfect flow properties.
Formula 3 had the highest % compressibility. It
can be related to the high moisture content 2.75%.
Moisture content can affect the compressibility
index and flowability because the moist powder
mass will result in less free flowing powder.
High moisture content of the F3 can also be
caused by the amount of corn starch higher than
other formulas. Corn starch is hygroscopic so the
granules will be more humid. The results of each
formula met the requirements as good granules, the
moisture content of the granules were in the range
of 2% - 4%. These results can guarantee the
granules are stable during storage. Humidity of a
granule will affect the stability of the granules. The
higher the humidity, the higher the potential for
microbes to live so stability become shorter. When
the moisture content too much can lead to sticky
and hard flowing granules, but small water
content will produce a dry granule and easy fragile.
Fines content (particle size <100µm) from all
formulas were more than 20%. From all that
formula, the best formula chosen was determined
based on the evaluation has been done. Formula 2
was chosen formula because the formula 3 had a
high moisture content, it is feared the stability of
the granules is getting shorter. While the formula 1
and 2 based on the results of the statistical analysis,
flowability and angle of repose did not provide a
significant difference but in terms of the yield
obtained, compressibility and flowability, formula
2 was better than formula 1. Thus formula 2 with
ratio 1:1 for lactose and corn starch was a best
formula and chosen to do the next process.
CONCLUSION
Granules with filler ratio 1:1 for lactose and corn
starch can result in good physical properties of
granules.
REFERENCES
1. Ansel, H. C., Popovich, N. G., and Allen, L.
V. 1995. Pharmaceutical Dosage Form and
Drug Delivery System, 6
th
ed., Malvern:
Williams and Wilkins, p. 60-65.
2. Arifani, G. 2012. Pengaruh Tween 80
Terhadap Laju Disolusi Gendarusin A dalam
Granul Ekstrak Etanol 70% Daun Justicia
gendarussa burm. f. Untuk Sediaan Kapsul.
Skripsi. Fakultas Farmasi Universitas
Airlangga.
3. Aulton, M. E. 2002. Pharmaceutics : The
Science of Dosage Forms Design. London :
Churchill Livingstone
4. Banker, G. S. and Anderson, N. R. 1989.
Tablet, In: Lachman, L., Lieberman, H. A.
And Kanig, J. L. (Eds). Teori dan Praktek
Farmasi Industri, edisi ketiga, Vol II, Jakarta:
Universitas Indonesia.
5. Bhagawan, W.S. 2015. Formulasi dan Uji
Disolusi Granul Ekstrak Etanol 70%
Terfraksinasi Daun Gendaarusa. Thesis.
Fakultas Farmasi Universitas Airlangga.
6. Carstensen. 1977. Pharmaceutics of Solid
and Solid Dosage Forms. New York: John
Willey and Sons.
7. Carstensen, J.T. and Rhodes, C.T., 2000, Drug
Stability Principles and Practices, Third
Edition, Revised and Expanded, Marcel
Dekker, Inc., New York : 238 – 381.
8. Chakravarty, A. K., Dastiar, P. P. G., and
Pakrashi, S. C. 1982. Simple aromatic amines
from Justicia gendarussa 13C NMR Spectra
of the bases and their analogues. Tetrahedron.
Elsivier, 18 (12):1797-1802.
Table 2 : Physical Evaluation of Granules
Evaluation Formula 1 Formula 2 Formula 3 Requirement
Flowability (g/s) *3.28±1.08 6.04±1,80 6.48±1,32 4-10
g
/s (Good flowabilit
y
)
Angle of repose (
o
) *30.54±1.14
o
29.98±0,34
o
26.98±0,00
o
20-30
o
(Good flowabilit
y
)
fines (%) 24.15 25.97 21.09 < 20%
Compressibility (%) 12.00 10.00 11.99 5-12% (Perfect flowabilit
y
)
Moisture content (%) 1.82±0,02 2.08 ± 0,04 2.75 ± 0,02 2-4%
Yield (%) 61.63% 71.08% 78.40% -
Composition Ratio of Lactose and Corn Starch in Granule Capsule Formulation of 70
5

9. Dalimartha, S. 2001. Atlas Tumbuhan Obat
Indonesia, Jilid 1, Jakarta: Trubus Ariwidya.
10. Departement of Health. 2002. British
Pharmacopoeia. London: The Stationary
Office. p. 1003, A 241-242.
11. Departemen Kesehatan RI. 1995. Farmakope
Indonesia. Edisi IV. Jakarta : Dirjen POM.
12. Departemen Kesehatan RI. 1995. Materia
Medika Indonesia. Jilid VI. Jakarta:
Departemen Kesehatan RI.
13. Departemen Kesehatan RI. 2000. Parameter
Standar Umum Ekstrak Tumbuhan Obat.
Jakarta: Departemen Kesehatan RI.
14. Dhirendra, K., Lewis, S., Udupa, N., and Atin,
K. 2009. Solid Dispersion, India, Manipal
College of Pharmaceutical Science. p. 234-
246.
15. EMEA., 1999. Working Part on Herbal
Medicinal Products (HMPWP), Stability
testing of HD (Herbal Drug), HDP (Herbal
Drug Preparation), and HMP (Herbal
Medicinal Product), Guidelines 25 : 48.
16. Feher, M., and Schmidt, J. M. 2003, Property
Distributions: Differences Between Drugs,
Natural Products, And Molecules From
Combinatorial Chemistry, J. Chem. Inf.
Comput. Sci. 43, 1, 218-227
17. Gordon, R. E., Rosanske, T. W., Fonner, D. E.,
Anderson, N. R., and Banker, G. S. 1990.
Granulation Technology and Tablet
Characterization. In: Lachman, L.,
Lieberman, H. A., Schwartz, J. B. (Eds),
Pharmaceutical Dosage Forms: Tablets, 2
nd
ed,
Vol. 2, New York: Marcel Dekker, Inc.
18. Gunsel, W. C. and Kanig, J. L. 1976. Tablet,
In: Lachman, Lieberma, H. A. and Kanig J.
L., The Teory and Practice of Industrial
Pharmacy, 2
nd
Ed., Lea and Febiger,
Philadelphia.
19. Gupta, R. S., and Sharma, R. 2006. A Review
on Medicinal Plants Exhibiting Antifertility
Activity in Males. Natural Product
Radiance. Vol. 5 (5), p. 389-410.
20. Handa, S. S., Khanuja, S. P. S., Longo, G., and
Rakesh, D. D. 2008. Extraction Technologies
for Medicinal and Aromatic Plants. Itali:
International centre for science and high
technology. pp.21-25.
21. Harborne, J. B., 1987. Metode Fitokimia
Penuntun Cara Modern Menganalisis
Tumbuhan, Edisi kedua. Bandung: Institut
Teknologi Bandung Press.
22. Heyne. 1987. Tumbuhan Berguna
Indonesia. Jilid III. Terjemahan Badan
Litbang Kehutanan. Jakarta: Yayasan Sarana
Wana. hal 1759.
23. Kiren, Y., Deguchi, J., Hirasawa, Y., Morita,
H., and Prajogo, B. 2014. Justidrusamides A-
D, new 2-aminobenzyl Alcohol Derivatives
from Justicia gendarussa. Journal of Natural
Medicines. Vol. 68, pp. 754-758.
24. Kusumahyuning, R., Soebagyo., Sulihtyowati,
S. 2005. Pengaruh Laktosa dan Povidon dalam
Formula Ekstrak Kaempferia galanga L.
Secara Granulasi Basah. Majalah Ilmu
Kefarmasian. Vol. II, No. 16, 110-115.
25. Lachman, L., Lieberman H.A., Kanig J.L.
1994. Teori dan Praktek Farmasi Industri
edisi III. Jakarta : UI Press.
26. Lieberman, H.A., Lachman, L., Schwartz, J.B.
1990. Pharmaceutical Dosage Forms. New
York : Marcel Dekker.
27. Ncube, N. S., Afolayan, A. J., and Okoh, A. I.
2008. Assessment Techniques of
Antimicrobial Properties of Natural
Compounds of Plant Origin: current methods
and future trends. African Journal of
Biotechnology. Vol 7, pp.1797-1806.
28. Prajogo, B. E. W., A. Khoiril, IGP Santa dan
Soeharno. 1997. Efek Ekstrak Diklormetan
dan Ekstrak Metanol Daun Gendarussa
vulgaris Ness pada Spermatogenesis tikus,
Simposium PERHIPBA IX, Universitas
Gadjah Mada, Yogyakarta.
29. Prajogo, B. E. W., NS. Matty, IGP. Santa and
PS. Onny. 1998. Efek Ekstrak Diklormetan
dan Ekstrak Metanol Daun Gendarussa
vulgaris Ness pada Aktivitas Enzim
Spermatozoa Kelinci. Symphosium
POKJANAS TOI 8 VIII Universitas Brawijaya,
Malang.
30. Prajogo, B. E. W. 2002. Aktivitas Antifertilitas
Flavonoid Daun Gendarusa Vulgaris Ness.
Penelitian Eksperimental Pencegahan
Penetrasi Spermatozoa Mencit dalam Proses
Fertilisasi In Vitro. Disertasi. Program Pasca
Sarjana Universitas Airlangga Surabaya.
31. Prajogo, B. E. W., Dudi, S., dan Mulya, H. S.
2007. Analisis Gendarusin A pada tanaman
Budidaya Justicia gendarussa Burm f. Jurnal
Farmasi Indonesia. 3: 176-180.
32. Prajogo, B. E. W., Pramesti, D., Musta’ina, S.,
Winarso, H., Radjaram, A., Suharjono., Zaini,
N. C., Flourisa, J., Anggraeni, M. 2011.
Clinical Trial: The Use of Justicia
gendarussa Burm. f. as Male Contraception.
APCRSHR 6. Yogyakarta: Indonesia.
33. Prajogo, B. E. W. 2014. Autentik Tanaman
Justicia gendarussa Burm. f. Sebagai Bahan
Baku Obat Kontrasepsi Pria. Surabaya:
Airlangga University Press dengan LP3
UNAIR.
34. Pratama, B. O. 2012. Penentuan Standar
Umum Ekstrak Etanol 70% Daun Justicia
gendarussa Burm. f. Skripsi. Fakultas Farmasi
Universitas Airlangga.
35. Radji, M., Oktavia, H., Suryadi, H. 2008.
Pemeriksaan Bakteriologis Air Minum Isi
Ulang Di Beberapa Depo Air Minum Isi Ulang
6

di Daerah Lenteng Agung dan Srengseng
Sawah Jakarta Selatan. Majalah Ilmu
Kefarmasian, Vol 5(2) : 101-109
36. Rajakumar, N., and Shivana, M. B. 2009.
Ethno-medical Application of Plants in the
Eastern Region of Shimoga Distric. Journal
Ethnopharmacology. Vol 126, pp.64-73.
37. Rizqa, O, D., 2010. Standardisasi Simplisia
Daun Justicia gendarusssa Burm f. Dari
Berbagai Tempat Tumbuh (Daerah
Mojokerto lahan 1, Mojokerto Lahan II,
dan Ponorogo). Skripsi, Fakultas Farmasi
Universitas Airlangga, Surabaya.
38. Rowe, R. C., Paul, J. S., Sian, C. O. 2009. The
Handbook of Pharmaceutical Excipients. 6
th
Ed, London: Pharmaceutical Press and
American Pharmacist Association.
39. Sari, M.A. 2010. Pengembangan Formula
Granul Ekstrak Etanol 70% Daun Justicia
gendarussa Burm.f. Sebagai Sediaan
Fitofarmaka. Skripsi, Fakultas Farmasi
Universitas Airlangga, Surabaya.
40. Staniforth, J. N. 1988. Powder Flow. In:
Aulton, M., Pharmaceutica: The Science
Dosage Form Design, New York: Churchill
Livingstche.
41. Sucker, H. 1982. Test Methods for
Granulates, In Pharm Ind, 44
th
Ed, number 3,
Switzerland.
42. United State Pharmacopoeia Convention.
2003. The United State Pharmacopeia 26-
National Formulary.
43. United States Pharmacopeia Convention.
2002. The United State Pharmacopeia 25-
National Formulary.
44. Wells, J. L. and Aulton, M. E. 1988.
Preformulation, in Aulton, M. E., Editor,
Pharmaceuticals The Sciences Dosage Form
Design. London: Churcill Livingstone.
45. WHO. 1999. WHO Monograph on Selected
Medicinal Plants. Vol.I, Geneva: WHO.
46. Zheng, Jack. 2009. Formulation and
Analytical Development for Low Dose Oral
Drug Products. New Jersey: John Wiley &
Sons, Inc.
Composition Ratio of Lactose and Corn Starch in Granule Capsule Formulation of 70
7
