
Cell Cycle Arrest Activity of Alkaloid Fraction of Litsea Cubeba
Lour. Heartwoods Towards Hela Cancer Cell
Aminah Dalimunthe
1*
, Poppy Anjelisa Zaitun Hasibuan
1
, Denny Satria
2
1
Department of Pharmacology,
2
Department of Pharmaceutical Biology
Faculty of Pharmacy, University of Sumatera Utara, Medan, Indonesia
Keywords: Alkaloid Fractions, Cell cycle, HeLa, Litsea Cubeba Lour. Heartwood.
Abstract: Cervical cancer therapy with chemotherapeutic agents is limited because of drug resistance problem and
toxic effect on normal tissue leads to immunosuppression and cardiotoxicity. This study was to investigated
cell cycle arrest activity towards HeLa cell lines of Litsea cubeba Lour. heartwood alkaloid fraction. Litsea
cubeba Lour. heartwood powder was extracted by maceration method with ethanol 96% and fractionated
with n-hexane and chloroform at pH 3,7 and 9. The cytotoxic study was using MTT method and analysis
cell cycle was using flow cytometry method. The IC50 of ethanol extract, n-hexane and chloroform
fractions at pH 3,7 and 9 at were 156.24 ± 2.96; 67.23 ± 0.63; 175.92 ± 2.40; 52.46 ± 0.34; and 94.81 ± 2.16
µg/mL respectively. The chloroform fractions at pH 7 concentration 25 and 10 µg/mL were caused
accumulation in G2-M phase (33.84 and 29.08%). The results reveal that Litsea cubeba Lour. heartwood
alkaloid fraction provides effective as cell cycle arrest. Our further study is to assess the mechanism of
alkaloid fraction in inhibit metastasis in cervical cancer.
1 INTRODUCTION
Cancer is one of the high incidence dangerous
diseases in human and presently there is a
considerable number of new anticancer agents
from natural products (Sharma, et al., 2011).
According to WHO data, cancer is one of the
leading cause of death worldwide especially
cervical cancer (Berrington and Lall, 2012).
Cervical cancer theraphy with chemotherapeutic
agents is limited cause of drug resistance and
toxic side effect on normal tissue leads to some
effects such as immunosuppression and
cardiotoxicity (Jemal, et al., 2010; Tyagi, et al.,
2004).
Attarasa (Litsea cubeba (Lour,) is a plant from
Lauraceae family which contain many essential
oils which used as antideppressants,
antiinflammation, antioxidant, pesticide,
antimicrobial, anticancer on breast cancer and
neuro pharmacology. The methanol extract from
attarasa fruits showed to be active on HeLa cell
lines which cause apoptosis through activation of
caspase 3/7 (Trisonthi, et al., 2014; Piyapat, et al.,
2013). There are more than forty isoquinoline
alkaloids that contained in Litsea genus which are
active as antibacterial agents against
Staphylococcus aureus (Feng, et al., 2009). The
heartwoods of Litsea cubeba contained high level
of phenolic and flavonoid and found to be active as
antioxidant and has anti breast cancer activity
which causes cell cycle inhibition. Alkaloids
compound which isolated from heartwood have
antioxidant activity with DPPH and ABTS
methods (Dalimunthe, et al., 2016; Dalimunthe, et
Dalimunthe, A., Zaitun Hasibuan, P. and Satria, D.
Cell Cycle Arrest Activity of Alkaloid Fraction of Litsea cubeba Lour. Heartwoods Towards HeLa Cancer Cell.
DOI: 10.5220/0009844000002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

al., 2017; Dalimunthe, et al., 2018). The aim of this
study was to assess cell cycle arrest activity of
alkaloid fraction of Litsea cubeba Lour.
heartwoods on HeLa cells.
2 MATERIALS AND METHODS
2.1 Fractions Preparation
Fresh heartwoods of Litsea cubeba Lour. was
collected from Balige subdistrict, Sumatera Utara
province, Indonesia. The air-dried and powdered
heartwoods of Litsea cubeba (Lour,) (1 kg) were
repeatedly macerated with ethanol 96% (3x3 d, 7.5
L), The filtrate was evaporated to give a viscous
extract. Viscous extract was fractionated with n-
hexane and continue with chloroform at pH 3,7
and 9 (Rosidah, et al., 2018; Dalimunthe, et al.,
2018; Satria, et al., 2015).
2.2 Cytotoxicity assay
Extract and alkaloid fractions were submitted for
cytotoxicity test. In that way, HeLa cell line was
grown in RPMI medium containing 10% Fetal
Bovine Serum (Gibco), 1% penicillin-
streptomycine (Gibco), and fungizone 0.5%
(Gibco) in a flask in a humidified atmosphere (5%
CO
2
) at 37
o
C. The inoculums seeded at 1 x
10
4
cells/mL at an optimal volume of 0.1 mL per
well. After 24 h incubation, the medium was
discharged and treated by fractions. After
incubation for 24 h, the cells were incubated with
0.5 mg/mL MTT for 4 h at 37
o
C. Viable cells
reacted with MTT to produce purple formazan
crystals. After 4 h, SDS 10% as stopper (Sigma) in
0.01N HCl (Merck) was added to dissolve the
formazan crystals. The cells were incubated for 24
h in room temperature and protected from light.
After incubation, the cells were shaken, and
absorbance was measured using microplate reader
at λ 595 nm. The data which were absorbed from
each well were converted to percentage of viable
cells (Hasibuan, et al., 2015 and Nurrochmad, et
al., 2014).
2.3 Cell Cycle Inhibition Assay
HeLa cells (7.5x10
5
cells/well) were seeded into 6-
well plate and incubated for 24 h. After that, the
cells were treated and then incubated for 24 h.
Both floating and adherent cells were collected in
conical tube using trypsin 0.025%. The cells were
washed thrice with cold PBS and centrifuged at
2500 rpm for 5 min. The supernatant was
separated, while the sediment was collected and
fixed in cold 70% ethanol in PBS at 4
o
C for 1 h.
The cells were washed thrice with cold PBS and
resuspended then centrifuged at 3000 rpm for 3
min and PI kit (containing PI 40 µg/mL and
RNAse 100 µg/mL) added to sediment and
resuspended and incubated at 37
o
C for 30 min. The
samples were analyzed using FACScan flow
cytometer. Based on DNA content, percentage of
cells in each of stage in cell cycle (G1, S and
G2/M) were calculated using ModFit Lt. 3.0.s
(Harahap, et al., 2018 and Satria, et al., 2017).
2.5 Statistical Analysis
The results were presented as means ± SD.
2.5.1 Results
Inhibitory Concentration 50% (IC
50
)
MTT method was used to determine cell viability
after incubation for 24 h. In every treatment extract
and alkaloid fractions were shown in Table 1.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
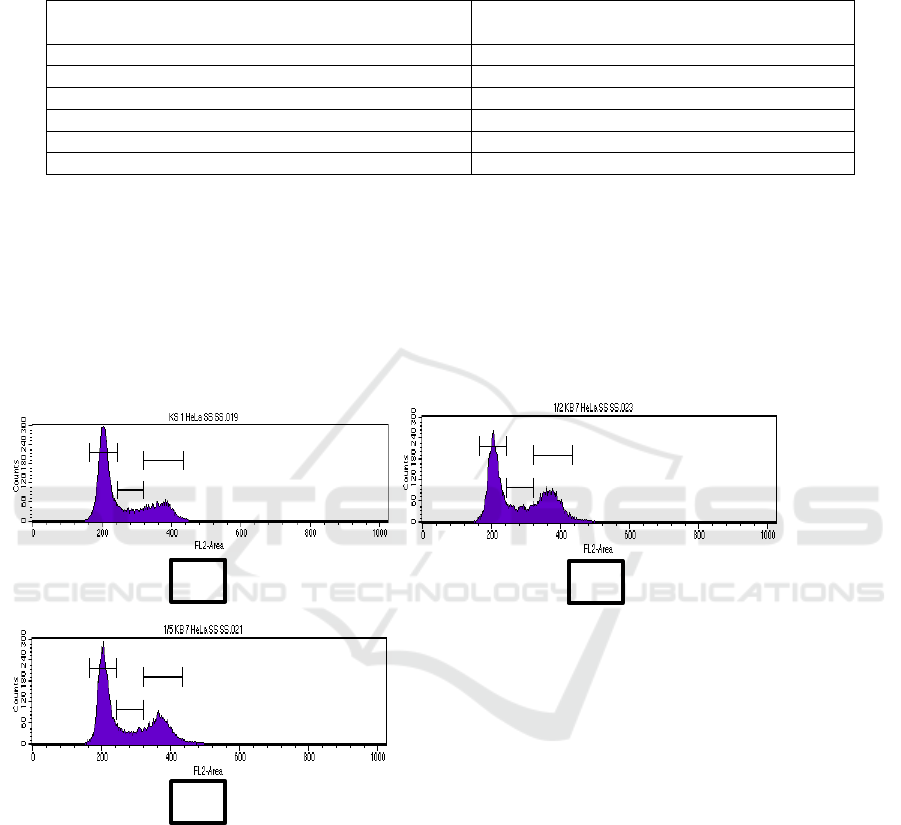
Table 1. IC
50
value of extract and alkaloid fractions of Litsea cubeba heartwood with MTT assay (Mean ± SD,
3 times of replication)
Treatment
IC
50
(µg/mL)
Ethanol Extract
156.24 ± 2.96
n-hexane Fraction
67.23 ± 0.63
Chloroform Fraction pH 3
175.92 ± 2.40
Chloroform Fraction pH 7
52.46 ± 0.34
Chloroform Fraction pH 9
94.81 ± 2.16
Cisplatin
24.01 ± 0.31
2.4 Effect on Cell Cycle
To evaluate the effect of chloroform fraction at pH
7 (CF-7) to increase cell death by modulating cell
cycle, we concentrated on it for further studies
using flow cytometry method.
The effect of CF-7 at 25 and 10 µg/mL is given in
Figure 1. Whereas treatment of CF-7 at 25 and 10
µg/mL caused cell accumulation at G
2
/M phase
(33.84% and 29.08%) and for control cell
(17.78%).
Figure 2: Percentage of cell cycle phase of HeLa cells were treated for 24h. (a) Control cell, (b) 25 µg/mL (1/2 IC
50
), (c) 10
µg/mL (1/5 IC
50
).
R1
M1
GO-G1
S-phase
G2-M
M5
GO-G1
S-phase
G2-M
R1
M1
GO-G1
S-phase
G2-M
M5
GO-G1
S-phase
G2-M
R1
M1
GO-G1
S-phase
G2-M
M5
GO-G1
S-phase
G2-M
a
b
c
Cell Cycle Arrest Activity of Alkaloid Fraction of Litsea cubeba Lour. Heartwoods Towards HeLa Cancer Cell
3

3 DISCUSSION
The cytotoxicity estimate of herbal is correlated to
content of active compound in these plants
including Litsea cubeba Lour. Alkaloids as major
compound have main role in cytotoxicity effect
(Yadav, et al., 2010). Litsea genus is rich in
isoquinoline alkaloids and for Litsea cubeba Lour
has been found two alkaloids (+)-N-
(methoxy-carbonyl) N-norlauroscholtzine and (+)-
N-(methoxy-carbonyl)-N-norglaucine (Feng, et al.,
2009). Alkaloids are the compound which
potentially in inhibits the cancer proliferation for
the example berberine is an isoquinoline alkaloid
which inhibits proliferation of multiple cancer cell
line by inducing cell cycle arrest at G
0
/G
1
or G
2
/M
phases and by apoptosis (Sun, et al., 2009; Eom, et
al., 2010; Burgeiro, et al., 2011). Inhibition of
tumor invasion and metastasis is the mechanism of
action of berberine (Tang, et al., 2009; Ho, et al.,
2009). Evodiamine is a quinolone alkaloid inhibits
topoisomerase enzyme, induces DNA damage,
exhibit G
2
/M phase arrest (Liao, et al., 2005; Kan,
et al., 2004; Huang, et al., 2004).
ACKNOWLEDGEMENTS
We gratefully thank to Research Center University
of Sumatera Utara through Hibah Talenta ““Hibah
Penelitian Dasar” Research Grant 2018 “No:2590/
UN5.1.R/PPM/2018” for financial support in the
study.
REFERENCES
Berrington D and Lall N (2012). Anticancer activity
of certain herbs and spices on the cervical
epithelial carcinoma (HeLa) cell line. eCAM,
Article ID 564927.
Burgeiro Z, Gajate C, Dakir EH, Villa-Pulgar´ın JA,
Oliveira PJ. (2011). Involvement of
mitochondrial and B-RAF/ERK signaling
pathways in berberine-induced apoptosis in
human melanoma cells. Anti-Cancer Drugs,
22(6), 507–18.
Dalimunthe A, Achmad S, Satria D. (2016).
Phenolic, flavonoid content and antioxidant
activities of ethylacetate extract of litsea
cubeba (lour.) pers. barks. Der Pharma
Chemica, 8, 466-468.
Dalimunthe A, Hasibuan PAZ, Satria D. (2017).
Cell cycle arrest activity of Litsea cubeba
Lout. heartwood and fruit extracts against
T47D breast cancer cells. Asian J Pharm Clin
Resc, 10(11), 404-6.
Dalimunthe A, Hasibuan PAZ, Silalahi J, Sinaga
SF, Satria D. (2018). Antioxidant activity of
alkaloid compounds from Litsea cubeba Lour.
Orient J Chem, 34(2), 1149-52.
Eom KS, Kim HJ, So HS, Park R, Kim TY.
(2010). Berberine-induced apoptosis in human
glioblastoma T98G Cells Is mediated by
endoplasmic reticulum stress accompanying
reactive oxygen species and mitochondrial
dysfunction. Biol Pharm Bull, 33(10), 1644–9.
Feng T, Rong-Ting Z, Qin-Gang T, Xiang-Yun Z.
(2009). Two new isoquinoline alkaloids from
Litsea cubeba. Z Naturforsch, 64, 871-4.
Harahap U, Hasibuan PAZ, Sitorus P, Arfian N,
Satria D. (2018). Antimigration activity of an
ethylacetate fraction of Zanthoxylum
acanthopodium DC. fruits in 4T1 breast cancer
cells. Asian Pac J Cancer Prev, 19(2), 565-9.
Hasibuan PAZ, Harahap U, Sitorus P, Satria D
(2016). Ethylacetate extract of Zanthoxylum
acanthopodium DC. fruit against doxorubicin-
resistanced T47D cells. Der Pharma Chemica,
8(20), 172-174.
Hasibuan PAZ, Jessy C, Denny S (2015).
Combination effect of ethylacetate extracts of
Plectranthus ambonicius (Lour.) Spreng. with
doxorubicin againts T47D breast cancer cells. Int
J Pharm Pharm Sci, 7, 155-9.
Ho YT, Yang JS, Li TC. (2009). Berberine
suppresses in vitro migration and invasion of
human SCC-4 tongue squamous cancer cells
through the inhibitions of FAK, IKK, NF-κB, u-
PA and MMP-2 and -9. Cancer Letters, 279(2),
155–62.
Huang YC, Guh JH, Teng CM. (2004). Induction of
mitotic arrest and apoptosis by evodiamine in
human leukemic Tlymphocytes. Life Sciences,
75(1), 35-49.
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

Jemal A, Siegel R, Xu J, Ward E (2010). CA Cancer
J Clin,
Kan SF, Huang WJ, Lin LC, Wang PS. (2004).
Inhibitory effects of evodiamine on the growth
of human prostate cancer cell line LNCaP. Int J
Canc, 110(5), 641–51.
Liao CH, Pan SL, Guh JH. (2005). Antitumor
mechanism of evodiamine, a constituent from
Chinese herb Evodiae fructus, in human
multiple-drug resistant breast cancer NCI/ADR-
RES cells in vitro and in vivo. Carcinogenesis,
26(5), 968–75.
Nurrochmad A, Lukitaningsih E, Meiyanto E (2011).
Anti cancer activity of rodent tuber (Thyphonium
flagelliforme (lodd.) Blume on human breast
cancer t47d cells. International Journal of
Phytomedicine, 3, 138-146.
Piyapat T, Miyagawa K, Tamura H. (2013).
Induction of apoptosis in HeLa cells by methanol
extract of Litsea cubeba fruit residue from
essential oil extraction. J Life Sci. 7(9), 928-34.
Rosidah, Hasibuan PAZ, Haro G, Puteri M, Satria
D. (2018). Antioxidant activity of alkaloid
fractions of Zanthoxylum acanthopodium DC.
fruits with 1,1-diphenyl-2-picrylhydrazyl
assay. Asian J Pharm Clin Res, 11(1), 33-4.
Satria D, Furqan M, Hadisahputra S, Rosidah
(2015). Combinational effects of ethylacetate
extract of Picria fel-terrae Lour. and
doxorubicin on T47D breast cancer cells. Int J
Pharm Pharm Sci, 7, 73-6.
Satria D, Silalahi J, Haro G, Ilyas S, Hasibuan
PAZ. (2017). Antioxidant and antiproliferative
activities of an ethylacetate fraction of Picria
fel-terrae Lour. herbs. Asian Pac J Cancer
Prev, 18(2), 399-403.
Sharma J, Pitchaiah G, Satyawati D, Rao JV,
Kumar-Vikram HS (2011). In vitro anticancer
activity of methanolic extract of roots of
Glochidion zeylanicum Gaertn. Int J Res
Pharmaceut Biomed Sci, 2, 760-764.
Sun Y, Xun K, Wang Y, Chen X. (2009). A
systematic review of the anticancer properties of
berberine, a natural product from Chinese herbs.
Anti-Cancer Drugs, 20(9), 757–69.
Tang F, Wang D, Duan C. (2009). Berberine inhibits
metastasis of nasopharyngeal carcinoma 5-8F
cells by targeting rho kinase-mediated ezrin
phosphorylation at threonine 567. J Bio Chem,
284(40), 27456 – 66.
Trisonthi P, Akihiko S, Hisashi N, Hirotoshi T.
(2014). A new diterpene from Litsea cubeba
fruits: structure elucidation and capability to
induce apoptosis in HeLa cells. Molecules, 19,
6838-50.
Tyagi AK, Agarwal C, Chan DCF, Agarwal R
(2004). Oncology Reports, 11, 493-499.
Yadav VR, Sahdeo P, Bokyung S, Ramaswamy K,
Bharat BA (2010). Targetting inflammatory
pathways by triterpenoids for prevention and
treatment of cancer. Toxins, 2, 2428-66.
Zihlif M, Afifi F, Abu-Dahab R, Majid A, Somrain
H, Saleh M (2013). The antiangiogenic activities
of ethanolic crude extracts of four Salvia species.
Complementary & Alternative Medicine, 13,
358-65.
Cell Cycle Arrest Activity of Alkaloid Fraction of Litsea cubeba Lour. Heartwoods Towards HeLa Cancer Cell
5
