
Kinetic Studies of Purified Bromelain from Pineapple (Ananas
comosus [L.])Merr) Core with Hidroxyapatite and CM Sephadex C-50
Ion Exchange Chromatography
Nofa Rahayu Desi Putri
1
, Sumi Hudiyono
1
and Siswati Setiasih
1
1
Department of Chemistry, Faculty of Mathematics and Natural Sciences, Universitas Indonesia, Depok, Indonesia
Keywords: Pineapple core, bromelain, specific activity,Michaelis-Menten constant, maximum reaction velocity.
Abstract: Bromelain is a protease enzyme that functions to break peptide bonds. in the health field bromelain can
function as anti-inflammatory, anti-thrombotic and fibrinolytic. Here we purposed a new purification step to
collect enzyme from pineaplle. The purification of the enzyme from Ananas comosus was carried out by
precipitation with varying concentration of acetone and followed by column chromatography using
hidroxyapatite ion exchage and CM sephadex C-50 resin. The enzyme activities were evaluated using casein
as substrate. The highest specific activity of bromelain was gained from acetone fractination as 51,51 U/mg
in the range of 50-80% saturation with purity level of enzyme as 11 times from its crude extract. The
highest specific activity from last fraction which run on hidroxyapatite ion-exchange column
chromatography was 87,49 units/mg with purity level of enzyme resulted 19 times higher compared to the
crude extract. Later on, the purification with CM sephadex C-50 resulted in increasing the specific activity
to 200 U/mg with purity level of enzyme as 45 times higher from its crude extract. Hydrolysis of various
casein concentration with purified bromelain was carried out at optimum reaction condition of pH 7,0 and
37
0
C. The results obtained revealed the Km and Vmax value were 0,94% (w/v) and 0,023 U/min
respectively. From the various purification steps that have been done, it can be observed the increasing of
bromelain specific activity from each stage.
1. INTRODUCTION
Bromelain is a complex mixture of compounds that
differ from one another namely thiol endopeptidase
and other components that are not yet fully
characterized such as phosphatase, glucosidase,
peroxidase, cellulase, glycoproteins, carbohydrates,
some protease inhibitors, and calcium organically
bound, among others. Bromealin is non-toxic
compounds and a proteolytic enzyme that can
catalyze the hydrolysis reaction of protein.
Bromelain is a collective name for proteolytic
enzymes or proteases found in tissues including the
stem, fruit, and leaves of pineapple plants, the family
Bromeliaceae. (A.D Rowan 1990)
Bromelain is usually distinguished as bromelain
stem (EC. 3.4.22.32) or bromelain fruit (EC.
3.4.22.33) depending on the source. This proteolytic
enzyme has unique functions useful to the food,
pharmaceutical and cosmetic industries. As a drug,
bromelain has been used for the treatment of various
diseases, including thrombosis, rheumatoid arthritis,
inflammatory diseases such as atherosclerosis,
cancer treatments and others (Ketnawa et al., 2012) (
Jeung, A 1980)
In the process of isolation and purification of
enzymes, to obtain fractions with proteolytic activity
and high purity, it is necessary to know the
properties of the enzymes to be isolated. This is
needed so that in the process there is no
denaturation. Some techniques are often used such
as sedimentation, filtering solvent extraction, affinity
chromatography, ionic exchange and gel filtration.
Although this methods has a high concentration of
power but still has a low purity level. (M.A Desai,
2000)
Therefore, in this study protein isolation and
purification were carried out using various methods
to obtain increased purity and high activity of
bromelain from pineapple core.
Putri, N., Hudiyono, S. and Setiasih, S.
Kinetic Studies of Purified Bromelain from Pineapple (Ananas comosus [L.])Merr) Core with Hidroxyapatite and CM Sephadex C-50 Ion Exchange Chromatography.
DOI: 10.5220/0009843300002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

2. MATERIALS AND METHODS
2.1 Materials
The materials used for the process of isolation and
fractionation by precipitation include, Phosphate
buffers pH 7, prepared from NaH
2
PO4 (Merck) and
Na
2
HPO
4
(Merck), Acetone for precipitation. For
ion column exchange chromatography,
hydroxyapatite resin and CM sephadex C-50 are
required, Tris–HCl buffers, NaCl. Furthermore, for
the test of bromelain activity with Kunitz method
(Kunitz, 1947 ) and for the determination of protein
content by Bradford method (Bradford, 1976)
2.2 Methods
2.2.1 Preparation of Crude Extract
Core was cut into pieces and weighed as much as
500 gram, then blended and filtered using a filter
cloth. Furthermore, core solution that has been
obtained centrifuged at 6000 rpm for 15 minutes at
4°C. Supernatant was filtered and the filtrate taken
as a crude extract of bromelain
2.2.2 Bromelain Purification from Crude
Extract by Acetone Precipitation
Bromelain crude extract obtained proceed to the
purification process by precipitation method using
acetone. The concentration level was divided into
three fractions, fraction 1 (0-20)%, fraction 2 (20-
50)% and fraction 3 (50-80)%. Acetone was added
to the crude extract at 4°C. The extracts was kept for
overnight. Extract was centrifuged with a speed of
6000 rpm for 15 minutes at 4°C. The precipitate
obtained re-suspended using 0.2 M phosphate buffer
pH 7. The filtrate was resumed to higher
concentration levels.
2.2.3 Measurements of Protein Content and
Enzymatic Activity
Protein content was measured using Bradford
method and the standard used was bovine serum
albumin (Bradford, 1976). All measurements were
performed in duplicate. All measurements are done
in duplicate. While enzyme activity was measured as
follows: 1% casein (w / v) at 0.9 M Tris - Hcl buffer
(pH 8.0) was used as a substrate. Aliquots of 100 µL
of sample were added to a centrifuge tube containing
1.9 mL of casein buffer solution. The mixture was
held for 30 minutes in a water bath at 37 ° C.
Furthermore, 3 ml of trichloroacetic acid (TCA)
solution was added and after 30 minutes at 4 ° C.,
the mixture was centrifuged at 4000 rpm. for 10
minutes (Kunitz, 1947). The supernatant absorbance
was determined at 280 nm using a UV / visible
spectrophotometer (Shimadzu UV-2450)
2.2.4 Hydroxyapatite Chromatography
Following acetone precipitation, the sample was
applied to a hydroxyapatite column (50 cm × 3,3 cm
size), pre-equilibrated with using 0.05 M phosphate
pH 7buffer containing at a flow rate of 5 ml/4 min.
The enzyme was eluted by a linear gradient of 50–
400 mM phosphate buffer. The bound enzyme was
eluted by a linear gradient of 50 – 400 mM NaCl
in phosphate buffer . Each fraction was assayed for
protein and enzyme activity.
2.2.5 CM Sephadex C-50 Chromatography
Fractions with the highest specific activity will be
purified by CM sephadex C-50 ion chromatography.
The sample was applied to a cm sephadex c-50
column (50 cm × 3 cm size), pre-equilibrated with
using 0.05 M Tris - Hcl pH 8 buffer containing at a
flow rate of 5 ml/4 min. The enzyme was eluted by a
linear gradient of 50– 400 mM Tris-Hcl buffer. The
bound enzyme was eluted by a linear gradient of 50
– 400 mM NaCl in phosphate Tris - Hcl buffer .
Each fraction was assayed for protein and enzyme
activity.
2.2.6 Kinetic Studies
Determination of kinetic parameters such as
Michaelis-Menten constant (Km), maximum
reaction speed (Vmax) is determined by measuring
and distributing enzyme activity data at optimum pH
and temperature as a function of substrate
concentration, based on the Lineweaver-Burk
method (Alves et al., 2014) (Lehninger, 1982).
3. RESULT AND DISCUSSION
3.1 Acetone Precipitation
In this study, acetone (p.a) was used as the enzyme
precipitator. Acetone has been widely used for the
process of protein deposition and bromelain enzyme
(Sharma et al 2014); (Rowan et al., 1990), (Heinicke
& Gortner 1957) with high values of specific
enzyme activity. The addition of acetone to the
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
pineapple solution is then stirred until homogeneous
to precipitate the protein, including the bromelain
enzyme contained in the pineapple. The principle of
this protein precipitation process is to reduce water
activity in core pineapple solution (Scopes, 1994).
The dielectric constant of acetone (20.7) is smaller
when compared to the air dielectric constant (80.1).
It will be more of the protein contained in the air.
Acetone is less polar than air, the more confusing it
is from proteins as well because the polar air surface
of polarity, mixed with less polar acetone, will be
more interested in interacting with the same protein
molecules wiped with acetone. Acetone capable of
interacting with the hydrophobic part of the protein
also further increases the reduced solubility of the
mixture and increases the precipitation protein
The largest proteolytic activity, protein content, and
specific activity of enzyme were obtained from the
third fraction of bromelain enzyme (50-80%
saturation level of acetone) with a value of
proteolytic activity 7,717 Units and protein content
0.149 mg. Specific activity of 51.513 U / mg. The
purity level obtained is 11 times purer than the crude
enzyme
3.2 Hidroxyapatite and CM Sephadex
C-50 Ion Exchange
Chromatography
Ion exchange chromatography is an advanced
purification step for separating bromelain from other
proteins to obtain enzyme with higher specific
activity. Purification by ion exchange
chromatography uses the principle of charge
difference between proteins and charged groups
present in the column matrix. Proteins with the same
charge as the matrix will elute first from the column.
If the protein charge is different from the matrix,
then the protein will interact electrostatically on the
matrix and out of the column at different times
depending on the strength of the bond
After precipitation with acetone, the sample is
loaded onto a ion exchange chromatography
columns hidroxyapatite and collected fractions were
analyzed for proteins content and proteolytic
activity. This chromatography step resulted in a 15
fold purification. The pooled fractions was further
purified using CM sephadex C-50 chromatography.
In the second chromatographic step, the pooled
active fraction was loaded onto a CM Sephadex C-
50 ion-exchange column and the bound enzyme was
eluted with a linear gradient of 50-400mM NaCl.
The last step chromatography resulted in a 45 fold
purification (tabel 1)
The effluent (protein and eluent molecules
coming out of the column) is accommodated on
tubes that hold 5 mL of effluent on each tube. And
all fractions of protein uptake at 280 nm wavelength.
This absorbance measure aims to detect and separate
spreading proteins in each part of the column
chromatographic results (Burgess, 2008). All
fractions also measure their proteolytic activity by
Kunitz method.
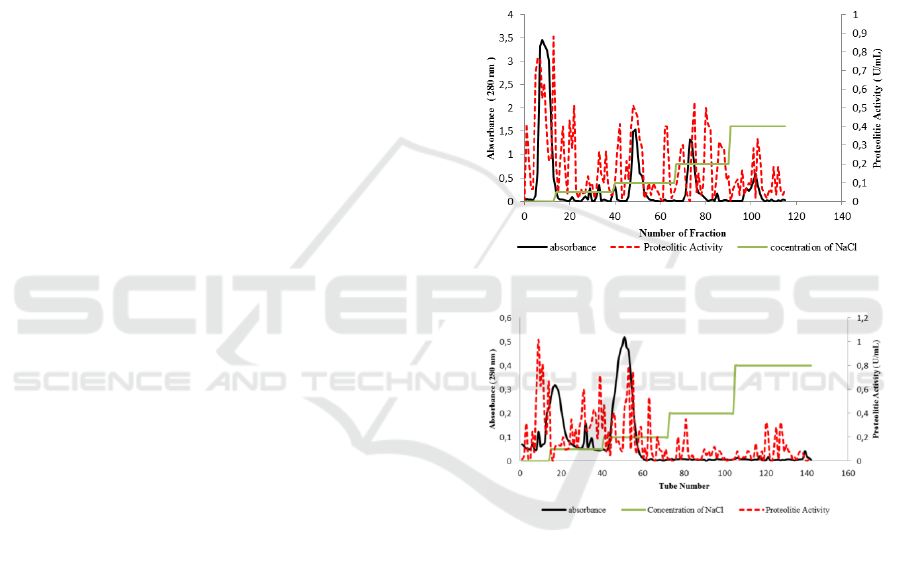
The following Figure shows that Elution profiles
of chromatography steps related to the purification
of bromelain from pineapple core.
Figure 1: Hidroxyapatite chromatography
Figure 2: CM Sephadex C-50 chromatography
At the elution stage, the bond between the
protein and the resin will be replaced by the ions
from the salt eluent, the salt ion ions binding to the
resin and the protein molecules will be released out
of the column. The protein molecule that binds to
the matrix will release more mixture through the
lower salt, and the higher the salt concentration, the
stronger the binding protein can also get out of the
column. Separation occurs in protein molecules
based on different times, depending on the strength
of the molecular charge attached to the resin.
Kinetic Studies of Purified Bromelain from Pineapple (Ananas comosus [L.])Merr) Core with Hidroxyapatite and CM Sephadex C-50 Ion
Exchange Chromatography
3
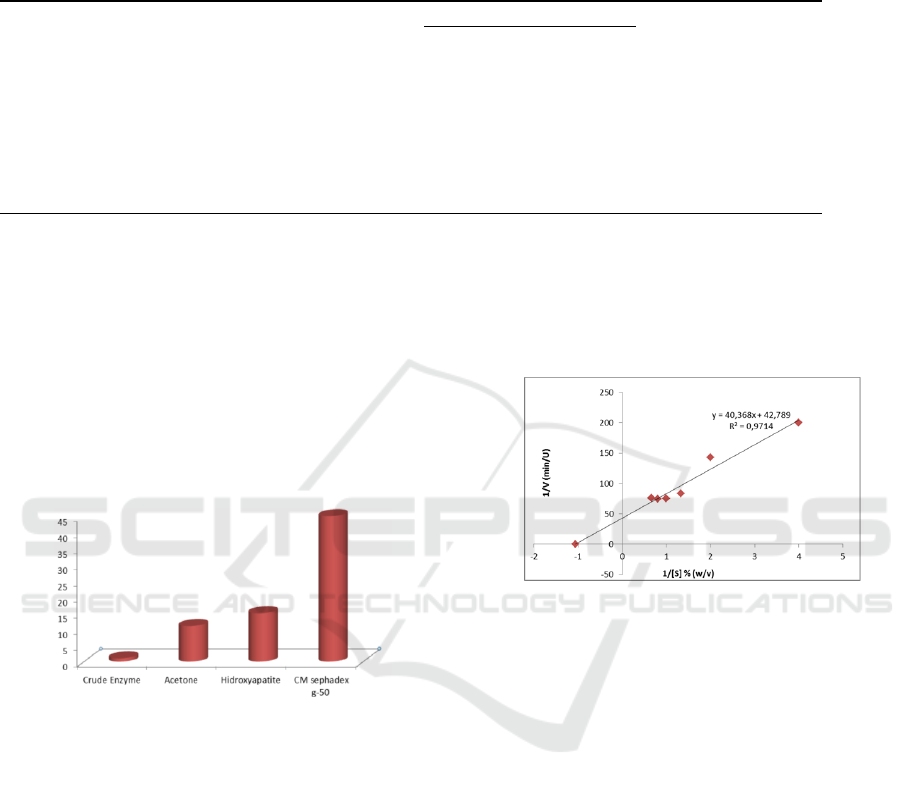
Table 1 Summary of purification and yields of bromelain
Step
Volume
Proteolitc
Activity
(U/mL)
Protein
Content
(mg/mL)
Total
Specific
Activity
(U/mg )
Fold
Proteolitc
Activity
(U)
Protein
Content
(mg)
Core solution
350
0,507
0,171
177,45
59,85
2,968
-
Crude Enzyme
200
0,733
0,163
146,60
32,60
4,490
1
Acetone
850
7,717
0,149
6559,45
126,65
51,513
11
Hidroxyapatite
85
2,116
0,031
179,86
2,635
68,258
15
CM Sephadex
C-50
45
0,400
0,002
18,00
0,09
200,00
45
Enzyme solution of each fraction were
proteinolytic activity and protein levels. Table 1
shows the value of proteolytic activity of each
fraction obtained in the purification stage. The
highest proteolytic activity was obtained in fraction
CM Sephadex C-50 with a value of 200 Units and
protein levels of 0,09 mg (figure 3). From the data of
proteolytic activity and protein levels, the highest
specific activity was obtained by a purity level
reached 45 times.
Figure 3 : an increase in purity levels of bromelain after
going through various stages of purification
3.3 Kinetic Studies
The Michaelis-Menten (Km) constant, maximum
reaction velocity (Vmax) is determined by passing
data of activity enzyme at pH and temperature
optimum as a function of substrate concentration,
based on the Lineweaver-Burk method plot as
shown in Figure 4
The Michaelis-Menten (Km) constant is the
amount of substrate required to achieve half of the
maximum rate of reaction. This constant can also be
used to show the enzyme specificity to a substrate
(Corzo et al., 2012). While Vmax is the maximum
rate of enzymatic reactions in the enzyme state that
has been saturated by the substrate. In this study, the
Michaelis-Menten (Km) constant for fraction AP1
purification with CM sephadex c-50 was 0.943% (w
/v) casein with a maximum velocity value (Vmax)
0.0234 U / min.
Figure 4 : Lineweaver-Burk Plot for Casein Hydrolysis by
Bromelain Fraction AP1 CM sephadex
4 CONCLUSIONS
Bromelain was successfully purified through a series
of purification steps, starting from extraction of
crude extract and precipitation of bromelain with
acetone and purification with chromatography
column ion exchange. The result of bromelain after
precipitation by acetone and purification with
hidroxyapatite and CM Sephadex C-50 gave higher
specific activity than before crude enzyme
ACKNOWLEDGEMENTS
This work was funded by Hibah Kompetensi
Publikasi Internasional Terindeks Untuk Tugas
Akhir Mahasiswa (PITTA), Universitas Indonesia
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

REFERENCES
A.D.,Rowan Buttlet, D.J. & Barrett, A.J., 1990. The
cysteine proteinases of the pineapple plant. Biochem.
J, 266, pp.869–875.
Alves, T., Rocha, I., Maria, G., Aoyama, H., Garrard, I., &
Tambourgi, E. B. 2014. Extraction and Preliminary
Characterization of Bromelain from Curaua (Ananas
erectifolius L. B. SMITH) Purple and White.
Chemical Engineering Transactions, 37, 769–774.
https://doi.org/10.3303/CET1437129
Bradford, M. B. 1976. A rapid and sensitive method for
the quantitation of micrograms quantities of protein
utilizing the principle of protein-dye binding.
Analytical Biochemistry, 72, 248–254.
Heinicke, R.M. & Gortner, W.A., 1957. Stem
bromelain-A new protease preparation from
pineapple plants. Economic Botany, 11(3),
pp.225–234.
Jeung A. Encyclopedeia of Common Natural Ingredients
Used in Foods, Drugs, and Cosmetics. New York, NY:
John Wiley & Sons;1980:74-76
Ketnawa, S., Chaiwut, P. & Rawdkuen, S., 2012.
Pineapple wastes: A potential source for
bromelain extraction. Food and Bioproducts
Processing, 90(3), pp.385–391. Available at:
http://dx.doi.org/10.1016/j.fbp.2011.12.006.
Kunitz, M. 1947. Crystalline soybean trypsin inhibitor II.
General properties. The Journal of General
Physiology, 30, 291–310.
Lehninger, A. L. 1982. Bases of Biochemical
.Jakarta: Erlangga.
M.A. Desai. 2000. Downstream Processing of Proteins:
Methods and Protocols (first ed.), Humana Press, New
Jersey
Scopes, R. K. 1994. Protein Purification: Principles and
Practice. 3rd ed. New York: Springer Advanced Text
in Chemistry.
Sharma, N. 2013. Kinetic study of free and immobilized
protease from Aspergillus sp. IOSR Journal of
Pharmacy and Biological Sciences, 7(2), 86–96.
https://doi.org/10.9790/3008-0728696
Kinetic Studies of Purified Bromelain from Pineapple (Ananas comosus [L.])Merr) Core with Hidroxyapatite and CM Sephadex C-50 Ion
Exchange Chromatography
5
