
The Study of the Antibacterial Activity of Asam Gelugor (Garcinia
Atroviridis) Against Methecillin-resistant Staphylococcus Aureus
(MRSA), Streptococcus Pneumoniae and Klebseilla Pneumoniae
May Florence Dela Cruz Bacayo
1
*, Nurul Shahira Sajali
1
, Wong Charng Choon
1
, Santosh Fattepur
1
,
Kiran Chanabasappa Nilugal
1
, Jiyauddin Khan
1
, Fadli Asmani
1
, Eddy Yusuf
1
1
School of Pharmacy, Management and Science University, 40100 Shah Alam Malaysia
Keywords: Antibacterial Activity, Garcinia Atroviridis Extracts, Zone of Inhibition.
Abstract: The curative ability of the endemic plants for different disorders has been described by traditional medicine
practitioners. Some herbs have been reported to have antibacterial activity such as Garcinia Atroviridis. The
antibacterial activity of Garcinia Atroviridis (Asam Gelugor) extracts was evaluated against Methicillin-resistant
Staphylococus aureus, Streptococcus pneumoniae and Klebseilla pneumoniae. Asam Gelugor fruits (600 grams)
were collected. The powder form were successively extracted with with 99.8 % of methanol. The extract was
filtered and dried using the rotatory evaporator at a temperature not exceeding 50ºC. The antibacterial activity
was determined by disc diffusion method for the zone of inhibition, minimum inhibitory concentration (MIC)
and minimum bactericidal concentration (MBC). The zone of inhibition were compared with that of ampicillin,
vancomycin, and gentamicin antibiotic disc. The test by using disc diffusion method shows highest inhibition
against the Klebsiella pneumoniae (15.33 ± 1.53) in 100% extract. Based on the result for MIC, inhibition is at
50mg/ml until 0.05 mg/ml against MRSA, while the MIC is positive in all concentration of extract against
Klebsiella pneumoniae but the MIC result is negative in all concentration of the extract against Streptococcus
pneumoniae. MBC result showed that there is bacterial growth in 500mg/ml of extract against MRSA while no
bacterial growth in extract against Klebsiella pneumonia, bacterial growth is positive in the extract against the
Streptococcus pneumoniae. The results shows that Asam Gelugor (Garcinia Atroviridis) may serve to the
development of a new antibacterial agent against these type of bacteria.
1 INTRODUCTION
Disease can be cure not only by a processed medicine
or medicine that can we can get at the market but
home remedies can also be used to treat the disease
furthermore, it is cheaper than the marketed drug.
Example of home remedies are ginger, turmeric,
garlic, black pepper, tamarind that can treat illness
such as infection or to reduce body temperature. Back
to ancient era, they use endemic plants to treat several
illness. Thus, plants play a major role in the treatment
of diseases. A very common example was morphine
from the opium poppy and most of the sources of the
drugs were from plant, animal and microorganism.
(Evans, W.C. Trease and Evans Pharmacognosy.,
2009)
Curative ability of many endemic plants has been
described by the practitioners of traditional medicine
for centuries. The increasing antibacterial activity of
various medicinal plants were reported from the
different parts of the world. The World Health
Organization estimates that plant extracts or their
active constituents are used as folk medicine in
traditional therapies of 80% of the world’s population.
(World Health Organization, WHO).
Herbs are widely exploited in the traditional
medicine and their curative potentials are well
documented. About 61 % of new drugs that were
developed between 1981 and 2002 were of natural
Bacayo, M., Sajali, N., Choon, W., Fattepur, S., Nilugal, K., Khan, J., Asmani, F. and Yusuf, E.
The Study of the Antibacterial Activity of Asam Gelugor (Garcinia atroviridis) Against Methecillin-resistant Staphylococcus Aureus (MRSA), Streptococcus Pneumoniae and Klebseilla
Pneumoniae.
DOI: 10.5220/0009843000002406
In Proceedings of BROMO Conference (BROMO 2018) - Symposium on Natural Product and Biodiversity, page 1
ISBN: 978-989-758-347-6
Copyright
c
2022 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
1

products focusing on infectious diseases and cancer
therapy. Unfortunately, the discovery rate of active
novel chemical entities is declining. Thus, natural
products from plants may give a new source of
antibacterial activity with possibly novel mechanism
of action. As the effects of plant extracts have been
studied by many researchers. (Nayan et al, 2011)
Antibacterial agents are the well-known weapons
to fight bacterial infections and helps to improve the
quality of human life since its introduction. But, due to
the emergence of drug-resistant bacteria, these
antibacterial agents have become less effective. It is
very essential to investigate newer drugs derived from
natural sources for the prevention and treatment of
bactrial infections as well as to combat antibiotic
resistance.
A large rainforest of Peninsular Malaysia is the
haven of Garcinia Artroviridis, known as Asam
Gelugur, Asam Gelugo or Asam Keping in Malay.
This species grows throughout in the rainforest and
widely cultivated especially in the northern states.
Asam Gelugor is commonly used for its weight
reduction properties (Ensiklopedia Pengubatan Herba,
2013). The tree grows for about 20 meters high and
has extended trunk, greyish smooth bark and hanging
branches. The floret are darkish red with a yellowish
to orange fruits borne singly on the ends of the twigs.
The plant contains fruit acids such as citric acid,
tartaric acid and ascorbic acid, that have antioxidant
properties. Phytochemical investigations have isolated
garcinia acid and its γ-lactone, atroviridin,
atrovirisidone, atrovirinone, and some organic acids.
(Amran et al, 2009). Hence, this study aims to
investigate the antibacterial activity of Garcinia
Atroviridis (Asam Gelugor) against MRSA,
Streptococcus pneumoniae and Klebsiella
pneumoniae.
2 METHODOLOGY
2.1 Extraction Process
Asam Gelugor fruits were collected and cut into small
pieces (600g). The oven dried fruits were grind until
its powder form which were extracted with 99.8%
methanol using a Soxhlet extractor. The solution was
filtered and dried using the rotatory evaporator at a
temperature not exceeding 50ºC.
2.2 Disc Diffusion Method for
Determination of Zone of Inhibition
The petriplates were inoculated with sterile swab
dipped into the inoculums. The excess inoculum from
the swab was removed by gently pressing and rotating
the swab firmly against the side of the tube in order to
remove excess fluid in the swab.
The sterile swab was streaked all over the surface
of the petriplate three times for a lawn of growth,
rotating the petriplate through an angle of 60° after
each application. The sterile swab was passed round
the edge of the agar surface. After the streaking, allow
the petriplates to dry in a room temperatures for a 5
minutes, with the petriplates lid closed. A narrow hole
was the bore in the petriplates and the extract were
added in the hole. The petriplates were placed in an
incubator at 37°C within 30 minutes
After 24 hours of incubation, use a metric ruler to
measure the diameter of the zone (including the
diameter disc) without opening the lid and record the
diameter in mm.
2.3 Minimum Inhibitory Concentration
(MIC)
Agar well diffusion method and the micro-broth
dilution technique were employed to determine the
minimum inhibitory concentration (MIC) for each
extract and test organism. A reconstituted extract of
500 mg/mL concentration was serially diluted in two-
fold up to 0.05 mg/mL. A 100 μL volume of each
dilution was introduced into duplicate wells in the
Mueller Hinton Agar plates that is pre‑inoculated with
test bacterial strain; and incubated at 37 °C for 24 h.
The minimum inhibitory concentration was taken and
recorded as the lowest concentration of the extract
showing measurable inhibition zone. For the
micro‑broth dilution technique, a 100 μL volume of
each dilution of the extract was introduced into
duplicate tubes of 2.0 mL Mueller Hinton broth
(MHB) seeded with 100 μL of the standardized
suspension of the test bacterial strain. Incubation was
at 37 °C for 24 hours and MIC was taken as the lowest
concentration of the extract that made the culture show
no visible growth.
2.4 Minimum Bactericidal
Concentration (MBC)
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
2
A modified agar well diffusion technique was
employed in the determination of the minimum
bactericidal concentration (MBC). A 2 mm diameter
agar disc cut out from the inhibition zone of the last
three consecutive wells in each dilution showing
inhibition was inoculated into a fresh sterile nutrient
broth medium. The broth cultures were incubated at 37
°C for 24 hours after which 100 μL was spread over a
fresh sterile MHA. The MHA culture was in turn
incubated at 37 °C for 24 hours and the least
concentration of the extract showing no growth was
taken as the MBC. An MBC which coincided with or
was next to the MIC value was considered bactericidal
while those that differed markedly were considered
bacteriostatic.
3 RESULT AND DISCUSSIONS
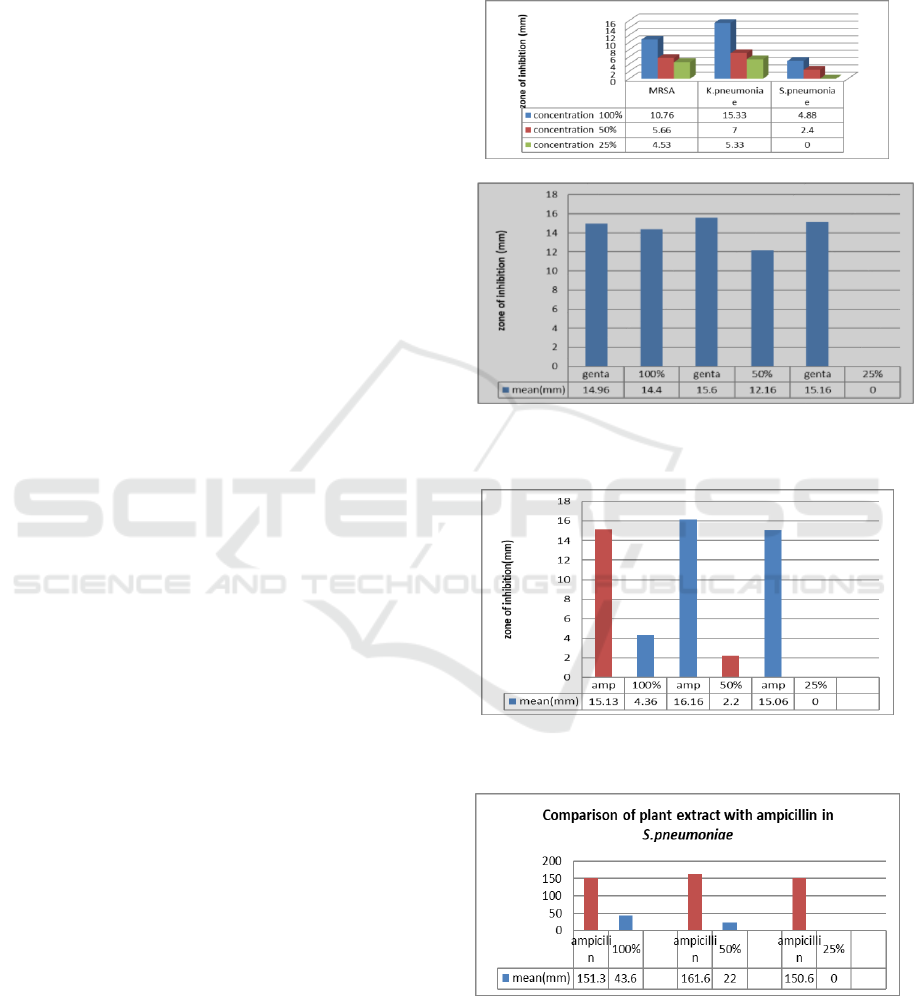
The antibacterial activity of Garcinia Atroviridis has
been measured by the different test employed on it.
The disc diffusion method was used to measure the
zone of inhibition of the extract shows highest
inhibition against Klebsiella pneumoniae (15.33 ±
1.53) in 100% concentration of the extract followed by
inhibition against MRSA (10.76 ± 4.13) then
Streptococcus pneumoniae (4.88 ± 0.44). The negative
result is shown the inhibition of 25% concentration of
the extract against Streptococcus pneumoniae.
The statistical tool one-way ANOVA showed that
the antibacterial activity of the fruit extracts is
significant when compared with positive control with
the P value (p< 0.05) against MRSA, S. pneumoniae
and K. pneumoniae.
MIC results showed at 50mg/ml until 0.05 mg/ml
against MRSA, while the MIC is positive in all
concentration for inhibition against Klebsiella
pneumoniae but the MIC result is negative in all
concentration against Streptococcus pneumoniae.
The MBC result showed that there is bacterial
growth in 500mg/ml of extract against MRSA while
no bacterial growth in the lowest concentration of
extract against Klebsiella pneumoniae. The bacterial
growth is positive in the lowest concentration of
extract against the Streptococcus pneumoniae.
The antibacterial activity of Asam Gelugor fruit
extract which was shown in this study is attributed to
the presence of the different active constituents of the
plant. (Zakaria, 2011)
Table 1: Zone of inhibition vs bacterial species with different
concentration of G. Atroviridis plant extract
Figure 1: Comparison of zone of inhibition of plant extract
vs gentamicin in Klebsiella pneumoniae
Figure 2: Comparison of zone of inhibition of plant extract
vs ampicillin in Streptococcus pneumoniae
Figure 3: Comparison of zone of inhibition of plant extract
vs ampicillin in Streptococcus pneumoniae
The Study of the Antibacterial Activity of Asam Gelugor (Garcinia atroviridis) Against Methecillin-resistant Staphylococcus Aureus
(MRSA), Streptococcus Pneumoniae and Klebseilla Pneumoniae
3

Table 2: Summary of G. Atrovidis plant extract zone of
inhibition against the 3 strains of bacteria
BACTERIA
MRSA
K.PNEUMONIAE
S.PNEUMONIAE
SIG
CONCENTRATION
MEAN (mm) ± SD
100%
10.76
± 4.1
15.33 ± 1.53
4.8 ± 0.44
0.05
50%
5.66 ±
1.57
7.00 ± 1.00
2.43 ± 0.40
0
25%
4.53 ±
0.50
5.33 ± 0.58
0
0
4 CONCLUSION
The in-vitro antibacterial study of Garcinia Atrovidis
against the selected gram-negative and gram-positive
organisms conclude that Garcinia Atrovidis extract
strongly inhibit the growth of Klebsiella pneumoniae
as well as the standard antibiotic (Gentamicin). The
plant extract has the potential to treat infection caused
by Klebsiella pneumoniae.
ACKNOWLEDGMENT
The author is very grateful and thankful to the research
committee and lecturers of Management and Science
University, Malaysia for providing all the needed
equipment and materials as well as the continuous
guidance and support until the completion of this
research project.
REFERENCES
Amran A A, Zaiton Z, Faizah O, Morat P, (2009), Effects of
Garcinia atroviridis on serum profiles and atherosclerotic
lesions in the aorta of guinea pigs fed a high cholesterol
diet, 2009; 50 (3) : 295
Awatif Al-Judaibi, Ashwag Al-Zahrani, Khadijah A.
Altammar, Salmah Binti Ismail and Nadia T.
Darweesh,(2014), Comparative Study Of Antibacterial
Activity Of Plant Extracts From Several Regions Of
Asia, 139-147, 2014
Sawsan T. Abu Zied and Somaia A. L. Eissa ,(2000)
Comparative Study On Antibacterial Activities Of Two
Natural Plants Versus Three different intracanal
Medications.
Gislene G. F. Nascimento; Juliana Locatelli, Paulo C.
Freitas,Giuliana L. Silva,(2000), Antibacterial Activity
Of Plant Extracts And Phytochemicals On Antibiotic
Resistant Bacteria, 1678-4405
Nayan R. Bhalodia and V. J. Shukla,(201), Antibacterial and
antifungal activities from leaf extracts of Cassia fistula l.:
An ethnomedicinal plant, 104–109.
Fuursted, Kurt(1997), Comparative Study of Bactericidal
Activities, Postantibiotic Effects, and Effects on
Bacterial Virulence of Penicillin G and Six Macrolides
against Streptococcus pneumoniae, p. 781–784, 0066-
4804/97
Liñares, J., Ardanuy, C., Pallares, R. and Fenoll, A. (2010),
Changes in antimicrobial resistance, serotypes and
genotypes in Streptococcus pneumoniae over a 30year
period. Clinical Microbiology and Infection, 16: 402–
410.doi 10.1111/j.14690691.2010.03182.x
Z. A. Zakaria,(2011), Antimicrobial activity of the aqueous
extract of selected Malaysian herbs, pp. 5379-5383,
DOI: 10.5897/AJMR11.874.
Todar’s online textbook of bacteriology.
William Charles Evans, Daphne Evans, George Edward
Trease (2009), Trease and Evans Pharmacognosy,
Saunders/Elsevier Ltd.
Sana Mukhtar and Ifra Ghori,( 2012), Antibacterial Activity
Of Aqueous And Ethanolic Extracts Of Garlic,Cinnamon
And Turmeric Against Escherichia Coli ATCC 25922
And Bacillus Subtilis DSM 3256, 0976-4550
Gislene G. F. Nascimento,Juliana Locatelli,Paulo C. Freitas,
Giuliana L. Silva, (2000).
Kumar A , Tantry BA, Rahiman S, Gupta U,(2011),
Comparative study of antimicrobial activity and
phytochemical analysis of methanolic and aqueous
extracts of the fruit of Emblica officinalis against
pathogenic bacteria, Sep;31(3):24650.
Marcos J. Salvador; Paulo S. Pereira; Suzelei C. França;
Regina C. Candido3\; Izabel Y. Ito; Diones A.
Dias,(2003), Comparative study of antibacterial and
antifugal activity of callus culture and adult plants
extracts from Alternanthera Maritima (Amaranthaceae)
34:131-136.
Satyanshu Kumar, Shelly Sharma, Sunil Kumar
Chattopadhyay,(2013), The potential health benefit of
polyisoprenylated benzophenones from Garcinia and
related genera: Ethnobotanical and therapeutic
importance, Volume 89, September 2013, Pages 86–125
Ibrahim Jantan,(2004), Medicinal Plant Research in
Malaysia: Scientific Interests and Advances, Jurnal Sains
Kesihatan Malaysia 2(2) 2004: 27-46.
HABIBAH ZAINAL ABIDIN, June 2005, The Evaluation,
Production, And Derivatisation Of Garcinia Acid In
Garcinia Atroviridis.
Permana, Dharma; Lajisa, Nordin Hj.; Shaaria, Khozirah;
Alib, Abdul M.; Mackeenb,
Mukram M.; Kitajimac, Mariko; Takayamac, Hiromitsu and
Aimic, Norio.: A new prenylated hydroquinone from the
BROMO 2018 - Bromo Conference, Symposium on Natural Products and Biodiversity
4

roots of Garcinia atroviridis Griff ex T. Anders
(Guttiferae). Z. Naturforsch. 58bb, 332D335 (2003).
Tisdale, Eric J.; Kochman, David A. and Theodorakis,
Emmanuel A.: Total synthesis of atroviridin.
Tetrahedron Letters 44 (2003) 3281–3284 Pergamon.
Achmadi, S.S. Hayati (Indonesia). (2001), Institut Pertanian
Bogor (Indonesia). Fakultas Matematika dan Ilmu
Pengetahuan Alam. The potency of potassium
hydroxycitrate derived from gelugur fruit (Garcinia
atroviridis) in reducing body weight and cholesterol
levels in rats, v. 8(1) p. 23-26
Jena, B. S.; Jayaprakasha, G. K.; Singh, R. P.; Sakariah, K.
K.: Chemistry and biochemistry of (-)-hydroxycitric acid
from Garcinia. Journal of Agricultural and Food
Chemistry 2002, 50, 1, p.10-22.
Shahab Qureshi, MD; Chief Editor: Michael Stuart
Bronze,(2015), Klebsiella Infections Treatment &
Management
http://emedicine.medscape.com/article/219907treatment
Shahab Qureshi, MD; Chief Editor: Michael Stuart
Bronze,(2015), Klebsiella Infections,
http://emedicine.medscape.com/article/219907overview.
R. Podschun and U. Ullmann,(1998), Klebsiella spp. as
Nosocomial Pathogens: Epidemiology, Taxonomy,
Typing Methods, and Pathogenicity Factors, Clin
Microbiol Rev. 1998 Oct; 11(4): 589–603.
Mohammed Rahmatullah, Md. Nur Kabidul Azam, Md.
Mizanur Rahman, Syeda Seraj, Mostafi Jumrut Mahal,
Sadia Moin Mou, Dilruba Nasrin, Zubaida Khatun,
Farhana Islam, Majeedul H. Chowdhury,( 2011) A
Survey of Medicinal Plants Used by Garo and Non-Garo
Traditional Medicinal Practitioners in Two Villages of
Tangail District, Bangladesh, American-Eurasian Journal
of Sustainable Agriculture, 5(3): 350-357, 2011 ISSN
1995-0748.
Jan van de Kassteelea, Marga G. van Santen-Verheuvelb,
Femke D. H. Koedijkc, Alje P. van Damd,e, Marianne
A. B. van der Sandec,f and Albert J. de Neeling, (2011),
New Statistical Technique for Analyzing MIC Based
Susceptibility Data, Antimicrob. Agents Chemother.
March 2012 vol. 56 no. 3 1557-1563.
Centers for Disease Control,The American Academy of
Family Physicians. apriotti, T. Dermatology Nursing,
Jan. 26, 2004.
Johnson, L. Infections in Medicine, 2005.
WebMD Feature: "Drug-Resistant Staph Spreads Across
U.S.
Nahida Tabassum and Mariya Hamdani, Plants used to treat
skin diseases, Pharmacogn Rev. 2014 JanJun; 8(15): 52–
60. doi: 10.4103/09737847.125531.
Dr. Khalid Gadd, (2013), Ensiklopedia Pengubatan Herba,
Terapi Alternatif Kedoktoran Islam, Al Hidayah House
of Publishers Sdn.Bhd.
The Study of the Antibacterial Activity of Asam Gelugor (Garcinia atroviridis) Against Methecillin-resistant Staphylococcus Aureus
(MRSA), Streptococcus Pneumoniae and Klebseilla Pneumoniae
5
