
Effect of Quercetin on Growth and Mortality of High Fat-fed
Drosophila Melanogaster
Nur Nazihah Binti Jainurin
1
, Melanie Lim Boon Jin
1
, Ameilia Zuliyanti Siregar
2
,
M. Mobin Siddique
1*
1
Environmental & Life Sciences, Faculty of Science, University of Brunei Darussalam.
2
Faculty of Agriculture, Universitas Sumatra Utara, Medan, 20155, Sumatera Utara, Indonesia.
Email:
*
Ameilia@usu.ac.id
Keywords: Effect, quercetin, growth and mortality, high fat-fed, Drosophila melanogaster
Abstract: Association of obesity with high fat diet is no longer debatable. Obesity causes hyperlipidemia and
upregulates the generation of several metabolic by-products such as reactive oxygen species (ROS). Excess
generation of ROS is toxic to the cell that often induces DNA damage and eventually induces cell death.
Accumulation of lipid along with high level of ROS increase mortality and impair organisms’ growth. Herein,
we have used Drosophila melanogaster as a model organism to assess the accumulation of lipid by commonly
used dietary fat (commonly known as ghee) in Asia and its effect on the growth and mortality. Synthetic
polyphenol, quercetin was used along with the high fat diet to address if this particular polyphenol can be
used as a protective health supplement to minimize lipid-related toxicity. Our study suggests that high fat diet
impairs the growth of D. melanogaster as detected by measuring total protein, whereas this is not affected by
quercetin. It also appears that quercetin alone can induce high level of lipid accumulation and this is further
enhanced in presence of dietary high fat (ghee) in these experimental flies.
1 INTRODUCTION
Drosophila melanogaster, commonly known as fruit
fly, is one of the widely used experimental model in
life sciences. For decades, this dipteran insect is being
used to investigate the genetic basis of inheritance
and hereditary disorders due to the simplicity in the
genome structure (Sobels and Vogel, 1976, Adams et
al., 2000, Palu et al., 2017). In recent years, D.
melanogaster has shown to be a useful model
organism in studying human disorders such as
Diabetes, Alzheimer’s, Parkinson and most recently,
neuro-associated disease (Zhu et al., 2014, Lau et al.,
2015, Vanhauwaert and Verstreken, 2015, Zhao et al.,
2015, Prussing et al., 2013, Lenz et al., 2013). Most
biological architectures and mechanisms found in the
fruit fly which affects the development and lifespan
of the organism, are nearly similar to those in human.
The fly offers many advantages as an investigative
biological tool due to its rapid rate of proliferation and
relatively inexpensive and easy to culture in
laboratories.
Several research findings suggest that D.
melanogaster can be used as a model for obesity or
obesity related disorders (Ruden et al., 2005,
Rovenko et al., 2015, Pospisilik et al., 2010,
Padmanabha and Baker, 2014). The rising incidence
of obesity is believed to be due to consumption of
high fat and high carbohydrate containing diets.
Though several other factors are responsible for this
condition, health researchers mainly pointing to the
increased uptake of fat and carbohydrates. Obesity
often leads to the more complicated diseases which
are the consequences of metabolic imbalance and
increased oxidative stress. Hence, polyphenolic
compounds are extensively used in health
supplements that mainly act as anti-oxidants and
prevent the oxidative stress-induce damages in the
body.
In this experiment, we have used D. melanogaster
to assess the effect of commonly used dietary animal
fat (ghee) along with a synthetic polyphenol
(quercetin) on their growth, ability to metabolize
lipid, and mortality.
Binti Jainurin, N., Boon Jin, M., Siregar, A. and Siddique, M.
Effect of Quercetin on Growth and Mortality of High Fat-fed Drosophila Melanogaster.
DOI: 10.5220/0010043904150419
In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), pages 415-419
ISBN: 978-989-758-496-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
415

2 MATERIAL AND METHODS
2.1 Establishing Drosophila
melanogaster culture
Wild-type Drosophila melanogaster were captured
from the local fruit market in Brunei. The flies were
cultured in a clean, cylindrical, plastic container at
room temperature. The entire experiment was
conducted at the University of Brunei Darussalam.
2.2 Preparation of Drosophila’s
Standard Diet
We have used our formulated diet that we have
optimized at the beginning of the experiment. The
diet contained 100g of banana (Hotil Banana), 25g of
rice flour, 4g of agar and 1g of bakery yeast (Mauri-
pan) as a source of protein. After blending these
ingredients in 100ml of water, the mixture was boiled
briefly and poured into the feeding containers. In
order to observe the effect of different diets to the
growth and mortality of D. melanogaster, 7 % animal
fat (v/ v, Ghee, Q. B. B) and 0.1% quercetin were
added.
2.3 Experimental Flies Separation and
Larval Collection for Protein and
Lipid Assays
Parental flies (F-generation) were separated into a
new container once the F
1
generation pupae had
emerged in the experimental box. New food was
introduced to the new container upon separation of
flies. The number of dead F flies in the container was
monitored regularly for 14 days to check the mortality
rate the parental flies. During this observation, all the
flies were fed with fresh food every 3 days to avoid
the mixing of F1 flies with the parental flies. F1 flies
were collected in batches of same age and used for
protein extraction. The third-instar larvae (final stage
before pupal stage) were obtained from the containers
containing the F1 adult generation. 20 larvae from
each experimental unit were isolated and used for
biochemical analysis.
2.4 Protein Extraction and
Quantification
Protein Extraction from Adult Flies: The preserved
flies in the Eppendorf tubes were homogenised in
300µl of RIPA buffer mixed with protease inhibitor
by using sterile plastic pestles while keeping the
homogenate on ice. The homogenized samples were
incubated on ice for 20 minutes for complete lysis of
the cell membrane. Precipitation of grinded sample
was minimised by vortex. The homogenate was
centrifuged at 12, 000 rpm for 15 min at 4⁰C and the
supernatants containing cytosolic protein were
transferred in a new tube. 250 µl of supernatant
containing cytoplasmic protein was transferred into a
new tube and stored in -80⁰C.
Protein Extraction from Larvae: Slightly different
technique was implemented to extract protein from
larval samples. Prior to the extraction, the larvae were
washed in PBS at least twice and incubated for 5min.
This step allowed the removal of any food residues
that might present on the external surface during
larval collection. The cells in larval samples were
lysed by the same method in carried out in the adult
samples. However, the lysed samples were
centrifuged at the speed of 2, 000 rpm for 5 min in
4⁰C.
Protein Quantification of Adult Flies and Larval
Samples: Total protein content was quantified by
Bradford Reagent (BioRad) according to the
manufacturer’s protocol. Bovine Serum Albumin
(BSA) was used as standards and the absorbance of
the samples and standards was recorded using a
microplate reader at OD595nm. Standard curve
derived from the BSA standard was used to quantify
the protein samples.
Lipid Quantification: 20 µg of protein sample was
loaded into a clean microfuge tube (Eppendorf tube)
and was spun-down briefly to bring all the contents to
the bottom of the tube. The sample was stained with
100 µl of Oil Red-O (ORO) dye (Sigma) that
selectively binds to neutral lipids. The tube was
inverted twice to ensure proper mixing of the dye with
the protein samples and then centrifuged at maximum
speed (12, 000rpm) for 10 min at room temperature
(25⁰C). The supernatant was discarded entirely and
the pelleted samples were washed with distilled water
to remove any residual unbound ORO dyes. Second
centrifugation of sample was applied at maximum
speed for another 10 min at room temperature. The
supernatant was discarded and 120 µl of isopropanol
was dispensed into the reaction. This allowed the
bounded ORO to be released from neutral lipids. The
sample was vortexed briefly followed by incubation
at room temperature for 10 min. Sample was
centrifuged again at maximum speed for 10 min. The
supernatant containing the ORO dye was eluted out
and transferred to a 96-microplate for quantitative
analysis. In microplate, 50 µl of the supernatant from
each sample was transferred to the well in duplicates
while 50 µl of isopropanol was used as blank. Oil-red-
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
416
O absorbance was recorded at OD515nm using a
microplate reader. Lipid content was presented upon
normalisation with total protein content measured
from Bradford assay.
3 RESULT
3.1 Effects of Different Diets on the
Growth of D. melanogaster
In order to assess the growth of the experimental
organisms in different growth media, we have
cultured Drosophila melanogaster in high fat diet
(7% ghee or 7% olive oil) with or without quercetin
(0.1%) while normal diet-fed D. melanogaster were
used as a control. The effects of quercetin was also
investigated in high carbohydrate containing diet
using 0.75M sucrose (Fig.1).
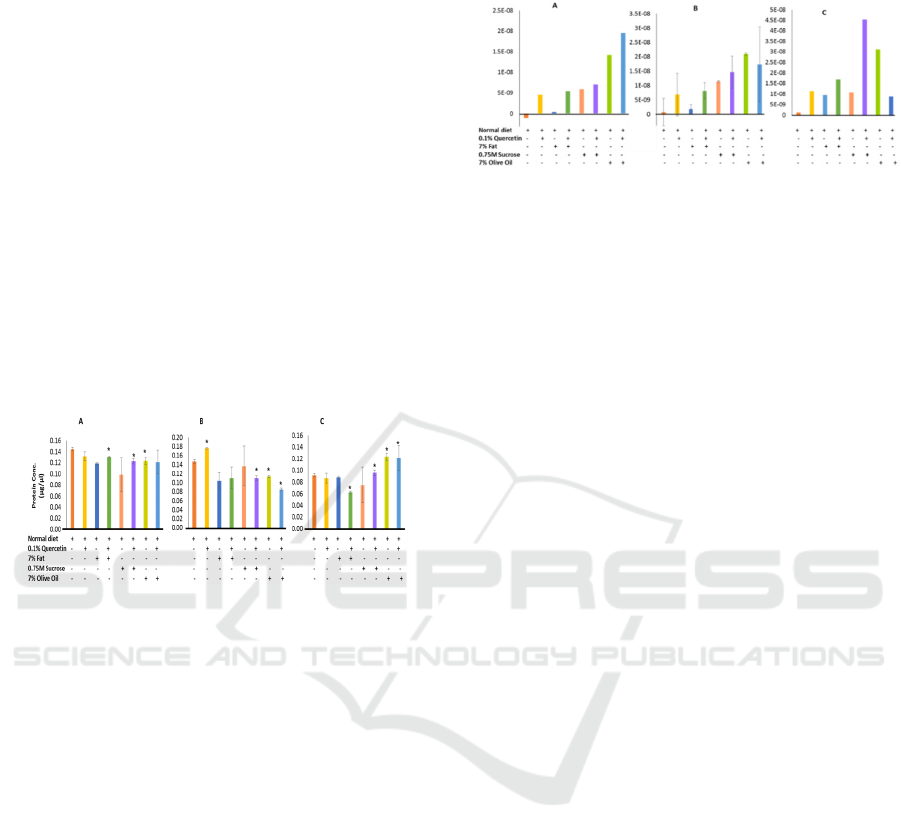
Figure 1: Protein contents per parental adult Drosophila, F
generation, (A); F1 generation (B); and F2 larva (C)
cultured in different diets (as indicated). *P<0.05
In F-, and F2-progenies, protein contents were
not affected by quercetin compared to the control,
but a significant increase was observed in F1 flies
(Fig.1 B). High fat diet significantly reduced the
amount of protein in F1 flies, suggesting a long term
treatment with high fat diet might impair their
growth. A similar trend was observed in sucrose +
quercetin and olive oil containing diets (Fig. 1 B).
Interestingly, we have not observed any
difference in terms of larval protein contents between
quercetin and high fat diet with control series (Fig.1
C). However, larval growth was significantly affected
in the quercetin containing high fat diet (P<0.05). In
order to avoid any residual effect of the normal diet
on the parental fly stocks (F generation) in their early
life cycles, we have used F2 larvae to confirm our
findings on the effect of these media on their growth.
These F2 larvae were derived from F1 flies, both
progenies were exclusively reared in the experimental
media as indicated in the figures. Sucrose + quercetin
and olive oil containing diets significantly increased
the amount of proteins in these larvae (Fig.1 C).
3.2 Amount of Neutral Lipids
Figure 2: Absorbance (OD 515nm) of the Oil-red-O stained
samples after normalizing with total protein. (A) F
generation, (B) F1 generation. and (C) F2 larvae.
Next, we proceeded to estimate the fat contents in
these model organisms. In f, f1, and f2 progenies,
cytosolic lipid accumulation was not observed in
normal diet feeding groups. F and f1 flies grown in
high fat diet alone accumulated a marginal amount of
lipids (fig. 2. A & b). Interestingly, high fat (animal
fat, ghee) diet induced highest amount of lipid in
these flies only in presence of quercetin, whereas
quercetin alone induced remarkably high level of
lipid. Both sucrose and olive oil induced significantly
high level of lipids with or without quercetin (fig. 2 a
& b).
The f2 larvae grown in different experimental
growth media. In all the experimental media,
accumulation of neutral lipids were significantly
higher compared to the control diet. High fat diet, as
expected, induced significantly high level of lipid
accumulation compared to the control. As like f ad f1
flies, we have also observed that quercetin alone
induced lipid accumulation similar to the high fat fed
larvae and the amount of neutral lipid was highest
when the larvae were grown in a combined diet of
quercetin and high fat (fig. 2c). This could be due to
the combined effect of quercetin and ghee as both of
them are able to induce lipid accumulation
independently as we have observed. In these larvae,
sucrose + quercetin and olive oil containing diets
induced significantly high level of neutral lipids,
whereas quercetin + olive oil containing diet tends to
reduce the lipid contents (fig. 2 c).
3.3 Mortality in Different
Experimental Diets
The above initial findings led to observe the mortality
of these flies grown in different media. High fat-fed
drosophila had significantly higher mortality rate
while the quercetin alone reduced their mortality in
the parental stock (f and f1) (fig.3).
Effect of Quercetin on Growth and Mortality of High Fat-fed Drosophila Melanogaster
417
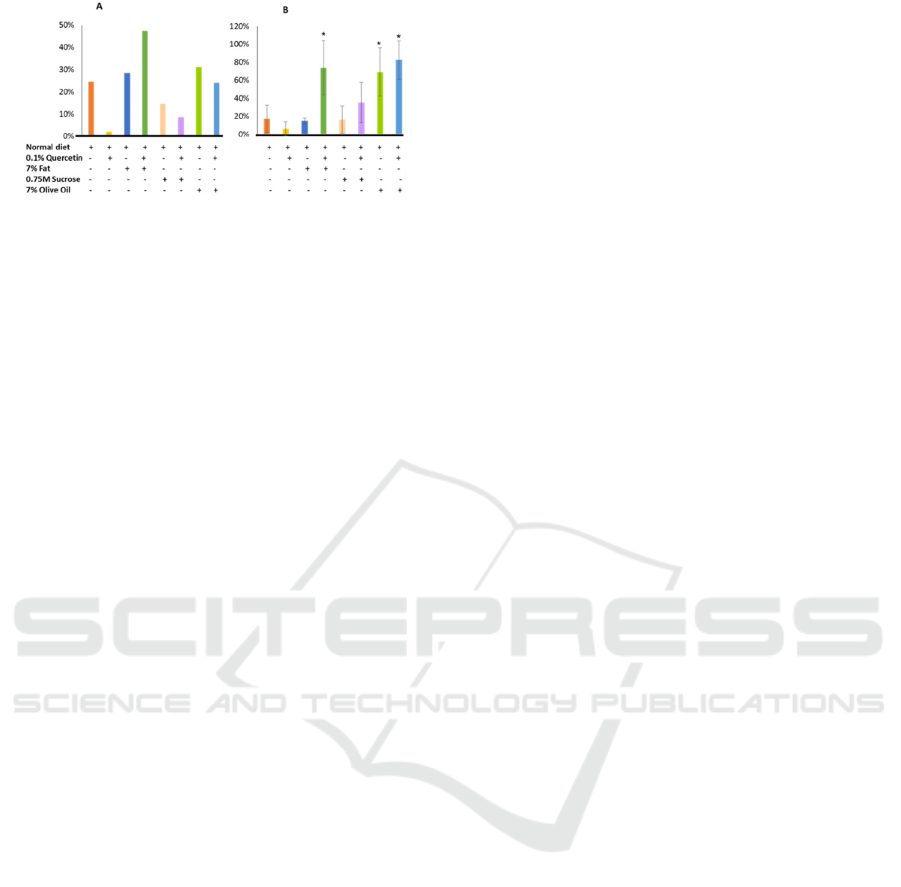
Figure 3: Percentage of mortality observed during the first
two weeks of culture. (A) F generation, (B) F1 generation.
However, the trend was different when we have
done similar study with F1 flies. In this experimental
unit, it has been observed that quercetin induced
higher mortality compared to the control, whereas
high fat diet did not. Combined diet high fat and
quercetin somehow induced significantly very high
percentage of mortality (Fig.3. B). In all those
experimental series, addition of sucrose did not
increase their mortality significantly. Unsaturated fat,
olive oil, induced high mortality rate as observed in
animal fat (ghee) treated group.
4 DISCUSSION
Ghee, a commonly used animal fat, is produced from
milk that contain a mixture of saturated and
unsaturated milk fat. The effects of ghee on human
body is still under debate with conflicting findings.
As this is being used in several parts of Asia for
traditional dishes, we were interested to investigate
the effect of ghee in our experimental wild-type
Drosophila model. Excess lipid accumulation often
induces higher lipid metabolism that eventually
causes lipid peroxidation and generate reactive
oxygen species (ROS). Antioxidants are receiving
increased attention due to their property to remove
ROS from the body. Hence, we have used quercetin,
a known antioxidant, to assess if this synthetic
polyphenol is able to minimize the lipid-mediated
oxidative stress in Drosophila reared with 7% ghee
containing diet.
In this study, we have observed that cytosolic
neutral lipid accumulation was remarkably high in
quercetin treated flies and larvae. This might be due
to the quercetin-mediated enhanced metabolism that
allows these D. melanogaster to utilize dietary
carbohydrate for de novo lipogenesis. The process
might be further enhanced in presence of high level
of dietary fat (ghee). Quercetin is known to possess
beneficial effects on human and we have observed a
similar phenomenon where it reduced mortality of the
experimental models (D. melanogaster). This study
raised the possibility that quercetin may also induce
lipogenesis apart from its anti-oxidative property. The
data presented here are based on our initial findings.
In order understand the metabolic consequences,
similar experiments need to be done using different
experimental conditions such as varied
concentrations of quercetin, carbohydrate, and animal
fat.
REFERENCES
Adams, M. D., Celniker, S. E., Holt, R. A., Evans, C. A.,
Gocayne, J. D., Amanatides, P. G., Scherer, S. E., Li, P.
W., Hoskins, R. A., Galle, R. F., George, R. A., Lewis,
S. E., Richards, S., Ashburner, M., Henderson, S. N.,
Sutton, G. G., Wortman, J. R., Yandell, M. D., Zhang,
Q., Chen, L. X., Brandon, R. C., Rogers, Y. H., Blazej,
R. G., Champe, M., Pfeiffer, B. D., Wan, K. H., Doyle,
C., Baxter, E. G., Helt, G., Nelson, C. R., Gabor, G. L.,
Abril, J. F., Agbayani, A., An, H. J., Andrews-
Pfannkoch, C., Baldwin, D., Ballew, R. M., Basu, A.,
Baxendale, J., Bayraktaroglu, L., Beasley, E. M.,
Beeson, K. Y., Benos, P. V., Berman, B. P., Bhandari,
D., Bolshakov, S., Borkova, D., Botchan, M. R., Bouck,
J., Brokstein, P., Brottier, P., Burtis, K. C., Busam, D.
A., Butler, H., Cadieu, E., Center, A., Chandra, I.,
Cherry, J. M., Cawley, S., Dahlke, C., Davenport, L. B.,
Davies, P., De Pablos, B., Delcher, A., Deng, Z., Mays,
A. D., Dew, I., Dietz, S. M., Dodson, K., Doup, L. E.,
Downes, M., Dugan-Rocha, S., Dunkov, B. C., Dunn,
P., Durbin, K. J., Evangelista, C. C., Ferraz, C.,
Ferriera, S., Fleischmann, W., Fosler, C., Gabrielian, A.
E., Garg, N. S., Gelbart, W. M., Glasser, K., Glodek,
A., Gong, F., Gorrell, J. H., Gu, Z., Guan, P., Harris,
M., Harris, N. L., Harvey, D., Heiman, T. J.,
Hernandez, J. R., Houck, J., Hostin, D., Houston, K. A.,
Howland, T. J., Wei, M. H., Ibegwam, C., Et Al. 2000.
The Genome Sequence Of Drosophila Melanogaster.
Science 287: 2185-95.
Lau, H. C., Lee, I. K., Ko, P. W., Lee, H. W., Huh, J. S.,
Cho, W. J. & Lim, J. O. 2015. Non-Invasive Screening
For Alzheimer's Disease By Sensing Salivary Sugar
Using Drosophila Cells Expressing Gustatory Receptor
(Gr5a) Immobilized On An Extended Gate Ion-
Sensitive Field-Effect Transistor (Eg-Isfet) Biosensor.
Plos One 10. E0117810.
Lenz, S., Karsten, P., Schulz, J. B. & Voigt, A. 2013.
Drosophila As A Screening Tool To Study Human
Neurodegenerative Diseases. J Neurochem 127: 453-
60.
Padmanabha, D. & Baker, K. D. 2014. Drosophila Gains
Traction As A Repurposed Tool To Investigate
Metabolism. Trends Endocrinol Metab 25: 518-27.
Palu, R. A. S., Praggastis, S. A. & Thummel, C. S. 2017.
Parental Obesity Leads To Metabolic Changes In The
F2 Generation In Drosophila. Mol Metab 6: 631-639.
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
418

Pospisilik, J. A., Schramek, D., Schnidar, H., Cronin, S. J.,
Nehme, N. T., Zhang, X., Knauf, C., Cani, P. D.,
Aumayr, K., Todoric, J., Bayer, M., Haschemi, A.,
Puviindran, V., Tar, K., Orthofer, M., Neely, G. G.,
Dietzl, G., Manoukian, A., Funovics, M., Prager, G.,
Wagner, O., Ferrandon, D., Aberger, F., Hui, C. C.,
Esterbauer, H. & Penninger, J. M. 2010. Drosophila
Genome-Wide Obesity Screen Reveals Hedgehog As A
Determinant Of Brown Versus White Adipose Cell
Fate. Cell 140: 148-60.
Prussing, K., Voigt, A. & Schulz, J. B. 2013. Drosophila
melanogaster As A Model Organism For Alzheimer's
Disease. Mol Neurodegener 8: 35.
Rovenko, B. M., Perkhulyn, N. V., Gospodaryov, D. V.,
Sanz, A., Lushchak, O. V. & Lushchak, V. I. 2015.
High Consumption Of Fructose Rather Than Glucose
Promotes A Diet-Induced Obese Phenotype In
Drosophila Melanogaster. Comp Biochem Physiol A
Mol Integr Physiol 180: 75-85.
Ruden, D. M., De Luca, M., Garfinkel, M. D., Bynum, K.
L. & Lu, X. 2005. Drosophila Nutrigenomics Can
Provide Clues To Human Gene-Nutrient Interactions.
Annu Rev Nutr 25: 499-522.
Sobels, F. H. & Vogel, E. 1976. The Capacity Of
Drosophila For Detecting Relevant Genetic Damage.
Mutat Res, 41: 95-106.
Vanhauwaert, R. & Verstreken, P. 2015. Flies With
Parkinson's Disease. Exp Neurol, 274, 42-51.
Zhao, X., Sun, X., Cai, S., Ran, D., Yan, Y. & Pei, Z. 2015.
Role Of Alpha-Synuclein In Cognitive Dysfunction:
Studies In Drosophila melanogaster. Mol Med Rep 12:
2683-8.
Zhu, Z. J., Wu, K. C., Qian, Z. M., Yung, W. H. & Ke, Y.
2014. Drosophila Models For Studying Iron-Related
Neurodegenerative Diseases. Sheng Li Xue Bao 66: 47-
54.
Effect of Quercetin on Growth and Mortality of High Fat-fed Drosophila Melanogaster
419
