
Utilization of Pumpkin Seeds (Cucurbita Moshcata D.)
in the Making of Fermented Drink
Eveline
1*
and Paloma
2
1
Lecturer, Food Technology Department, Faculty of Sains and Technology, University of Pelita Harapan,
Jl. MH. Thamrin Boulevard 1100 Lippo Village, Kelapa Dua, Karawaci, Tangerang, Indonesia
2
Alumnus, Food Technology Department, Faculty of Sains and Technology, University of Pelita Harapan,
Jl. MH. Thamrin Boulevard 1100 Lippo Village, Kelapa Dua, Karawaci, Tangerang, Indonesia
Keywords: fermented_beverage, Lactobacilus_plantarum, pumpkin_seeds, skim_milk, Streptococcus_thermophilus
Abstract: Pumpkin (Cucurbita moschata Duchesne) is a type of Indonesian plants whose flesh is widely used as
traditional processed foods (dodol, kolak, chips), while the utilization of the seeds is not optimal. Pumpkin
seeds have been reported to contain bioactive compounds and functional nutrients (vitamins, fiber,
antioxidants) that can be increased by the fermentation process. This study aims to produce fermented
pumpkin seeds. Initially, the ratio of pumpkin seeds to water (1:3, 1:4, 1:5 [w/v]) and skim milk concentration
(5%, 10%) was determined on LAB fermentation of Lactobacillus plantarum : Streptococcus thermophilus
1:1. Analysis of pH, total of titrated acids (TTA), and total LAB showed a ratio of 1:3 and 10% skim milk to
provide products that meet the standards, which in a sequence are 4.34, 0.84%, 1.5×10
9
cfu/ml. The selected
formulation was then used to determine the ratio of L. plantarum and S. thermophilus (1:1, 1:2, 2:1). The
ratio of 1:1 is selected as the ratio that yields pH, TTA, and total LAB according to the standard, which in a
sequence are 4.27, 0.84%, 2.2×10
9
cfu/ml. The products with preferred formulation contains proximate
according to the standard (83.62% water, 0.84% ash, 2.30% milk fat, 4.00% protein, 9.24% carbohydrate [by
difference]), phenolic 643.27 mg GAE/l, flavonoid 612.00 mg GAE/l; as well as increased antioxidant activity
by 55.81% after fermentation (IC
50
291694.47 mg/l). The product is considered acceptable by panelists in
hedonic (4.05 of 7.0) with a good level of safety based on toxicity test of 786.90 ppm (low toxic) and free of
coiliform microorganisms.
1 INTRODUCTION
Pumpkin (Cucurbita moschata Duchesne) or more
commonly known by the name of “Parang”
Pumpkin/Calabash originated from America (Peru
and Mexico). This type of Indonesian plant is
included in the Cucurbitaceae family which is quite
potential, about 20-21 ton/hectare (Data Badan Pusat
Statistik dalam Kumala and Nurlaela, 2015).
Pumpkin is widely used as traditional processed
foods, such as dodol, kolak, and chips (Usmiati, et al.,
2005). The part of pumpkin that can be utilized in
addition to the fruit flesh is its seeds.
Research by Atuonwu and Akobundu (2010); El-
Aziz and El-Kalek (2011); Primawati (2007) states
that pumpkin seeds potentially have antioxidant
activity of 47.01%. Pumpkin seeds are a good source
of protein (39.25%) and is widely used as a diabetes
drug in mice in the African region (Teugwa, et al.,
2013), as well as worm medicines and medications
for functional bladder disorder in North America and
Mexico (El-Aziz and El-Kalek, 2011). Utilization of
pumpkin based on its functional potential, needs to be
increased again especially in the field of food such as
lactic acid fermented beverage containing lactic acid
and probiotics so as to provide health benefits for the
body (Vasudha and Mishra, 2013). According to
Wardani (2011); Mardianto, (2015); and Kencana
(2015), the fermentation process also increases the
antioxidant (flavonoids and phenolics) which add
value to fermented beverage products.
Ratio determination research of pumpkin seeds to
water. According to Ariyanto, et al. (2015), Primurdia
and Kusnadi (2014), and Usmiati and Utami (2008),
separately reveals the best ratio that can be used in the
manufacture of grain fermented beverages is 1:4 and
1:5; therefore in this study tested both these ratios and
also as a comparison is the ratio of 1:3. The addition
of skim milk is also a factor in the successful
Eveline, . and Paloma, .
Utilization of Pumpkin Seeds (Cucurbita Moshcata D.) in the Making of Fermented Drink.
DOI: 10.5220/0010040301870193
In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), pages 187-193
ISBN: 978-989-758-496-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
187

manufacture of lactic acid fermented beverages.
Research done by Rachelia (2014) and Leo (2013)
found that 4% and 5% of skim milk can optimize the
fermentation process from peanut and tolo beans,
while Sari (2014) found 10% skim milk to make
fermented beetroot better. Therefore, in this study, it
is used 5% and 10% skim milk. The selected
formulation of the ratio and concentration of skim
milk was used in the next phase of the study (the
determination of culture ratio of Lactobacillus
plantarum and Streptococcus thermophilus) by pH
analysis, total titrated acid (TTA), and total lactic acid
bacteria (LAB) compared with the standard BSN
(2009), CODEX (2003), KEBS (2013), PNS (2007),
FSANZ (2014), and JETRO (2011).
Determination of treatment level of culture ratio
of L. plantarum and S. thermophilus based on
research done by Sari (2014) which found the best
amount of lactic acid and cell. This study used a ratio
level of 1:2, 1:1, and 2:1. The selected ratio was
determined through total LAB analysis, pH value,
total titrated acid also compared with the standard
BSN (2009), CODEX (2003), KEBS (2013), PNS
(2007), FSANZ (2014), and JETRO (2011). The
products with selected formulations are further
chemically tested (antioxidant, phenolic, flavonoid)
and microbiology (coliform), and evaluated by
proximate, toxicity, and hedonic test, so that this
pumpkin fermented beverage will meet the standard
of fermented beverages and may be accepted by the
panelists nutritionally functional and sensory.
2 MATERIALS AND METHODS
2.1 Materials
The main materials: pumpkin dried seeds, aquades,
skimmed milk powder, sugar, S. thermophilus, L.
plantarum. Man Rogosa Sharpe Broth (MRSB), Man
Rogosa Sharpe Agar (MRSA), Butterfield’s Phospate
Buffered, Lauryl Tryptose Broth (LTB), and Brilliant
Green Lactose Bile Broth (BGLBB). Analysis
materials: NaCl, 0.1 N NaOH, phenolphtalein
indicator, Folin-Ciocalteau reagent, sodium
carbonate, AlCl
3
, 25% HCl, DPPH, selenium, boric
acid, 98% H
2
SO
4,
35% NaOH, 0.02 N HCl, pH 4
buffer, pH 7 buffer, ethanol, dye solution, ascorbic
acid standard solution, HPO
3
-HOAc solution and
70% alcohol.
2.2 Methods
Phases of the research consisted of the phase of
making pumpkin seeds juice, phase 1 and phase 2.
Phases of pumpkin seeds juice extracts include
peeling skin from pumpkin seeds, seeds destruction
with blender, ratio of seeds:water = 1:3, 1:4, 1:5
(w/v), filtration and waste removal. Pumpkin seeds
juice according to each ratio was then used in phase 1
research. Phase 1 research (Figure 1) begins with the
addition of skim milk (5% and 10%) and sugar (5%)
in pumpkin seeds juice according to each ratio (1:3,
1:4, 1:5 [w/v]). Pasteurization was done (80°C, 15
minutes) and cooled to 45°C. Starter L. plantarum
(age 12 hours) and S. thermophilus (age 10 hours)
with a ratio of 1:1 were inoculated as much as 5% and
incubated at 42°C for 12 hours. Pumpkin seeds
fermented beverage was then analyzed for pH
(AOAC, 2005), total titrated acids (AOAC, 2005),
and total LAB (Wehr and Frank, 2004). The selected
formulation of the ratio of the pumpkin seeds to water
and the concentration of skim milk was used in the
next phase of the research through the comparison of
the results of each test parameter to standard BSN
(2009), CODEX (2003), KEBS (2013), PNS (2007),
FSANZ (2014), and JETRO (2011).
Phase 2 research (Figure 1) was done by
inoculating Starter L. plantarum (age 12 hours) and S.
thermophilus (age 10 hours) with a ratio of 1:1, 1:2,
and 2:1. The incubation conditions in the
fermentation process and the test analytical
parameters were performed the same as in the
previous phase. The culture ratios results that meet
the standard BSN (2009), CODEX (2003), KEBS
(2013), PNS (2007), FSANZ (2014), and JETRO
(2011) determined as the selected ratio. The
fermented beverages of the selected formulation and
ratio were then tested chemically (antioxidantt
activity [Nahak and Sahu, 2011], phenolic [Conde, et
al., 1997], flavonoids [Lamien-Meda, et al., 2008]) as
well as microbiological tests (coliform [BSN, 2006]).
Furthermore, it was evaluated proximately (water,
ash, fat, protein, carbohydrate by difference [AOAC,
2005]), toxicity [Lisdawati, et al., 2006], and hedonic
(scale 1-7).
2.3 Experimental Design
The experimental design of the Phase 1 research was
Completely Randomized Design of two factors. The
first factor (the ratio of pumpkin seeds to water)
contains three levels (1:3 [A1], 1:4 [A2], 1:5 [A3])
with three repetitions. The second factor (skim milk
concentration) contains two levels (5% [B1] and 10%
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
188

[B2]) with three repetitions. In the Phase 2 research
culture ratios of L. plantarum and S. thermophilus
was Completely Randomized Design one factor that
contained three levels (1:2 [A1], 1:1 [A2], and 2:1
[A3]) with repetition three times.
Pumpkin seeds
↓
peeling skin from pumpkin seeds
↓
Seeds destruction with blender on
(Seeds: Water ratio 1:3, 1:4, dan 1:5 (w/v))*
↓
Filtration
↓ →Waste (Remove)
Pumpkin seeds juice
↓
Add skim milk (5 and 10%) *and sugar (5%)
↓
Pasteurization in 80℃ for 15 minutes
↓
Cool down to 45℃
↓
Inoculate cultur L. plantarum dan S. thermophilus 5%
(1:1, 1:2, 2:1) **
↓
Incubate at 42℃ for 12 hours
↓
Pumpkin seeds fermented beverage
↓
Analysis
↓
p
H
↓
TTA
↓
LAB value
↓
determination of the best formulation
↓
Analysis
1.
Coliform
2. Toxicity
3. Proximate
4. Antioxidant activity
5. Phenolic content
6. Flavonoids content
7. Hedonic
↓
Pumpkin seeds fermented beverage
Note:
* = Phase I
** = Phase II
Figure 1. Research Flowchart
3 RESULT
3.1 Phase 1
The first phase research was conducted to determine
the best ratio of pumpkin seeds to water (1:3, 1:4. 1:5)
and skim milk concentration (5% and 10%) through
pH value analysis, total titrated acid value, and total
LAB based on comparison with standard BSN
(2009), CODEX (2003), KEBS (2013), PNS (2007),
FSANZ (2014), and JETRO (2011). L. plantarum and
S. thermophilus cultures were 5% inoculated at
optimum age of 12 hours (1.6×10
9
cfu/ml) and
10 hours (1.8×10
8
cfu/ml), respectively.
The result of statistical test between ratio of
pumpkin seeds and skim milk concentration showed
that both interact (p<0.05) influenced the pH value of
pumpkin seeds fermented. A number of nutrients in
the pumpkin seeds and milk lactose decompose as the
fermentation process becomes lactic acid and other
organic acids are formed (Wood, 1997; Retnowati
and Kusnadi 2014), thus lowering the pH value from
6.65 (control) to 4.09-4.49 (Table 1). The pH value of
all samples were still within the maximum standard
of 4.5 (FSANZ, 2014).
Results of the statistical test ratio of pumpkin
seeds and the concentration of skim milk showed no
interaction (p>0.05) influenced the TTA value of
pumpkin seeds fermented beverage, but each had
significant effect (p<0.05). Table 2 shows the lactic
acid and other organic acids are increasing with the
concentration of the pumpkin seeds juice and the
increasing concentration of skim milk. Energy
sources of pumpkin seeds and skim milk nutrients
increase the formation of lactic acid and other organic
acids during fermentation (Dewi, et al., 2013;
Mulyani, et al., 2013; Retnowati and Kusnadi, 2014;
Yanuar and Sutrisno 2015). During fermentation, the
TTA control value (0.33%) increased to 0.58-0.84%,
the value is still included in the standard of BSN
(2009) 0.2-0.9%; the sample ratio of 1:5 does not
meet the standards of PNS (2007) and CODEX
(2003) which more than 0.6%; and only 1:3 ratio and
skim milk 10% that meets KEBS standard (2013) 0.7-
0.9%.
Results of statistical test between ratio of pumpkin
seeds and skim milk concentration showed no
interaction to total LAB (p>0.05). The pumpkin juice
ratio did not affect the total LAB (p>0.05), whereas
the concentration of skim milk affected the total LAB
(p<0.05). Table 2 shows the total log value of LAB
during fermentation having increased from log 6.68
to ~log 8-9 both in the seeds ratio sample and on the
skim milk concentration sample. According to
Utilization of Pumpkin Seeds (Cucurbita Moshcata D.) in the Making of Fermented Drink
189

Vasudha and Mishra (2013), the fermentation process
will increase total lactic acid bacteria. Li, et al. (2012)
added that fermentation for 18-24 hours with more
than one culture can result in total lactic acid bacteria
of about 10
8
-10
9
cfu/ml (log 8-9). All test samples
meet the standard minimum of 10
7
cfu/ml (CODEX,
2003; PNS, 2007) and minimum 10
6
cfu/ml (BSN,
2009; FSANZ, 2015; JETRO, 2011).
Based on the analysis of pH value, TTA, and total
LAB compared with BSN (2009), CODEX (2003),
KEBS (2013), PNS (2007), FSANZ (2014), and
JETRO (2011), then the best formulation of pumpkin
seeds fermented beverages is the ratio of pumpkin
seeds to water and skim milk 1:3 and 10%. This
formulation has the results of analysis of each
parameter that meets all standards that is, pH 4.34,
TTA 0.84%, total LAB 1.5×10
9
cfu/ml; and will used
for phase 2 research in determining the best culture
ratio between L. plantarum and S.
thermophilus.
Table 1. Phase 1 Test Results (pH)
Pumpkin Seeds
:
Water
Skim Milk
(%)
pH
1:3
5 4.09±0.02
a
10 4.34±0.03
c
1:4
5 4.13±0.01
b
10 4.36±0.02
c
1:5
5 4.37±0.03
c
10 4.49±0.00
d
Note: - Different notation showed there was significant
difference (p<0.05)
Table 2. Phase 2 Test Results (TTA and Total LAB)
TTA
(%)
Total LAB
(Log)
Pum
p
kin S
e
ed : Wate
r
1:3 0.84±
0
.08
a
9.12±0.05
a
1:
4
0.69±0.11
b
9.01±0.11
a
1:5 0.58±0.15
c
9.05±0.0
6
a
Skim Milk (%)
5 0.62±0.15
a
8.99±0.05
a
1
0
0.78±0.1
0
b
9.15±0.0
4
b
Note: - Different notation showed there was significant
difference (p<0.05)
- No comparison between parameter analysis
3.2 Phase 2
Phase 2 research was conducted to determine the best
culture ratio between L. plantarum and S.
thermophilus through analysis of pH value, TTA, and
total LAB based on comparison with standard BSN
(2009), CODEX (2003), KEBS (2013), PNS (2007),
FSANZ (2014), and JETRO (2011). The results of
statistical test of culture ratio L. plantarum and S.
thermophilus showed an influence on TTA pumpkin
seeds fermented beverage (p<0.05). Table 2 shows
that the 1:1 ratio yields the highest TTA value
(0.84%) and meets all TTA value standards that is,
BSN (2009) 0.2-0.9%; CODEX (2003) and PNS
(2007) more than 0.6%; KEBS (2013) 0.7-0.9%. The
ratio of 1:2 and 2:1 both have no significant effect on
TTA value (0.60% and 0.66%) and significantly
affect the 1:1 ratio. According to Chandan and Kilara
(2013), the amount of Lactobacillus and
Streptococcus should be balanced so that
Lactobacillus can constantly provide peptide supply
decomposition result of Lactobacillus to
Streptococcus.
Results of statistical test of L. plantarum and S.
thermophilus showed that there was no effect on pH
value (p>0.05). Table 3 shows the entire sample
yielding pH values
4.27-4.3 and meeting the pH
standard FSANZ (2015) of 4.5. According to Zhang,
et al. (2011), pH of skim milk which tend to be neutral
and can serve as buffer can cause pH value of three
culture ratios not significantly different. In addition,
the amount of H
+
ions only shows the acid content
dissociated in the sample and does not measure all the
acid content contained in the product such as total
titrated acids, so it can happen that the pH value is
significantly different but the TTA value is not
(Primurdia and Kusnadi, 2014).
The result of statistic test of culture ratio of L.
plantarum and S. thermophilus also did not show any
influence to total LAB (p>0.05). Table 2 shows the
three culture ratios result total LAB in the range of
log 9.28-9.34 that include in standard of CODEX
(2003) and PNS (2007), that is more than 10
7
cfu/ml.
Based on the analysis of pH, TTA, and total LAB
compared with BSN (2009), CODEX (2003), KEBS
(2013), PNS (2007), FSANZ (2014), and JETRO
(2011), then the best ratio of L. plantarum and S.
thermophilus as culture of pumpkin seeds fermented
beverage is a 1:1 ratio. This ratio has the results of
analysis of each parameter that meets all standards,
that is pH 4.27, TTA 0.84% (significantly different
from 1:2 and 2:1 ratio), total LAB 2.2×10
9
cfu/ml.
Pumpkin seeds fermented beverage with the selected
formulation of seeds ratio and the concentration of
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
190

skim milk (1:3; 10%) and the selected culture ratio
(1:1) were then chemically analyzed (antioxidant,
phenolic, flavonoid activity), microbiologically
(coliform), proximate, toxicity, and hedonic.
Table 3. Phase 2 Test Result (pH, TTA, and Total LAB)
Culture
Ratio
(ST : LP)
pH
TTA
(%)
Total LAB
(Log)
1:1
4.27±0.02
a
0.84±0.04
b
9.34 ± 0.02
a
1:2
4.30±0.05
a
0.60±0.03
a
9.30 ± 0.04
a
2:1
4.30±0.05
a
0.66±0.04
a
9.30 ± 0.04
a
Note: - Different notation showed there was significant
difference (p<0.05)
- No comparison between parameter analysis
Based on the analysis of pH, TTA, and total LAB
compared with BSN (2009), CODEX (2003), KEBS
(2013), PNS (2007), FSANZ (2014), and JETRO
(2011), then the best ratio of L. plantarum and S.
thermophilus as culture of pumpkin seeds fermented
beverage is a 1:1 ratio. This ratio has the results of
analysis of each parameter that meets all standards,
that is pH 4.27, TTA 0.84% (significantly different
from 1:2 and 2:1 ratio), total LAB 2.2×10
9
cfu/ml.
Pumpkin seeds fermented beverage with the selected
formulation of seeds ratio and the concentration of
skim milk (1:3; 10%) and the selected culture ratio
(1:1) were then chemically analyzed (antioxidant,
phenolic, flavonoid activity), microbiologically
(coliform), proximate, toxicity, and hedonic.
The antioxidant activity test is performed to find
out how much antioxidant compound in the pumpkin
seeds fermented beverage can prevent radical
formation, inhibit oxidation reaction, decrease
oxygen concentration, metal chelating agent, and act
as bond breaker to prevent hydrogen removal from
the substrate (Winarsi, 2007). The analysis was
performed by DPPH method which measures the
sample ability in binding to free radicals expressed in
IC
50.
The lower the IC
50
value, the higher the
antioxidant activity which means is a few amount of
sample is needed to bind 50% of the free radicals.
(Somawathi, et al., 2014). According to Putri and
Hidajati (2015), antioxidant activity has five
categories: very strong (IC
50
<50 mg/l), strong (IC
50
50-100 mg/l), moderate (IC
50
100-250 mg/l), weak
(IC
50
250-500 mg/l), and very weak (IC
50
>500 mg/l).
The results of antioxidant activity test (IC
50
)
pumpkin
seeds fermented beverage before and after
fermentation were 660142.2 mg/l and 291694.7 mg/l;
although relatively weak, antioxidant activity
increased due to increased lactic acid levels during
fermentation. In addition, probiotic bacteria can
produce vitamin C and E which can act as an
antioxidant (Kusumaningrum, 2011).
Tests of total phenolic compounds were
performed using the Folin-Ciocalcetau colorimetric
method (standard phenolic curve), while for total
flavonoids using the aluminum chloride colorimetric
method (standard flavonoid curve). The total test
results of phenolic and flavonoid pumpkin seeds
fermented beverage sequentially were 643.27 mg
GAE/l and 612 mg QE/l. The total phenolic and
flavonoid contained are interconnected with the
antioxidant activity obtained (Primurdia and Kusnadi,
2014).
Testing of coliform contamination (Gram
negative, not forming spores, rod shape, producing
acid and gas) is stated negatively on pumpkin seeds
fermented beverage, while the amount of coliform
allowed in fermented beverages is a maximum of 10
APM/ml (Badan Standarisasi Nasional, 2009). The
organic acid produced by LAB inhibits the growth of
coliform bacteria (Soccol, et al., 2013).
Proximate test in the form of moisture content, ash
content, fat, protein, and carbohydrate (by difference)
can be seen in Table 3. All test parameters include in
the standard of SNI except fat content but still fulfill
requirement of CODEX (2003) that is <15%.
Product toxicity test is performed as a preliminary
test in more complex toxicity testing. The Brine
Shrimp Lethality Test (BSLT) method is used to
produce LC
50
(Lethal Concentration 50) which is the
number of products needed to kill 50% of shrimp
larvae. According to Onzago, et al. (2014), LC
50
0-
100 ppm: strong toxic, LC
50
100-500 ppm: moderate
toxic, LC
50
500-1000 ppm: low toxic, and LC
50
>1000
ppm: non toxic. The pumpkin seeds fermented
beverage has LC
50
786,90 ppm (low toxic).
According to Prasetia and Intan (2013), LC
50
<1000
ppm can be caused by components such as flavonoids
contained in the sample so that the product is still safe
for consumption.
The hedonic test of pumpkin seeds fermented
beverage was conducted on 70 panelists to determine
the level of product acceptance. The test scale
consists of 7: extremely dislike (1), dislike (2),
moderately dislike (3), neutral (4), moderately like
(5), like (6), extremely like (7). Average yields of
each parameters results is 3.29, taste 4.01, texture 4.4,
and overall 4.05. Overall the panelists evaluate the
product in the neutral category (4.05), that means the
product can still be accepted by the panelists.
Utilization of Pumpkin Seeds (Cucurbita Moshcata D.) in the Making of Fermented Drink
191
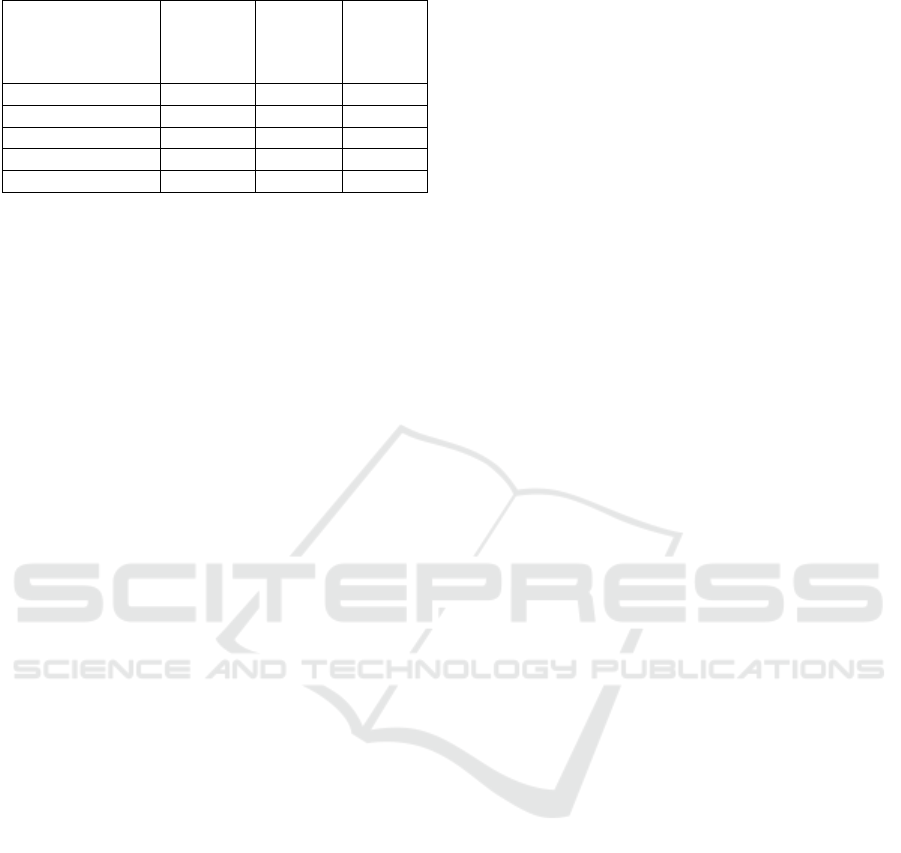
Table 4. Phase 2 Test Result (Proximate)
Parameters
Pumpkin
seeds
fermented
b
everage
SNI
Standard
(BSN,
2009)
CODEX
Standard
(CODEX,
2003)
Water (%) 83.62 - -
Ash (%) 0.84 max. 1.00 -
Fat
(
%
)
2.30 0.50-0.60 max. 15%
Protein
(
%
)
4.00 min. 1.00 min. 2.7
Carb. [b
y
diff]
(
%
)
9.24 - -
4 CONCLUSIONS
Preparation of pumpkin seeds fermented beverage
with a ratio of pumpkin seeds to water of 1:3,
addition of skim milk 10%, and culture ratio of L.
plantarum : S. thermophilus 1:1 result pH, TTA, and
total LAB that meet the standard. The pH, TTA, and
total LAB are respectively 4.27; 0.84%; 2.2×10
9
cfu/ml.
The products of this selected culture formulation
and ratio contains 83.62% water, 0.84% ash, 2.30%
fat, 4.00% protein, 9.24% carbohydrate, total
phenolic 643.27 mg GAE/l sampel, and total
flavonoids 612 mg QE/l. The product increased the
antioxidant activity by 55.81% after the fermentation
process, with IC
50
value after fermentation were
291694.7 mg/l. In hedonic, fermented beverage of
pumpkin seeds are still acceptable to consumers (4.05
of 7.00), and declared safe for consumption with low
toxicity and coliform-free microorganisms.
REFERENCES
Association of Official Analytical Chemist (AOAC). 2005.
“Official Methods of Analysis of AOAC International.”
AOAC International, Madison.
Ariyanto, N. O., Wiyanto, S. D., Hindarso, H., and
Aylianawati. 2015. Pengaruh rasio massa biji dan
volume air dan suhu ekstraksi terhadap ekstraksi biji-
bijian dalam pembuatan susu nabati. Jurnal Ilmiah
Widya Teknik 14(1): 20-25.
Atuonwu, A. C and Akobundu, E. N. T. 2010. Nutritional
and sensory quality of cookies supplemented with
defatted pumpkin (Cucurbita pepo) seed flour. Pakistan
Journal of Nutrition, 9: 672-677.
Badan Standarisasi Nasional (BSN). 2006. “SNI 01-
2332.1:2006: Cara uji mikrobiologi – Bagian 1:
Penentuan coliform dan Escherichia coli pada produk
perikanan.” Badan Standarisasi Nasional, Jakarta.
Badan Standardisasi Nasional (BSN). 2009. “SNI
7552:2009-Minuman susu fermentasi berperisa.”
Badan Standarisasi Nasional, Jakarta.
Chandan, R. C and Kilara, A. 2013. “Manufacturing Yogurt
and Fermented Milks”, Second Edition. John Wiley &
Sons, Inc, Chichester.
Codex Alimentarius Commission. 2003. “Codex Standard
for Fermented Milks.” Codex Alimentarius
Commission, Rome.
Conde, E. E., Cadahia, M. C., Garcia-Vallejo, B., Simon,
and Adrados, J. 1997. Low molecular weight
polyphenol in cork of Quercus suber. Journal of
Agriculture Food Chemistry: 2695-2700.
Dewi, E. C., Wulandari, S., and Sayuti, I. 2013. “Efektivitas
penambahan madu dan susu skim terhadap kadar asam
laktat dan pH yoghurt kacang hijau (Phaseolus
radiatus L.) dengan menggunakan inokulum
Streptococcus thermophilus dan Lactobacillus
bulgaricus.” Skripsi. Universitas Riau, Pekanbaru.
El-Aziz, A. B and El-Kalek, H. H. 2011. Antimicrobial
proteins and oil seeds from pumpkin (Cucurbita
moschata). Nature and Science 9(3): 105-119.
Food Standards Australia New Zealand (FRANZ). 2015.
“Standard 2.5.3: Fermented milk products.” Available
from
https://www.legislation.gov.au/Details/F2015L00413.
Accessed 1 Oktober 2016.
Japan External Trade Organization (JETRO). 2011.
“Specifications and Standards for Foods, Food
Additives, etc. Under the Food Sanitation Act
(Abstract) 2010.” Available from
https://www.jetro.go.jp/ext_images/en/reports/regulati
ons/pdf/foodext2010e.pdf. Accessed 1 Oktober 2016.
Kencana, A. H. 2015. “Aplikasi minuman fermentasi kulit
melinjo sebagai anti asam urat pada tikus wistar.”
Skripsi. Universitas Pelita Harapan, Tangerang.
Kenya Bureau of Standards (KEBS). 2013. “DKS 05-
941:2013-Specification for fermented (cutured) milks.”
Kenya Bureau of Standards, Nairobi.
Kumala, I and Nurlaela, L. 2015. Pengaruh penambahan
puree labu kuning dan lama pengocokan (agitasi)
terhadap sifat organoleptik es krim yoghurt. Boga 4(1):
202-210.
Kusumaningrum, A. P. 2011. “Kajian total bakteri
probiotik dan aktivitas antioksidan yoghurt tempe
dengan variasi substrat.”
Skripsi. Universitas Sebelas
Maret, Surakarta.
Lamien-Meda, A., Lamien, C. E., Compaore, M. M. Y.,
Meda, R. N. T., Kiendrebeogo, M., Zeba, B., Millogo,
J. F., and Nacoulma, O. G. 2008. Polyphenol content
and antioxidant activity of fourteen wild edible fruits
from Burkina Faso. Molecules 13: 581-594.
Leo, D. J. 2013. “Kajian minuman fermentasi sari kacang
tolo (Vigna unguiculata (L.) Walp) terhadap mikroflora
usus mencit”. Skripsi, Universitas Pelita Harapan,
Karawaci.
Li, S., Walsh, H., Gokavi, S., and Guo, M. 2012.
Interactions between Lactobacillus acidophilus strains
and the starter cultures, Lactobacillus bulgaricus and
Streptococcus thermophilus during fermentation of
goats milk. African Journal of Biotechnology 11:
11271-11279.
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
192

Lisdawati, V., Sumali, W., and Kardono, L. B. S. 2006.
Brine Shrimp Lethality Test (BLST) dari berbagai
fraksi ekstrak daging buah dan kulit biji mahkota dewa
(Phaleria macrocarpa). Buletin Penelitian Kesehatan
34(3): 111-118.
Mardianto. 2015. “Peranan minuman fermentasi daun
sirsak (Annona muricata L.) sebagai antikolesterol pada
tikus Sprague Dawley.” Skripsi. Universitas Pelita
Harapan, Karawaci.
Mulyani, S., Sudaryati, and Susanto, A. 2013. Kajian peran
susu skim dan bakteri asam laktat pada minuman
sinbiotik umbi bengkuang. Jurnal Teknologi Hasil
Pertanian 46-54.
Nahak, G and Sahu, R. K. 2011. Evaluation of antioxidant
activity in ethanolic extracts of five curcuma species.
International Research Journal of Pharmacy 2: 243-248.
Onzago, R.O., Kiama, S.G., Mbaria, J.M., Gakuya, D.W.,
and Nduhiu, J.G. 2014. “Evaluation of antimicrobial
activity and toxicity of vernonia hymenolepis (A.Rich)
traditionally used for toothache in Kenya.” Journal of
Phytopharmacology 3(1): 22-28.
Philippine National Standard (PNS). 2007. “PNS/BFAD
08:2007-Fermented milks-Specification.” Available
from
http://www.fda.gov.ph/attachments/article/153547/PN
S-BFAD%2008-2007-fermented%20milks-specs.pdf.
Accessed 1 Oktober 2016.
Prasetia, R and Intan, I. 2013. Uji toksisitas akut ekstrak
etanol buah lakum (Cayratia tryfolia) terhadap larva
Artemia salina Leach dengan metode brine shrimp
lethality test (BSLT). Prosiding Seminar Nasional
Kimia: 155-157.
Primawati, R. 2007. “Aktivitas antioksidan dan kadar
fenolik total biji semangka (Citrullus vulgaris schrad.)
dan biji labu kuning (Cucurbita moschata ex Poir).”
Skripsi. Universitas Kristen Satya Wacana, Salatiga.
Primurdia, E. G and Kusnadi, J. 2014. Aktivitas antioksidan
minuman probiotik sari kurma (Phoenix dactilyfera L.)
dengan isolat L. plantarum dan L. casei. Jurnal Pangan
dan Agroindustri 2(3): 98-109.
Putri, A. A. S and Hidajati, N. 2015. Uji aktivitas
antioksidan senyawa fenolik ekstrak metanol kulit
batang tumbuhan nyiri batu (Xylocarpus moluccensis).
UNESA Journal of Chemistry 4(1): 1-6.
Rachelia, M. Y. 2014. “Pemanfaatan bakteri asam laktat
dalam minuman fermentasi kacang tanah (Arachis
hypogaea L.)”. Skripsi, Universitas Pelita Harapan,
Karawaci.
Retnowati, P. A and Kusnadi, J. 2014. Pembuatan minuman
probiotik sari buah kurma (Phoenix dactilyfera)
dengan isolat Lactobacillus casei dan Lactobacillus
plantarum. Jurnal Pangan dan Agroindustri 2(2): 70-81.
Sari, Y. T. 2014 “Pemanfaatan sari bit merah (Beta
vulgaris L.) pada pembuatan minuman fermentasi
dengan bakteri asam laktat.” Skripsi. Universitas Pelita
Harapan, Tangerang.
Soccol, C. R., Pandey, A., and Larroche, C. 2013.
“Fermentation Processes Engineering in the Food
Industry”. CRC Press, Boca Raton.
Somawathi, K. M., Rizliya, V., Wijesinghe, D. G. N. D.,
and Madhujith, W. M. T. 2014. Antioxidant activity and
total phenolic content of different skin coloured brinjal
(Solanum melongena). Tropical Agricultural Research
26(1): 152-161.
Teugwa, C. M., Boudjeko, T., Tchinda, B. T., Mejiato P.
C., and Zofou, D. 2013. Anti-hyperglycaemic globulins
from selected Cucurbitaceae seeds used as antidiabetic
medicinal plants in Africa. BMC Complementary and
Alternative Medicine. 13:63.
Usmiati, S and Utami, T. 2008. Pengaruh bakteri probiotik
terhadap mutu sari kacang tanah fermentasi. J.
Pascapanen 5(2): 27-36.
Usmiati, S., Setyaningsih, D., Purwani, E. Y., Yuliani, S.,
and Maria, O. G. 2005. Karakteristik serbuk labu
kuning (Cucurbita moschata). Jurnal Teknologi dan
Industri Pangan 16 (2): 157-167.
Vasudha, S and Mishra, H. N. 2013. Non dairy probiotic
beverages. International Food Research Journal 20(1):
7-15.
Wardani, A. K. 2011. “Pengaruh fermentasi menggunakan
bakteri Lactobacillus bulgaricus terhadap kandungan
fenol total dan aktivitas antioksidan jus buah naga
merah (Hylocereus polyrhizus).” Skripsi, Universitas
Jember, Jember.
Winarsi, H. 2007. “Antioksidan Alami dan Radikal Bebas”.
Penerbit Kanisius, Yogyakarta.
Wehr, H. M and Frank, J. F. 2004. “Standard Methods for
Examination of Dairy Products”. American Public
Health Association, Washington.
Yanuar, S.E and Sutrisno, A. 2015. Minuman probiotik dari
air kelapa muda dengan starter bakteri asam laktat
Lactobacillus casei. Jurnal Pangan dan Agroindustri
3(3): 909-917.
Zhang, T., Zhang, C., Li, S., Zhang, Y., and Yang, Z. 2011.
Growth and exopolysaccharide production by
Streptococcus thermophilus ST1 in skim milk. Brazilian
Journal of Microbiology: 1470-1478.
Utilization of Pumpkin Seeds (Cucurbita Moshcata D.) in the Making of Fermented Drink
193
