
Simulation of Stress Salinity Conditions in the Phase of Germination
of National and Local Aceh Peanut Variety
Halimursyadah
1*
, Agam Ihsan Hereri
2
and Melika Bosniana
3
1,2,3
Department of Agrotechnology, Agriculture Faculty, University of Syiah Kuala
Keyword: Saline condition, variety, peanut, viability and vigor
Abstract: The degradation of agricultural land in Indonesia due to salinization has become one of the national issues.
Mapping of saline land in Indonesia has not been widely implemented, but has identified many farms that
are saline. The tsunami in Aceh in 2004 increased the salinity of the land (DHL 2-40 dS / m) damaging
more than 120,000 ha of agricultural land. The decrease in peanut production is followed by the limited land
and environmental factors required for peanut cultivation. The use of improved varieties and local varieties
with good cultivation techniques will increase the doubling of peanut productivity and is the means used for
planting on land with limited water. This research was conducted at Seed Technology Laboratory of
Agricultural Faculty of Syiah Kuala University, Darussalam Banda Aceh in April 2017. The purpose of this
research is to know the viability and vigor to grow several varieties of peanut seeds at various levels of
salinity stress with some concentration of NaCl solution. This study used a complete randomized design
with three replications. The first factor is varieties of Local Aceh, Talam 2, Kancil and Kelinci. The second
factor was control salinity (0 dSm
-1
, 1 g L
-1
=1.89 dSm
-1
, 3 g L
-1
=3.90 dSm
-1
, 5 g L
-1
=4.93 dSm
-1
, and 7 g L
-
1
=5.77 dSm
-1
. The parameters observed are maximum growth potential, germination, vigor index,
simultaneity growth, speed growth, time required to achieve total germination 50%, root length of normal
seedling, and dry weight of normal seedling. The results showed that the varieties of Talam 2 and Kelinci
showed more tolerance to salinity stress compared with Kancil and Local Aceh based on all parameters
observed. While on the factor of NaCl concentration of 3 g L
-1
has shown decreased viability and vigor of
seed growth in all peanut varieties. There is a significant interaction between varieties with NaCl
concentrations in viability and vigor based on maximum growth potential, germination, speed growth, time
required to achieve total germination 50% and dry weight of normal seedling.
1 INTRODUCTION
Peanut (Arachis hypogaea L.) plays an important
role in improving people's nutrition. Fat content
composition 45.15% and 23.97% protein has made
this commodity has the potential as a raw material
agroindustri (Danuwarsa, 2006). However, peanut
productivity tends to fluctuate due to the diminished
productive cultivation area. Production and total area
of peanut harvest in 2015 is 605 thousand tons with
harvest area of 454 thousand ha, while in 2016
production is 510 thousand tons with harvested area
of 393 thousand ha (BPS, 2016). This condition has
caused a deficit in this commodity and attempted to
import peanuts as much as 150-200 thousand tons in
line with the high demand.
Efforts can be made to overcome the lack of
production is using of peanut varieties that are able
to adapt to conditions of marginal areas such as high
salinity. Local Aceh varieties still dominate over
50% of the peanut growing area, followed by Gajah
and Kelinci varieties, each released in 1950 and
1987. Each variety has its own resistance to sub
optimum conditions such as saline conditions. The
data reveal that newly released varieties in 2010
(Talam 1) and 2012 (Takar 1 and Takar 2) are
produced for adapting to saline soil conditions
(Kasno and Harnowo, 2014).
High salinity in a field is caused by the intrusion
of sea water and heavy fertilization. Salinity
problems will arise when the concentrations of
NaCl, Na
2
CO
3
, Na
2
SO
4
and Magnesium salts present
on the land are in high state. NaCl salt is the most
dominant in which sodium ions (Na
+
) will
accumulate in the soil layer. This causes seeds
grown on land with high salinity, difficult or even
Halimursyadah, ., Hereri, A. and Bosniana, M.
Simulation of Stress Salinity Conditions in the Phase of Germination of National and Local Aceh Peanut Variety.
DOI: 10.5220/0010040101730178
In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), pages 173-178
ISBN: 978-989-758-496-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
173

not germinate at all. Water absorption by seed will
decrease with increasing osmotic pressure of the
solution or salt concentration in the medium. To
germinate seed requires water averaging more than
50% of the seed weight (Hasanuddin, 2015).
The results of Zakaria and Fitriani (2006)
suggest that seed sorting using NaCl solution with
1.5% concentration can be used as an alternative for
peanut seed sorting. However, the use of NaCl with
a 3.0% concentration may negatively affect the
viability and vigor of the seed. Hajar et al. (1993) in
his research stated that the growth of peanut seeds in
NaCl 5000 ppm still able to grow well, despite the
decrease in wet weight. The ability of seed
germination on different saline conditions between
plants even there is a noticeable variation among the
varieties of that same plant.
Plant performance during germination is often
used to assess plant tolerance to salinity (Khan et al.,
2000). Wijayanti et al. (2014) of 29 peanut strains
tested in saline condition showed that the salinity of
7 grams L
-1
NaCl had significant effect on
germination behavior, the number of live plants at 6
weeks after planting, the number of live plants at
harvest, the number of pods and dry weight pods.
This study aims to find out the viability and vigor of
growing several varieties of local Aceh and national
at various levels of salinity stress.
2 MANUSCRIPT OF
PREPARATION
This research was conducted in Agricultural Science
and Technology Laboratory of Agrotechnology
Study Program of Agriculture Faculty of Syiah
Kuala University from March to May 2017. The
materials used are Local Aceh varieties from Aceh
Barat Daya District, and Talam 2, Kancil and
Kelinci national varieties from Balai Penelitian
Tanaman Kacang-kacangan dan Umbi-umbian
(BALITKABI). Peanut seeds used had an average
initial germination of 79.0% (Local Aceh), 86.0%
(Talam 2), 82.0% (Kancil), 92.7% (Kelinci). The
total required seed is 1500 grains. Other materials
used are 20 g NaCl Pro analysis, paper merang and
aquades as much as 7 liters. The tools used are
analytical scales (Mettler PM 100), 1 liter volume
meter, electric oven, electro conductivity meter, and
germinator.
2.1 Preparation of Planting Media
The paper media substrate is moistened using a
NaCl solution according to a predetermined
concentration. Each NaCl solution consisting of
concentrations is filled into the container slowly,
which has previously been filled with paper on each
container until the paper is wet and field capacity.
This research used the Rolled Paper Test Method
Established in plastic based on International Seed
Testing Association (ISTA) rules. This method
begins by preparing a thin transparent plastic
measuring 20cm x 30cm overlaid on the table, and
then preparing 7 sheets of paper that have been
soaked with NaCl solution in accordance with the
concentration. A sheet paper substrate is placed
overlaid on preprinted plastic paper. Four varieties
of peanuts were added as many as 25 grains on
paper. The seeds are then covered with three sheets
of paper that have been soaked in a concentrated
NaCl solution. Then the rolled paper is placed in the
container and put into the germinator for 10 days.
Observations were made on the viability and
vigor parameters based on ISTA standard that is
maximum growth potential, germination,
simultaneity growth, relative growth rate, vigor
index, time required to achieve 50% relative total
germination, root length of normal primary sprout
and dry weight of normal sprouts.
2.2 Experimental Design
The experimental design used was a complete
randomized design (CRD) with three replicates and
there were two factors studied. The first factor of
peanut varieties consists of four levels: Local Aceh,
Talam 2, Kancil, and Kelinci. The second factor was
the concentration of NaCl consist of five level:
Control, 1 g L
-1
simulation with salinity level 1.89
dSm
-1
), 3 g L
-1
simulation with salinity level 3.90
dSm
-1
), 5 g L
-1
simulation with salinity level 4.93
dSm
-1
), 7 g L
-1
simulation with salinity level 5.77
dSm
-1
). The data obtained were analyzed using
Analysis of Variance (ANOVA), and continuous
differences test with Honestly Significant Difference
(HSD) at the test level of 0.05.
3 RESULT AND DISCUSSION
Table 1 shows that the correlation (r) of NaCl
treatment on the maximum potential growth
measuring parameter r = 0.892 and the percentage of
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
174
influence was 79.55%. At germination r = 0.969 and
the percentage of influence is 93.95%. On the index
vigor r = 0.956 and the percentage of influence r =
91.35%. In the simultaneity growth r = 0.915 and the
percentage of influence is 83.7%. At speed growth r
= 0.947 and the percentage of influence is 89.63%.
At time required to achieve total germination 50% r
= 0.974 and the percentage of influence is 94.82%.
At normal dry weight of normal seedling r = 0.962
and the percentage effect of 92.5%. While the root
length of the normal seedling r = 0.957 and the
percentage of influence of 91.62%. If the value of
correlation and determination closer to 1, that means
the relationship between the concentration of NaCl
to the viability and vigor parameters peanut seeds
very closely. The relationship of linear regression
equation can be seen in the following Table 1.
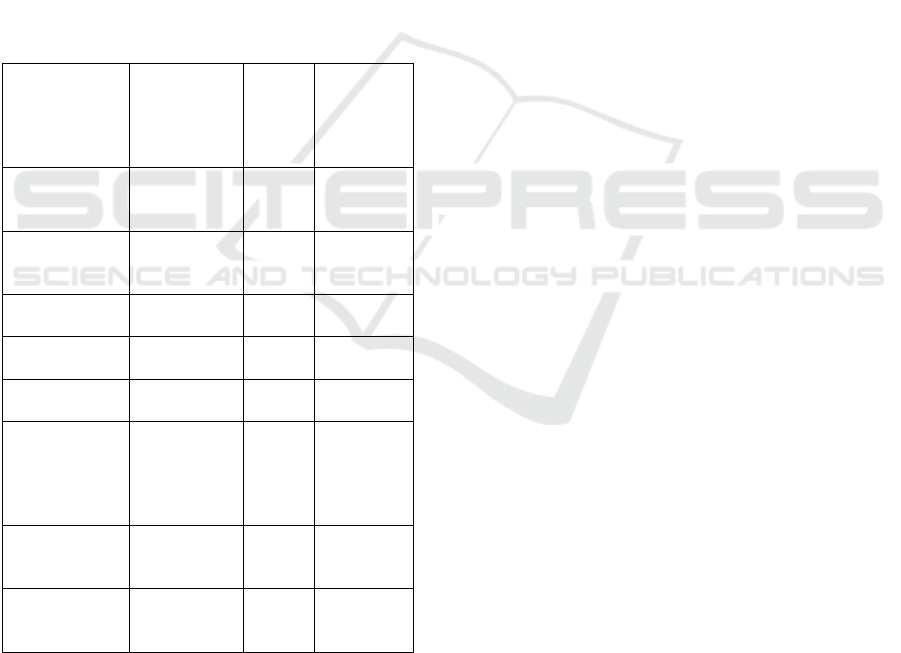
Table 1: Linier Regression Equation, Correlation
Coefficient and Determination Coefficient due to NaCl
concentration on viability and vigor of peanut seed
Parameters Linier
Regression
Equation
Corre-
lation
Coeffi
-cient
(
r
)
Determi-
nation
Coeffici-
ent
(
R
2
)
Maximum
growing
p
otential
(
%
)
y = -0.662x
+ 97.183
0.892 0.795
Germination
(%)
y = -
12.853x +
81.061
0.969 0.939
Vigor index
(
%
)
y = -2.127x
+ 13.273
0.956 0.913
Simultaneity
growth (%)
y = -7.900x
+ 46.413
0.915 0,837
Speed growth
(% etmal
-1
)
y = -5.162x
+ 30.858
0.947 0.896
Time required
to achieve
total
germination
50% (day)
y = 0.038x
+ 1.140
0.974 0.948
Dry weight of
normal
seedlin
g
(g)
y = -1.557x
+ 9.592
0.962 0.925
Root length of
normal
seedlin
g
(
cm
)
y = -2.565x
+ 20.092
0.957 0.916
Based on the results of the study on the viability
and vigor parameters of seed growth, the treatment
without NaCl solution resulted in the highest
viability and vigor of seed growth and the lowest in
the administration of NaCl solution with a
concentration of 7 g L
-1
. Peanut seeds are still
tolerant of NaCl solution with a concentration of 3 g
L
-1
. The provision of salinity stress is aimed at
knowing the resistance response of peanut varieties.
A NaCl solution with a concentration of 7 g L
-1
was the optimum dose to create a diversity of growth
and yield properties (Hajar et al., 1993). The
concentration has exceeded the peanut batch dose of
3.2 dSm
-1
(Yadav et al., 2011). Pohan (2005) also
reported that at a concentration of 3 g L
-1
NaCl has
inhibited peanut growth as indicated by root xylem
change.
This result was in line with Zakaria and Fitriani
(2006) research, the highest peanut vigor was
obtained in the treatment without NaCl, while the
lowest sprout vigor was obtained in NaCl treatment
with concentration of 1.5%. Salinity relationship
with peanut shell vigor shaped linear, where the
higher level of salinity then decrease the viability
and grow peanut seed vigor with value r = 0,98. The
effect of salinity on seed germination involves two
things: the high influence of osmotic pressure so that
the seeds are difficult to absorb water and chemical
or poisoning by specific ions that make up the salt.
According to Widoretno (2002), a seed will decrease
its germination if planted on less water-growing
media or drought stress conditions due to simulation
by salt. The addition of a salt solution (solute) to the
seed germination medium causes the occurrence of
plasmolysis (shrinkage due to fluid depreciation in
the cell) if the solute is increased, the cytoplasm is
not permeable to the solute either inside or outside
the cell. The potential of the vacuous aqueous
solution will be greater than the outer water potential
of the solution, so that the water diffuses outward, as
a result of the outflow of water. The middle vakuole
will shrivel up and protoplasm and the clinging cell
walls also shrink together with the vacuoles. If the
vacuole volume is so large then the protoplasm will
be separated from the cell wall and the seed will be
very sensitive to drought stress. Increasing the NaCl
concentration may inhibit the seeds imbibition
process because salt solubility can decrease the
osmotic pressure so that the seeds can not absorb
water from the growing environments needed for
enzyme activation for the germination process. Rini
et al. (2005) stated that salinity in planting medium
of seed can affect seed germination process because
it can decrease water potential in planting medium
thus inhibiting water absorption by germinating
seeds.
Although seeds can germinate under saline
conditions, the seeds germinate become abnormal.
The higher the concentration of NaCl, the higher the
seeds that germinate abnormally or the dead seed.
Simulation of Stress Salinity Conditions in the Phase of Germination of National and Local Aceh Peanut Variety
175

Erinnovita et al. (2008) suggest that salinity causes
some abnormalities in seeds and propagules during
germination. Inhibition of plant growth by salinity
can occur in two ways, namely by damaging the
cells that are growing and limiting the supply of
essential metabolic products.
If the salinity concentration increases continuously
then the tissue damage occurs, even the death of
seed or seed can germinate but grow abnormally
(Duan et al., 2004). The effects of NaCl on the
germination process include reducing the hydration
of the embryo and cotyledon, inhibiting and
reducing the appearance of radicles and plumules,
and reducing the growth of sprouts (Erinnovita et
al., 2008)
There is a very significant interaction between
peanut varieties treatment with salinity stress to
maximum growth potential, germination, and speed
growth, time required to achieve 50% total
germination, and dry weight of normal (Table 2).
Table 2: Mean of viability and vigor value due to
interaction between peanut varieties and NaCl
concentration
Peanut
varieties
NaCl concentration
(g
L
-1
)
Control 1 3 5 7
Maximum
p
otential
g
rowth
(
%
)
Local
Aceh
94,66
Aa
97,33
Aa
97,33
Ab
94,66
Aab
90,66
Ab
Talam 2 98,66
Aa
100
Aa
98,66
Ab
98,66
Ab
98,66
Ab
Kancil 93,33
Ca
90,66
BCa
82,66
ABa
88,00
BCa
77,33
Aa
Kelinci 100
Aa
100
Aa
100
Ab
100
Ab
100
Ab
HSD
0,05
10,47
Peanut
varieties
Germination (%)
Local
Aceh
66,67
Cab
66,67
Cab
36,00
BCab
2,67
A
b
a
0,00
Aa
Talam 2 93,33
Bb
94,67
Bb
9,33
Aa
8,00
Aa
0,00
Aa
Kancil 57,33
Ca
57,33
Ca
37,33
BCab
4,00
A
b
a
0,00
Aa
Kelinci 98,67
Cb
96,00
Cb
60,00
Bb
10,67
Aa
0,00
Aa
HSD
0,05
33,70
Peanut
varieties
Speed Growth (% etmal
-1
)
Local
Aceh
26,69
Db
23,82
Cb
8,25
Bb
0,41
Aa
0,00
Aa
Talam 2 40,79
Dc
39,45
D
d
7,09
Cab
2,31
Bb
0,00
Aa
Kancil 20,31
Ca
18,99
Ca
6,41
Ba
1,17
Aab
0,00
Aa
Kelinci 45,07 32,33 13,70 0,00 0,00
D
d
Cc Bc Aa Aa
HSD
0,05
1,81
Peanut
varieties
Time required to achieve total
germination 50% (day)
Local
Aceh
0,96
Aab
1,32
Ab
1,26
Ab
1,42
Aa
1,46
Aa
Talam 2 0,68
ABa
0,50
Aa
0,66
ABa
1,02
A
b
a
1,20
Ba
Kancil 1,47
Ab
1,49
Ab
1,48
Ab
1,50
Aa
1,50
Aa
Kelinci 1,49
Ab
1,46
Ab
1,46
Ab
1,47
Aa
1,93
Aa
HSD
0,05
0,54
Peanut
varieties
Dry weight of normal seedling (gram)
Local
Aceh
5,36
Ca
8,54
Da
3,84
Ba
0,26
Aa
0,00
Aa
Talam 2 11,68
Db
8,15
Ca
2,80
Ba
0,73
Aa
0,00
Aa
Kancil
6,56 Ca
8,27
Da
3,41
Ba
0,39
Aa
0,00
Aa
Kelinci 14,55
Dc
12,11
Cb
5,54
Bb
0,00
Aa
0,00
Aa
HSD
0,05
1,22
Description: The numbers followed by the same letter in the same
row (capital letter) and the same (lower case) columns are not
significant at the 5% level (HSD Test 0.05)
Increased salinity concentration adversely
affected seed viability and vigor that is in the
parameters of maximum growth potential,
germination rate, growth speed rate, time required to
achieve total germination 50% (T50) and root length
of normal root germination in all four varieties
studied. The results showed that Kelinci, viability
and vigor varieties grew better or had tolerant
properties than Local varieties, Talam 2 and Kancil
on increasing the concentration of NaCl to 3 g L
-1
.
Waskom (2003) states that soil salinity can
inhibit seed germination, irregular growth in
agricultural crops such as beans and onions.
Meanwhile, according to Noor (2004) high salt
solubility can inhibit the absorption of nutrients and
water by plants due to increased osmotic. In
particular, high salt levels can lead to plant
poisoning.
According to Adisyahputra et al., (2004) drought
stress in the seed germination phase will increase
with increasing salinity levels. The presence of salt
in the growing medium negatively affects the ability
of seed germinating. The mechanisms of salinity
influence on seed germination include two
mechanisms: (1) high osmosis media pressure so
that the seeds are difficult to absorb water and (2)
the toxic effects of salt-making ions (Albregts and
Howard, 1972).
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
176

Another possible influence of the NaCl salt
solution is suspected to be poisoning by Na
+
and Cl
-
ions. The ions generally can achieve sufficient
concentration of solution to cause osmotic problems
in plants without first having specific toxicities that
cause death are chloride and sulphate. Sodium will
affect soil properties if present in excessive state.
This resulted in the seeds difficult to absorb water so
that the germination process will be inhibited.
The presence of salt in the growing medium also
shows an adverse effect on germination, because of
its concentration on germination media, resulting in
changes in enzyme activity either directly or
reducing the potential for water. Bad influence of
salts for plants is generally indirectly through
increased osmotic pressure in groundwater making it
difficult for plants to absorb water, especially for
plant sprouts and roots. So the effect is the same as
dry land (Harnowo, 2002).
Sipayung (2003) stated that the level of stress
experienced by plants varies in different species with
unequal tolerance to different salt concentrations.
According to Yuniati (2004), the growth response to
salinity is considered the basis of evaluation for
tolerance. Different individuals will respond
differently to the salinity stress provided. Karajol
and Naik (2011) say that salinity-tolerant varieties
that germinate quickly under normal conditions like
as germinate under saline conditions. Varieties that
have higher germination rates have more salinity
tolerance opportunities. Inhibition of canopy and
root growth is a common response to salinity stress
and is an important indicator for assessing crop
tolerance. Root is the first organ exposed to salinity
stress so its role in tolerance is very important
especially in the process of water absorption.
Inhibition of canopy and root growth is a common
response to salinity stress and is an important
indicator for assessing crop tolerance. This
phenomenon can be a simulation of a condition of
water deprivation or drought that affects plant
compensation prolonging the rooting part.
4 CONCLUSIONS
Talam 2 and Kelinci showed more tolerance to
salinity stress compared with Kancil and Local Aceh
varieties based on all parameters of viability and
vigor of peanut seed. Increasing salinity at simulated
conditions above 3 g L
-1
or equivalent to 3.99 dSm
-1
resulted in a significantly decreased germination
value of four varieties.
REFERENCES
Adisyahputra, S. Ilyas dan Sudarsono. 2004. Penggunaan
polyethylene glycole untuk menguji tanggap kacang
tanah terhadap cekaman kekeringan pada tahapan
perkecambahan. Departemen Agronomi dan
Hortikultura. Institut Pertanian Bogor. Bogor.
Albregts, E. C. dan C. M. Howard. 1972. Influence of
temperature and moisture stress from sodium chloride
salinization on okra emergence. Crop Sci. 836-837.
Badan Pusat Statistik. 2016. Data produksi, luas panen,
dan produktivitas palawija di indonesia 2013 – 2016.
www.bps.go.id. [13 Oktober 2017]
Danuwarsa. 2006. Analisis proksimat dan asam lemak
pada beberapa komoditas kacang-kacangan. Buletin
Ilmu Pertanian. Vol 11 (1): 5-8
Duan, D., X. Liu, M.A. Khan, and B. Gul. 2004. Effect of
salt and water stress on the germination of
Chenopodium glaucum L. seed. Pak J. Bot. 36 (4) :
793-800.
Erinnovita, M. Sari, D. Guntoro. 2008. Invigorsi benih
untuk memperbaiki perkecambahan kacang panjang
(Vigna unguiculata Hask ssp sesquipendalis) pada
cekaman salinitas. Bul. Agro (36) 214-220.
Flowers, T.J. and S.A. Flowers. 2005. Why does salinity
pose such as a difficult problem for plant bredding.
Water Management. 78: 15-24.
Hajar, A.S., M.M. Heikal, Y.M. Maghrabi and R.A.
Abuzinadah. 1993. Responses of peanut to salinity
stress. K.A.U. Scie. (5): 5-13.
Halimursyadah, A.I. Hereri, dan A. Hafnizar. 2013.
Penggunaan polyethylene glycole sebagai media
simulasi cekaman kekeringan terhadap viabilitas dan
vigor beberapa varietas benih kacang tanah (Arachis
hypogaea L.) pada stadia perkecambahan. J. Floratek
8: 73 – 79.
Harnowo, D. 2002. Pertumbuhan kecambah kedelai akibat
cekaman salinitas. Jakarta: BPPT. 192-202
Hasanuddin. 2015. Pengujian model simulasi vigor
kekuatan tumbuh benih kedelai (Glycine max L.
merril) pada lahan salin. Jurnal Floratek 10(2):72-77.
Karajol, K., dan G.R. Naik. 2011. Seed germination rate
as a phenotypical marker for the selection of NaCl
tolerant cultivars in pigeon pea (Cajanus cajan (L.)
Millsp.). World J. of Sci. and Tech. 1(2): 1-8.
Kasno, A. dan D. Harnowo. 2014. Karakeristik varietas
unggul kacang tanah dan adopsinya oleh petani. Balai
Penelitian Tanaman aneka Kacang dan Umbi,
Malang.
Khan, M.A., I.A. Ungar and A.M. Showalter. 2000.
Effects of sodium chloride treatments on growth and
ion accumulation of the halophyte haloxylon
recurvum. Coummun. Soil Sci. Plant Anal. 31: 2763–
2774.
Marusoh, S.T. 2008. Uji cekaman garam (NaCl) pada
perkecambahan beberapa kultivar kedelai (Glycine
max (L.) Merril). Skripsi. Universitas Islam Negeri
Malang. Malang.
Munns. 2002. Comparative physiology of salt and water
stress. Plant Cell Environt. 25: 239-250.
Simulation of Stress Salinity Conditions in the Phase of Germination of National and Local Aceh Peanut Variety
177

Noor, M. 2004. Lahan rawa, sifat dan pengelolaan tanah
bermasalah sulfat masam. Raja Grafindo Persada.
Jakarta.
Pohan, FA. 2005. Uji ketahanan pada beberapa kultivar
kacang tanah (Arachis hypogea L.) terhadap salinitas.
Thesis.Program Pascasarjana.USU.
Purnomo. 2007. Keragaan varietas kacang tanah unggul
di lahan ultisol masam. peningkatan produksi kacang-
kacangan dan umbi-umbian mendukung kemandirian
pangan. Badan Penelitian Dan Pengembangan
Pertanian. Pusat Penelitian dan Pengembangan
Tanaman Pangan. Bogor.
Rini, D.S., Mustikowe., dan Surtiningsih. 2005. Respon
perkecambahan benih sorgum (Sorgum bicolor L.
Moerch) terhadap perlakuan osmoconditioning dalam
mengatasi cekaman salinitas. Jurnal Biologi 7(6) :307-
313.
Sipayung, R. 2003. Stres garam dan mekanisme toleransi
tanaman. Skripsi. Universitas Sumatera Utara. Medan.
Taufiq, A dan Purwaningrahayu R. D. 2013. Tanggap
Varietas kacang hijau terhadap cekaman salinitas.
Jurnal Penelitian Tanaman Pangan. 32(3):159-170
Waskom, R. 2003. Improved growth of salinity stressed
soybean after Inoculation with salt pre-treated
mycorrhizal fungi. Plant Physiology Elsevier.
http://www.science direct.com diakses pada tanggal 13
Agustus 2017.
Widoretno, W. 2002. Efektivitas polietilena glikol untuk
mengevaluasi tanggapan genotipe kedelai terhadap
cekaman kekeringan pada fase perkecambahan.
Hayati. 9(2): 33–36.
Wijayanti, W., Taryono dan Toekidjo. 2014. Keragaan 29
galur kacang tanah (Arachis hypogaea L.) pada
kondisi salin. Vegetalika. 3(4): 40 – 51.
Yuniati, R. 2004. Penapisan galur kedelai Glycine max
(L.) Merrill toleran terhadap NaCl untuk penanaman
di lahan salin. Makara Sains. 8(1): 22.
Yadav, S., I. Mohammad, A. Aqil dan H. Shamsul. 2011.
Causes of salinity and plant manifestations to salt
stress: A review. Journal Environtmental Biology
32:667–685.
Zakaria, S. dan C.M. Fitriani. 2006. Hubungan antara dua
metode sortasi dengan viabilitas dan vigor benih
kacang tanah (Arachis hypogaea L.) serta aplikasinya
untuk pendugaan ketahanan salinitas. Jurnal Floratek
(2): 1-11.
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
178
