
Study of Free Radical Scavenging Activity on Film with Addition of
Silver Nanoparticle Synthesized using Papaya Leaves and Fruits
Eveline
1*
, A. Herry Cahyana
2
, and Jessica
3
1
Lecturer, Food Technology Department, Faculty of Sains and Technology, University of Pelita Harapan,
Jl. MH. Thamrin Boulevard 1100 Lippo Village, Kelapa Dua, Karawaci, Tangerang, Indonesia
2
Senior Lecturers, Chemistry Department, Faculty of Mathematics and Natural Science, University of Indonesia,
Jl. Margonda Raya, Beji, Pondok Cina, Depok, Indonesia
3
Alumnus, Food Technology Department, Faculty of Sains and Technology, University of Pelita Harapan,
Jl. MH. Thamrin Boulevard 1100 Lippo Village, Kelapa Dua, Karawaci, Tangerang, Indonesia
Keywords: AgNPs, antioxidants, Carica_papaya, free_radical_scavenging, nanoparticles
Abstract: Silver nanoparticles (AgNPs) have been the focus of research in terms of catalyst, antimicrobial, and
biomaterial production due to the potential of proteins, amino acid residues, free radical anions (antioxidants),
and eukaryotic cell receptors. The synthesis of AgNPs using plant extracts such as leaf and papaya fruit is one
of the safest nanoparticles and has a wide metabolite for reduction, so it is timely to apply to food packaging
materials that have antioxidant activity. Initially, phytochemical compounds (phenolic and flavonoids) of
papaya leaves and fruit were each extracted with air and ethanol solvents (1:0, 1:1, 0:1). The papaya leaf was
selected as a better source of phytochemical compounds than papaya, while ethanol solvent was determined
as the best solvent, based on analysis on plant extract (solids content, total phenolic, total flavonoids,
antioxidant activity), and on AgNPs synthesis analysis (yield, level of inhibition, and characteristics of
AgNPs) with successive results of 1.52%, 659.65 mg GAE/g, 614.04 mg QE/g, 130.3 ppm, 15.76%, 82.44%,
and morphology according to nanoparticle criteria (round; 97.92 nm [SEM]; 79.92 nm [PSA]). AgNPs were
selected then homogenized with AgNPs nanoparticles (0.0, 0.4, 0.6, 0.8, 1.0%). The addition of 1%
nanoparticles gave the best characteristics of free radical resistant packaging films (4.5% starch and 1.5%
glycerol), 1.52 MPa tensile strength, 22.37% elongation, and 18.94% free radical inhibition (42 times greater
than control).
1 INTRODUCTION
Nanoparticles is a nanotechnology development that
has been interesting in recent years in various fields.
Not only in the fields of physics, chemistry, biology
and engineering, but also applications in the
environmental, biomedical, electronic, and optical
fields (Wahyudi, et al., 2011). Shefar (2007) added
that in the biomedical field, nanoparticles can
facilitate the entry of drug compounds into micro or
nanometer-sized tissue through blood transport,
manipulate temperatures when hypothermia, and
utilization in technique of Magnetic Resonance
Inmaging (MRI).
In addition to gold nanoparticles, silver
nanoparticles (AgNPs) are nanoparticles that are
often used in research. AgNPs have higher absorption
molar coefficients than other nanoparticles such as
iron, zinc, platinum, palladium nanoparticles. Optical
properties and measurements of AgNPs with better
UV-Vis spectrophotometers (Caro, et al., 2010). The
synthesis of AgNPs is increasingly developed
through chemical reduction that is environmentally
friendly, harmless, and relatively inexpensive, using
plant extract as a reducing agent (Sathishukumar, et
al., 2009).
Because of its potential as a micro particle that is
considered safe for the health of the body and
environmentally friendly, AgNPs are recommended
to also play a role in the field of food, such as the
manufacture of free radical food packaging products.
An antioxidant compound as an antidote to free
radicals inserted in nanoparticles package can be
extracted from leaves and papaya fruit (Carica
papaya L.). According to Duthie (2000), Zuhair, et al.
(2013), and Philip, et al., (2011); organic compounds
including phytochemical compounds that are
78
Eveline, ., Cahyana, A. and Lesmana, J.
Study of Free Radical Scavenging Activity on Film with Addition of Silver Nanoparticle Synthesized using Papaya Leaves and Fruits.
DOI: 10.5220/0010038600780083
In Proceedings of the 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and Technology (ICEST 2018), pages 78-83
ISBN: 978-989-758-496-1
Copyright
c
2021 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved

antioxidants in the plant part have the ability to be a
metal ion agent in the biosynthesis process. Jain, et al.
(2009) and Banala, et al. (2015) added that papaya
leaves can be a good source of free radical-free
compounds in the synthesis of AgNPs.
Handayani, et al., (2010) in his research extracted
antioxidant phytochemical compounds by using
water solvents (polar). Therefore, in this study, it is
designed that the extraction of plant phytochemicals
is used in different solvents of polarity to study if
other phytochemicals exist in plants that are insoluble
in water polarity and may be reducing agents in AgNP
synthesis; so the solvent used is water and ethanol
with a ratio of 1:0, 1:1, and 0:1. Analysis of plant
extract (solid content, total phenolic, total flavonoid,
antioxidant activity) and analysis of AgNPs (yield,
inhibition level, and AgNPs characteristics) were
performed to determine the best solvent ratio so that
further AgNPs of the selected ratio could be applied
in the manufacture of packaging film at the next
research stage.
AgNPs of the ratio yielding the best analysis were
then applied to the creation of the packaging film
(4.5% cassava starch and 1.5% glycerol) with
concentrations of 0.0, 0.4, 0.6, 0.8, 1.0%. The best
concentration determination was performed based on
tensile strength analysis, elongation, antioxidant
activity. The existence of this study is expected to be
a study that can continue to move the development of
nanoparticles not only in the area of food packaging
but also able to develop added value in other areas of
food technology.
2 MATERIALS AND METHODS
2.1 Materials
The materials: leaf and papaya fruit (Carica papaya
L.), methanol, distilled water, distilled water, tissue
paper, AgNO3 powder, glycerol, aluminum chlorite
2%, sodium hydydroxide solution (NaOH), and
Diphenyl-picryl-hydrazyl (DPPH).
2.2 Methods
The research consists of two stages (stages 1 and 2)
and is preceded by the sample preparation stage. The
sample preparation stage was performed to prepare
dried papaya leaves and fruit to be used in the
extraction, including: leaching each 30 grams of
leaves and papaya fruit with distilled water, cutting,
drying in the dryer cabinet (30°C, 24 hours), sieving
(40 mesh shifter).
Phase 1 study (Figure 1) began with the extraction
of dried papaya leaves and fruit (30 grams each) with
water solvent and ethanol ([1:0, 1:1, 0:1],
sample:solvent 1:8); extraction (Microwave Assited
Extraction [MAE], 450 watts, 13.5 minutes);
filtration (Whatman No. 1) resulting in extracts. The
analysis of the extract was carried out, ie: solids
content, total phenolic (Handayani, et al., 2011), total
flavonoids (Handayani, et al., 2014), antioxidant
activity (Nahak and Suhu, 2011 with Modification).
Meanwhile, 50 ml of AgNO
3
0.6 M solution was
prepared by dissolving 5 grams of AgNO
3
powder in
50 ml of distilled water. The extract and the AgNO3
solution were then homogenized (pH 8, 75-80°C, 3
hours) and centrifuged (5000 rpm, 15 min), the
resulting precipitate was applied to the watch glass
and dried (oven, 24 h, 60°C) AgNPs. AgNPs analysis
was performed: level of inhibition, and characteristics
of AgNPs (Scanning Electron Microscopy [SEM]
(Jain et al., 2009) and Particle Size Analyzer [PSA]).
Analysis of extracts and AgNPs is the basis for
determining the solvent ratio that produces AgNPs
with the best antioxidant activity with characteristics
appropriate to nanoparticle criteria according to
Albert et al. (2006).
In Phase 2 of the study (Figure 2), the synthesis of
AgNPs with the highest free radical retardant activity
in the first phase of the study was made as a silver
nanoparticles agent (0, 0.4, 0.6, 0.8, 1.0%) in the
making of free radical scavenging activity film (4.5%
cassava starch and 1.5% glycerol). Analysis of
inhibition ability (Nahak and Suhu, 2011 with
Modification) was performed to determine the best
concentration of AgNPs. Films with best inhibition
ability were then analyzed for tensile strength and
elongation (Zhong and Xia, 2008).
2.3 Experimental Design
The experimental design of phase 1 was a 1-factor
(solvent) Randomized Complete Design (solvent)
containing 3 levels (water, water: ethanol, ethanol 1:0
[A1], 1:1 [A2], 0:1 [A3]) with repetition 2 times. In
stage 1, a t-test of the two independent samples was
obtained which was the statistically highest yield on
each parameter of analysis to determine one part of
the plant (leaf or fruit) that had the best antioxidant
activity and characteristics as AgNPs. The Phase 2
study used Completely Randomized Design 1 factor
(AgNPs concentration) consisting of 5 levels (0.0
[A
1
], 0.4 [A
2
], 0.6 [A
3
], 0.8 [A
4
], 1.0% [A
5
]) with 2
times of repetition.
Study of Free Radical Scavenging Activity on Film with Addition of Silver Nanoparticle Synthesized using Papaya Leaves and Fruits
79
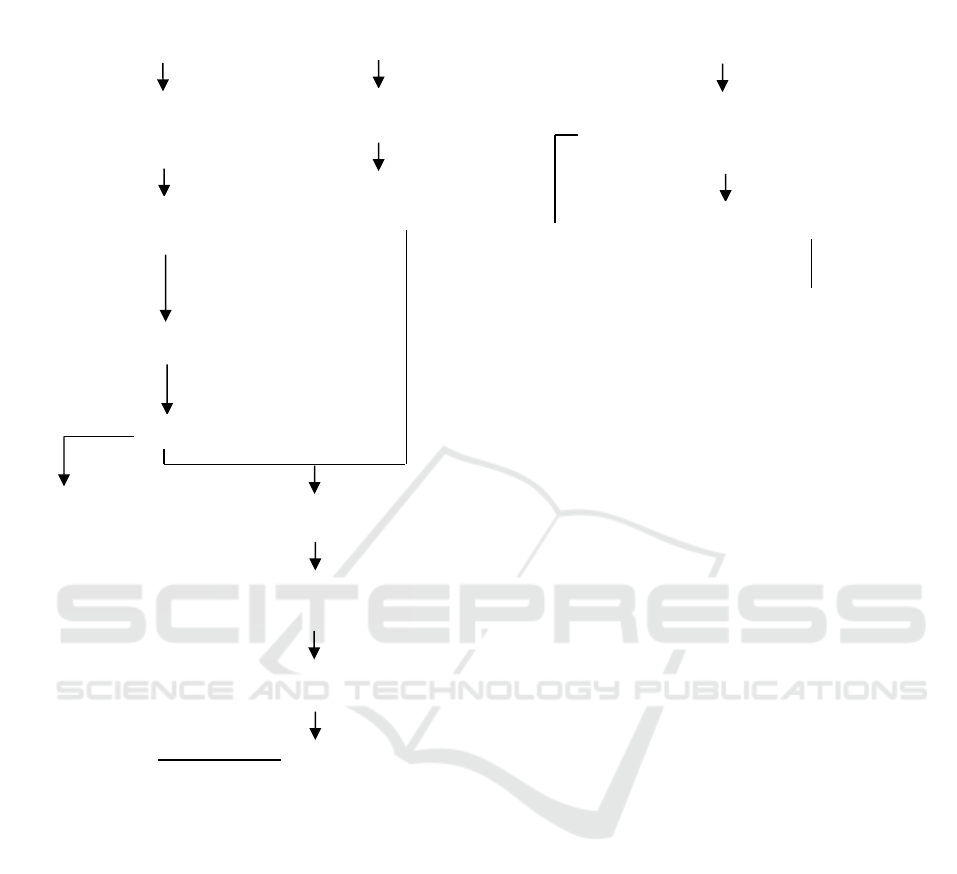
Figure 1. Phase 1 Research Flowchart
Figure 2. Phase 2 Research Flowchart
3 RESULT
3.1 Phase 1
Phase 1 study was conducted to determine the
extraction solvent (water and ethanol 1:0, 1:1, 0:1)
and to determine the part of the plant (leaf or fruit)
that can produce the best free radical activity of
AgNPs. The analysis of the extract (solid content,
total phenolic, total flavonoid, antioxidant activity)
and AgNPs analysis (inhibitory level) were
performed to achieve these objectives, the AgNPs
characteristics of selected plant and solvent sections
were then analyzed SEM and PSA.
The result of statistical test showed the influence
of solvent type to the solid content both on leaves and
papaya fruit (p<0.05). Table 1 shows that the solids
of the extract with the ethanol solvent were the
lowest, both on the leaves and on the papaya fruit
(1.52 and 0.79%). The presence of water in a solution
can increase the yield due to components other than
extracted phenolics, protein solubility and
carbohydrates in water (Zielinski, 2016). Statistical
results with t-test showed that there were differences
of solids content in leaf and fruit extract (p<0.05).
According to Saran and Choudhary (2013), papaya
fruit contains thiamine, riboflavin, nicain, tryptophan,
methionine, lysine, magnesium, and phosphoric acid
not contained in papaya leaves as a contribution of
solids to the extract.
The results of statistical test also showed that the
solvent ratio had an effect on phenolic content, both
on leaf extract and papaya fruit (p<0.05). Table 1
shows that ethanol solvent yields the highest total
Synthesis of AgNPs with the highest free radical
activity in the first phase of the study
Silver nanoparticles agent (0, 0.4, 0.6, 0.8, 1.0%)
in the making of free radical scavenging activity
film
(4.5% cassava starch, 1.5% glycerol)
Analysis:
- Inhibition ability
Films with best inhibition ability
Analysis:
- Tensile strength
- Elon
g
ation
Dried papaya leaves and fruits
(30 grams each)
Extraction with water solvent
and ethanol ([1:0, 1:1, 0:1],
sample:solvent 1:8)
Extraction using MAE,
450 watts,
13.5 minutes)
Filtration using Whatman No. 1
AgNO
3
powder
5 grams
Dissolving with
50 ml distilled water
50 ml of AgNO
3
0.6 M solution
AgNPs
Analysis
- Solids content
- Total phenolic
- Total flavonoids
- Antioxidant activity
Centrifugation
(5000 rpm, 15 min)
Homogenization
(pH 8, 75-80°C, 3 hours)
Resulting precipitate was applied to the
watch glass and dried (oven, 24 h, 60°C)
extracts
Analysis
- Level of inhibition
- Characteristicsof AgNPs
- Particle Size Analyzer
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
80

phenolic in papaya leaf and papaya extracts compared
to water solvents and water-ethanol mixtures (659.65
and 245.12 mg GAE/g). The ethanol solvent is a
semi-polar solvent which is advantageous for
phenolic and flavonoid extracts (Chirinos, 2007). The
results of t-test on total phenolic leaf and papaya fruit
revealed that papaya leaf extract contained higher
total phenolic than fruit extract (p<0.05). According
to Johnson, et al. (2008) papaya leaves contain
alkaloid components, antokuinon, catechol,
flavonoid, phenolic, saponin, steriods, tannins,
triterpenoids, polyphenols, pholenicins are
compounds that contribute to the total phenolics of a
material.
The result of statistic test showed that the solvent
ratio influenced the total flavonoids of leaf extract
and papaya fruit (p<0.05). Table 1 shows that ethanol
solvent yields leaf extract and papaya fruit with the
highest total flavonoids (614.04 and 135.48 mg
QAE/g). Wang, et al. (2008) and Velioglu, et al.
(1998) revealed that papaya leaves and fruits contain
biologically soluble flavonoids in ethanol because
flavonoids correlate to total phenolics due to
flavonoid dominance of phenolic groups in papaya
leaf and papaya extracts. T-test between leaf extract
and papaya fruit showed papaya leaf extract
containing more flavonoids than papaya (p<0.05).
Most of the biological activity of papaya leaves is
contributed by kaemferol and quercetin which are
more soluble in ethanol (Maisarah, et al., 2013).
The result of statistic test showed that the solvent
ratio had an effect on the free radical activity of
papaya leaf extract and papaya fruit (p<0.05). Table
1 shows the ethanol solvent yielding the lowest IC
50
value of both the leaf extract and papaya fruit (130.3
and 508.65 ppm). Do, et al. (2014) in his study also
found that ethanol solvents gave the lowest IC
50
values compared to the use of other solvents. This
means, ethanol solvents are able to extract more
compounds that potentially counteract free radicals.
Lie, et al. (2006) and Hou, et al. (2003) stated that
phenolic and flavonoid components contain hydroxyl
acting as proton donors in free radical (antioxidant)
retention. T-test of antioxidant activity of leaf extract
and papaya fruit showed leaf extract has greater
potential to counteract free radical, IC
50
value is
smaller than IC
50
papaya fruit. This is related to the
total phenolic and flavonoid yields that are also
higher in papaya leaf extracts, both of these
compounds contribute to the antioxidant activity of a
substance. Hanani, et al. (2005) added that if a
material has an IC50 value <200 g/ml, then the
antioxidant activity is strong.
The result of statistical test showed that the yield
of AgNPs was influenced by the solvent ratio of both
the leaf extract and the papaya fruit (p<0.05). Table 1
shows the leaf extract from the ethanol solvent
yielding the lowest yield of AgNPs (15.76%), while
the yield of AgNPs reached the highest value on fruit
extract (7.39%). T-test between yield of extract on
leaf and fruit showed that papaya leaf yield higher
rendement than papaya fruit (p<0.05). Phenolic
content and flavonoid in leaves are higher than in
fruits thus increasing the strength of Ag
+
reduction in
the process of forming nanoparticles and increasing
the number of nanoparticles formed (Do, et al., 2014).
Table 1. Phase 1 Test Result
Parameters
Wate
r
:Ethanol
Leaves Extract Fruit Extract
1:0 1:1 0:1 1:0 1:1 0:1
Solid Content
(
%
)
3.61±0.95
b
3.80±0.38
b
1.52±0.15
a
4.53±0.14
b
4.64±0.21
b
0.79±0.18
a
Total Phenolic (mg
GAE/g)
86.86±2.05
a
137.21±4.49
b
659.65±5.62
c
15.81±1.09
a
19.22±0.50
a
245.12±3.18
b
Total Flavonoid (mg
QAE/
g)
97.79±0.98
a
153.18±2.00
b
614.04±4.07
c
57.21±2.10
a
108.29±1.27
b
135.48±2.86
c
IC
50
(ppm) 278.42±6.00
c
210.63±6.65
b
130.30±0.90
a
1139.21±7.04
c
912.85±7.33
b
508.65±8.57
a
Yiel
d
(%) 10.66±0.39
a
13.11±0.41
b
15.76±0.45
c
3.39±0.37
a
5.67±0.39
b
5.67±0.39
b
Inhibition
(
%
)
40.95±1.21
a
55.88±0.95
b
82.44±0.66
c
36.52±1.37
a
43.66±1.33
b
53.17±0.22
c
Note: - Different notation showed there was significant difference (p<0.05)
- No comparison between parameter analysis
- Different notation not showed t-test result
The result of statistic test of solvent type shows
the solvent ratio influence the inhibition level of leaf
extract and papaya fruit (p<0.05). Table 1 shows the
two extracts with ethanol solvent having the highest
rates of inhibition compared to other solvents, both in
leaf extract and fruit extract (82.44 and 53.17%). This
is associated with greater antioxidant activity
resulting in extraction with alcohol solvents than
other solvents. According to Ahmad and Sharma
(2012), the mechanism of free radical retardation
Study of Free Radical Scavenging Activity on Film with Addition of Silver Nanoparticle Synthesized using Papaya Leaves and Fruits
81

activity starts from AgNPs cation which gets
electrostatic appeal from bioactive components of
plant extracts as a result of reduced cations, while the
phytochemicals covered by AgNPs and their
bioactivity rose synergistically:
AgNO
3
→ Ag
+
+ NO
3
-
; e + Ag
+
→ Ag
T-test inhibition level of leaf extract and papaya fruit
showed papaya leaf extract had greater inhibition rate
than fruit (p<0.05). It is associated with leaf extracts
that have a potent antioxidant activity greater than
fruit.
Based on the analysis of AgNPs extract and
nanoparticles, it was determined that nanoparticles
with papaya leaf extract extracted by ethanol have the
potential to counteract free radicals better than other
solvents. AgNPs were then analyzed using SEM and
PSA characteristics. The results of AgNPs
characteristic test with SEM showed agrous
morphology of 97.92 nm, spherical, and there were
coagulated particles (Figure 3). Test with PSA is a
strengthening test of AgNPs characteristics with
SEM. In the PSA test, AgNPs appears to be 79.92 nm
in size (Figure 3). In both the SEM and PSA tests, the
size of AgNPs nanoparticles is not out of nanoparticle
size requirements according to Albert, et al. (2006),
which is 1-100 nm.
Figure 3. AgNPs Morphology
3.2 Phase 2
The second phase of the research is the application
stage of AgNPs that has been synthesized in the
previous stage on the free radical container
packaging. The packaging film material formulation
comprises 4.5% starch, 1.5% glycerol, and the best
AgNPs in the addition of 0.0, 0.4, 0.6, 0.8, 1.0%. The
statistical test showed the effect of AgNPs
concentration on free radical inhibition of packaging
film (p<0.05). Table 2 shows a concentration of
AgNPs of 1% capable of inhibiting the largest free
radical (18.94%) and increased by 42 times compared
to control (0.45%). The higher concentration of
AgNPs is added, the film structure is more compact
and flexible (not rigid), and clear.
AgNPs with 1% concentration were then analyzed
tensile strength and elongation with consecutive
values were 1.52±0.07 Mpa and 22.37±4.12%.
Warkoyo (2014) said a film may have a strong
breakup if it is 31.65%. Therefore, further research is
needed on better formulations, especially of the type
of material and the concentration of hydrocolloids,
lipids, and edible composite films of free radical in
order to achieve the value.
Table 2. Phase 2 test Result
Concentration of A
g
NPs (%) Inhibition (%)
0.0 0.45 ± 0.36
a
0.4 4.88 ± 0.77
b
0.6 8.17 ± 0.63
c
0.8 14.77 ± 0.35
d
1.0 18.94 ± 0.36
e
Note: - Different notation showed there was
significant difference (p<0.05)
4 CONCLUSIONS
The papaya leaf with ethanol solvent was chosen as a
source of phytochemical and solvent compounds
capable of producing the best free radical extract and
AgNPs based on 1.52% solids, total phenolic 659.65
mg GAE/g, total flavonoids 614.04 mg QE/g,
antioxidant activity 130.3 ppm, AgNPs 15.76%, level
of inhibition AgNPs 82.44%, and morphology
according to nanoparticle criteria (round, 97.92 nm
[SEM]; 79.92 nm [PSA]). As much as 1% of the best
AgNPs are able to produce free radical-free films
with 1.52 MPa tensile strength, 22.37% elongation,
and able to inhibit free radicals by 18.94% (up 42
times compared to controls).
REFERENCES
Albert, M. A., Evans, C. W., and Ratson, C. L. 2006. Green
Chemistry and the Health Implications of
Nanoparticles. Green Chem 8: 417-432.
Banala, Reddy, R., Nagati, V. B., and Karnati, P. R. 2015.
Green Synthesis and Characterization of Carica Papaya
Leaf Extract Coated Silver Nanoparticles through X-
ray Diffraction, Electron Microscopy and Evaluation of
Bactericidal Properties. Journal of Biological Science
22:637-644.
Caro. C., Castillo, P. M., Klippstein R., Pozodan D., and
Zaderenco A. P. 2010. Silver Nanoparticles: Sensing
ICEST 2018 - 3rd International Conference of Computer, Environment, Agriculture, Social Science, Health Science, Engineering and
Technology
82

and Imaging Applications Silver Nanoparticle 2: 201-
223.
Chirinos, R., Rogez, H., Campos, D., Pedreschi, R., and
Larondelle, Y. 2007. Optimization of Extraction
Conditions of Antioxidant Phenolic Compounds from
Mashua (Tropaeolum tuberosum Ruiz & Pavon) Tuber.
Technol 55:217-225.
Do, Q. D., and Angkawijaya, A. E., Tran-Nguyen, P. L.,
Huynh, L. H., Soetaredjo, F. E., Ismadji, S., and Ju, Y.
2014. Effect of Extraction Solvent on Total Phenol
Content, Total Flavonoid Content, and Antioxidant
Activity of Limnophila Aromatica. Journal of Food
and Drug Analysis 22: 296-302.
Duthie, G., and Crozier, A. 2000. Plant-derived Phenolic
Antioxidants. Curr Opin Lipidol 11:43-47.
Hanani, E., Abdul, M., and Ryany, S. 2005. Identifikasi
Senyawa Antioksidan dalam Spons Callyspongia sp
dari Kepulauan Seribu. Majalah Ilmu Kefarmasian
Universitas Indonesia Vol. II (3): 127-133.
Handayani, D., Mun’im, A., and Ranti, A. S. 2014.
Optimation of Green Tea Waste Extraction Using
Microwave Assisted Extraction to Yield Green Tea
Extract. Herbal Magister, Faculty of Pharmacy
Universitas Indonesia 19: 29-35.
Jain, Devendra, Hemant Kumar Daima, Sumita
Kachhwaha, S. L. Kothari. 2009. Synthesis of Plant-
Mediated Silver Nanoparticles Using Papaya Fruit
Extract and Evaluation of their Anti Microbial
Activities. Digest Journal of Nanomaterials and
Biostructures 4: 723-727.
Johnson, U. E., Akwaji, P. I., Aniendi-Abasi, M., Effiong,
U. S., and O. E Effiom. 2014. Phytohemical
Composition, Antimicrobial Effect of Azadirachta
Indica and Carica Papaya Extracts on Fungi Isolated
from Gmelina Arborea Seedlings. International
Journal of Phytopathology 3: 109-115.
Lie, B. B., Smith, B., and Hossain, Md. M. 2006. Extraction
of Phenolics from Citrus Peels: I. Solvent Extraction
Method. Separation and Purufication Technology 48:
182-188.
Maisarah, A. M., Nurul, A. B., Asmah, R, and Fauziah, O.
2013. Antioxidant Analysis of Different Parts od Carica
Papaya. International Food Research Jounal 20: 1043-
1048.
Nahak, G, and Suhu, R. K. 2011. Evaluation of Antioxidant
Activity in Ethanolic Extract of Five Curcuma Species.
International Research Journal of Pharmacy 2: 243-
248.
Philip, D. Unni, C., Aromal, S. S., and Vidhu, V. K. 2011.
Murayya keonigii Leaf-Assited Rapid Green Syntesis of
Silver and Gold Particles, Spectrochemica Acta Part A:
Molecular and Biomolecular 78: 899-904.
Saran, P. L., and Choudhary, R. 2013. Drug Bioavailability
and Traditional Medicaments of Commercially
Available Papaya: A Review. Indian Agricultural
Research Institute 23: 613-621.
Sathiskumar, M., Snecha, K., Won, S. –W., Cho, C. W.,
Kim, S., Yun, Y., and –S. 2009, Cinnamon Zeylanicum
Bark Exract and Powder Mediated Green Synthesis of
Nanocrystalline Silver Particles and Its Bactericidal
Activity, Journal of Colloids and Surfaces B:
Biointerfaces
73: 332-338.
Shefar, A. 2007. The Application of Nanotechnology in the
Food Industry.New Jersey: Salvona Technology Inc.
Velioglu, Y. S., Mazza, G, and Oomah, B. D. 1998.
Antioxidant Activity and Total Phenolic in Selected
Fruits, Vegetables, and Grain Products. Journal of
Agricultural and Food Chemistry 46:4113-4117.
Wahyudi, T., Sugiyana D., and Helmy Q. 2011. Sintesis
Nanopartikel Perak dan Uji Aktivitasnya terhadap
Bakteri E. coli dan S. aureus, Arena Tekstil, 26:1-60.
Wang, J., Wu, F. A., Zhao, H., Liu, L., and Wu, Q. S. 2008.
Isolation of Flavonoids from Mulberry (Morus alba L.)
Leaves with Macroporous Resins. African Journal of
Biotechnology 7: 2147-2155.
Warkoyo., Rahardjo, B., Marseno, D. W., and Karyadi, J.
N. W. 2014. Sifat Fisik, Mekanik, dan Barrrier Edible
Film Berbasis Pati Umbi Kimpul (Xanthosoma
sagittifolium) yang diinkorporasi dengan Kalium
Sorbat. Argitech 34:115-127.
Zhong, Q. P., and Xia, W. S. 2008. Physicochemical
Properties of Edible and Preservative Films from
Chitosan/Cassava Starch/Gelatin Blend Plasticized
with Glycerol. Food Technol. Biotechnol. 46:262-269.
Zielinski, H., and Kozlowska, H. 2000. Antioxidant
Activity and Total Phenolics in Selected Area Grains
and their Different Morphological Fractions. Journal
Agriculture Food Chemistry 48:2008-2016.
Zuhair, R.A, A. Aminah, A.M. Sahilah, D. Eqbal. 2013.
Antioxidant Activity and Physicochemical Properties
Changes of Papaya (Carica papaya . cv. Hong Kong)
during Different Ripening Stage. Int. Food Res. J., 20:
1652-1659.
Study of Free Radical Scavenging Activity on Film with Addition of Silver Nanoparticle Synthesized using Papaya Leaves and Fruits
83
