
Development of Electronic Lab-book for
College Chemistry-Experiment - S
N
1 & S
N
2 Reactions -
Akira Ikuo,
Yuki Toyama, Yusuke Yoshinaga, and Haruo Ogawa
Department of Chemistry, Tokyo Gakugei University, Tokyo 184-8501, Japan
Keywords: CG, Visualization, Tert-Butyl Chloride, 1-Chlorobutane, Electronic Textbook, Chemical Experiment.
Abstract: We developed a computer graphics (CG) teaching material (TM) for university students, concerning
reactions involving drastic changes in the structure of the reactants in the following chlorination, for
example S
N
1: formation of tert-butyl chloride from tert-butanol and S
N
2: formation of 1-chlorobutane from
1-butanol. The CG-TM could clearly demonstrate the changes in the structures during the reaction by the
ball-and-stick model, in addition to the image of the energy change in terms of the reaction profile. An
electronic lab-book for chemical experiments in the students’ laboratory at the university was produced,
aiming at the integration of observable-level experiments, symbolic chemical equations, and the molecular
world. The lab-book displays pictures of apparatus, flow-chart of experimental procedures, and reaction
mechanisms with the CG-TM. A preliminary study on the effectiveness of the CG-TM suggested that
students were able to obtain images of S
N
1 and S
N
2 reactions.
1 INTRODUCTION
Based on the understanding of the observed
phenomena in chemical reactions (observable or
macro level), chemists try to imagine and explain
observations in terms of molecules (sub-micro or
molecular level). Observation and molecular level
models are then represented in terms of a
mathematical or chemical equation (representative
or symbolic level) (Gilbert, 2009 and Tasker, 2010).
Students’ difficulties and misconceptions in
chemistry often stem from inadequate or inaccurate
models at the molecular level (Kleinman, 1987). A
molecular structure visualized by computer graphics
(CG) allows for a deeper understanding of molecules
(Tuvi-Arad, 2006). However, CG teaching material
(TM) for objective reactions is not readily available,
because creating accurate CG requires molecular
structures based on X-ray crystallography
experiments or quantum chemistry calculations.
We are attempting to produce a CG-TM based on
quantum chemistry calculations, which provides
accurate and realizable images of the nature of a
reaction (Ikuo, 2006 and 2009). It has been reported
that molecular-level animations combined with
video clips of macroscopic phenomena enable
students to predict the outcome of a chemical
reaction better (Velazquez-Marcano, 2004). Many
electronic textbooks of chemistry are available, but
most of them are very similar to hard copies and
very few are related to chemical experiments
(Morvant, 2013). Moreover, a combination of the
CG movie of a reaction and an experiment is not
available. If the CG can be combined with a lab-
book of chemical experiments, students can observe
the reaction from three levels of thinking: molecular-
level CG, which enables students to obtain a realistic
image of a symbolic-level chemical equation, and
the phenomenon of an actual reaction. Our ultimate
goal is to produce an electronic lab-book for
chemical experiments, which integrates these three
levels of thinking.
Nucleophilic substitution (S
N
) reactions, in
which a nucleophile displaces another group or atom
from a compound, are among the basic reactions in
organic chemistry. Therefore, the S
N
reaction is
often adopted in TM on the university curriculum,
including appropriate schemes that aim to show
drastic changes in the molecular structure
(McMurry, 2001). Teaching materials or schemes
that enable students to provide realizable images of
the nature of a reaction need to be developed. We
have reported that an electronic lab-book with CG-
TM is a promising means to provide images of the
556
Ikuo, A., Toyama, Y., Yoshinaga, Y. and Ogawa, H.
Development of Electronic Lab-book for College Chemistry-Experiment - SN1 & SN2 Reactions -.
DOI: 10.5220/0006381305560561
In Proceedings of the 9th International Conference on Computer Supported Education (CSEDU 2017) - Volume 1, pages 556-561
ISBN: 978-989-758-239-4
Copyright © 2017 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
Walden inversion during the S
N
2 reaction (Ikuo,
2016).
This paper describes our work on the CG
visualization of the chlorination of both tert-butanol
and 1-butanol as an example of S
N
1 and S
N
2
reactions, in order to provide realistic images of the
mechanisms underlying both types of nucleophilic
substitution reactions. The CGs showing the
molecular world and experimental procedures for
the students’ laboratory at the university are
combined in the electronic lab-book in order to
integrate the observable-level experiment, symbolic-
level chemical formula, and the molecular world for
the nucleophilic substitution reaction (Scheme 1).
Scheme 1: Development of Electronic Lab-book.
2 METHOD
2.1 Development of Experimental
Program and Electronic Lab-book
There are two possible mechanisms underlying
nucleophilic substitution. In S
N
1 reactions (Scheme
2), a carbocation is first formed, which then reacts
with the nucleophile. The carbocation is planar, and
the anion can attack from either side. Consequently,
if the original molecule is optically active, a racemic
mixture of products is obtained.
Scheme 2: Mechanism Underlying Chlorination of tert-
Butanol, S
N
1 Reaction.
On the other hand, the S
N
2 reaction (Scheme 3) is a
concerted reaction in which the nucleophile
approaches from the left side of the central carbon as
the other group leaves to the right.
Scheme 3: Mechanism Underlying Chlorination of 1-
Butanol, S
N
2 Reaction.
In this case, the configuration of the molecule is
inverted. If the original molecule is optically active,
the product has the opposite activity, an effect
known as the Walden inversion (McMurry, 2001). A
schematic representation of the reaction is often used
in textbooks, but it is not always easy for the student
to obtain the configuration of the molecule or the
dynamics of the reaction. The CG-TM may provide
such an image.
The attainment targets and contents of an
experimental program are shown in Scheme 4.
Scheme 4: Attainment Target and Contents of an
Experimental Program.
In STEP1, the S
N
1 reaction mechanism is
introduced first; then, a learner is expected to grasp a
three-dimensional rearrangement image of the
reactant molecules during the reaction by watching
the CG and CG movie along with the chemical
equation and scheme. Subsequently, the
stereochemistry is studied using CG. Finally, the
reactivity is studied. The S
N
2 reaction is studied in a
similar manner. This step may take approximately
20 min, which can be assigned as homework.
In STEP2, the actual S
N
1 and S
N
2 reactions are
introduced. Chlorination of both tert-butanol for S
N
1
and 1-butanol for S
N
2 is conducted. The infrared
spectral data of the products and reactants are
compared. This step may take approximately 2.5 h
Macro Level Symbolic Level Sub-micro Level
S
N
1
Electronic Lab-
book
Experimental program
C(CH
3
)
3
OH + HCl → C(CH
3
)
3
Cl + H
2
O
CH
3
CH
2
CH
2
CH
2
OH + HCl → CH
3
CH
2
CH
2
CH
2
Cl + H
2
O S
N
2
C(CH
3
)
3
OH + HCl → C(CH
3
)
3
Cl + H
2
O
CH
3
CH
2
CH
2
CH
2
OH + HCl → CH
3
CH
2
CH
2
CH
2
Cl + H
2
O
Grasp image of S
N
1,S
N
2
Actual reaction of S
N
1,S
N
2
S
N
1: tert-BuOH →tert-BuCl
S
N
2: 1-BuOH →1-BuCl
Fixation of
knowledge,S
N
1,S
N
2
Attainment target Contents of study
1.5 Introduce reaction mechanism of S
N
2
1.6 Watch CG movie of S
N
2
2.1 Synthesis of tert-BuCl
2.2 Verification by IR
2.3 Synthesis of 1-BuCl
2.4 Verification by IR
3.1 Quiz
1.7 Study stereochemistry of S
N
2 with CG
1.8 Understand reactivity in S
N
2
1.1 Introduce reaction mechanism of S
N
1
1.2 Watch CG movie of S
N
1
1.3 Study stereochemistry of S
N
1 with CG
1.4 Understand reactivity in S
N
1
STEP1
STEP2
STEP3
Development of Electronic Lab-book for College Chemistry-Experiment - SN1 & SN2 Reactions -
557
for each reaction.
In STEP3, the learner is expected to integrate
his/her knowledge about the S
N
1 and S
N
2 reactions
by participating in a quiz.
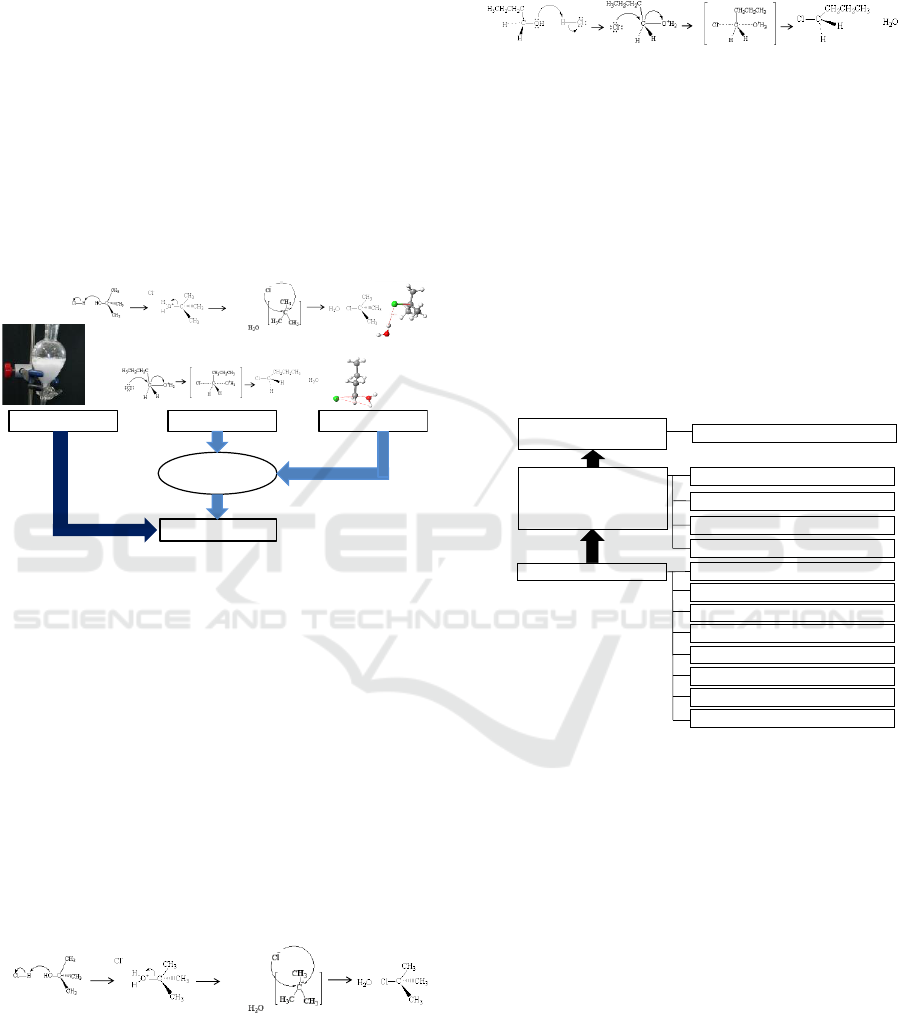
2.2 Quantum Chemistry Calculations
The structures of the reactants during the course of
the reaction were calculated as follows: the semi-
empirical molecular orbital calculation software
MOPAC (Stewart, 1989) with PM5 Hamiltonian in
the SCIGRESS (ver. 6.01, FUJITSU, Inc.) was used
in all the calculations for optimization of the
geometry by the Eigenvector following method, for
searching the transition state by using the program
with saddle point search, and for searching the
reaction path from the reactants to the products via
the transition state by intrinsic reaction coordinate
(IRC) calculation (Fukui, 1970) (Scheme 5).
The structure of the reacting molecules at the
transition state was confirmed by a single absorption
peak in the imaginary region: -649.8 cm
-1
(first part
of the reaction) and -154.24 cm
-1
(second part of the
reaction) in the chlorination of tert-butanol, S
N
1
reaction; and -422.94 cm
-1
in the chlorination of 1-
butanol, S
N
2 reaction.
Scheme 5: Procedure for Making CG Movie.
The structures of the initial state, transition state
and final state were obtained by the IRC calculation,
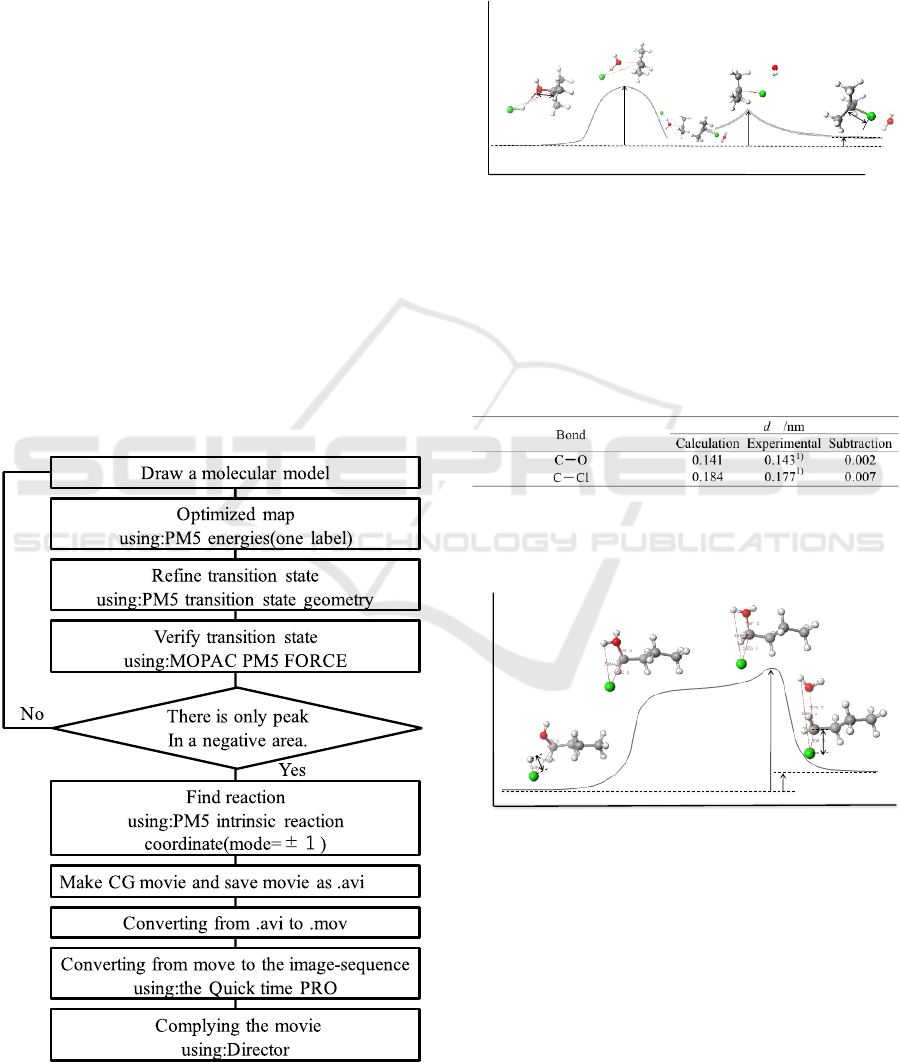
as shown in Figures 1 and 2. The Gibbs energy
changes and interatomic distances obtained by the
calculation were in good agreement with the
literature values, as seen in Tables 1 and 2.
Figure 1: Reaction Path for Chlorination of tert-Butanol,
S
N
1 Reaction. d: bond distance; ΔEa: activation energy
(Ea
1
=266.19 kJ mol
-1
, Ea
2
=60.27 kJ mol-
1
), ΔG: E
pro
‐
E
rea
1) Nihonkagakukai (CSJ) Ed., 1984. Kagaku binran
kisohen (Handbook of chemistry Basic) 3rd ed., Maruzen,
305.
Table 1: Interatomic Distances of Selected Atoms for
Chlorination of tert-Butanol, S
N
1 Reaction.
1) Nihonkagakukai (CSJ) Ed., 1984. Kagaku
binran kisohen (Handbook of chemistry Basic)
3rd ed., Maruzen, 717.
Figure 2: Reaction Path for Chlorination of 1-Butanol, S
N
2
Reaction. d: bond distance;
Δ
Ea: activation energy
(=623.05 kJ mol
-1
); ΔG: E
pro
‐E
rea
1) Nihonkagakukai
(CSJ) Ed., 1984. Kagaku binran kisohen (Handbook of
chemistry Basic) 3rd ed., Maruzen, 305.
d
d
Potential energy
Reaction coordinate
ΔG= 25.11 kJ mol
-1
(8.78 kJ mol
-1
)
1)
Ea
1
Ea
2
Potential energy
Reaction coordinate
d
d
ΔEa
ΔG=34.23 kJ/mol
(10.78 kJ/mol)
1)
CSEDU 2017 - 9th International Conference on Computer Supported Education
558
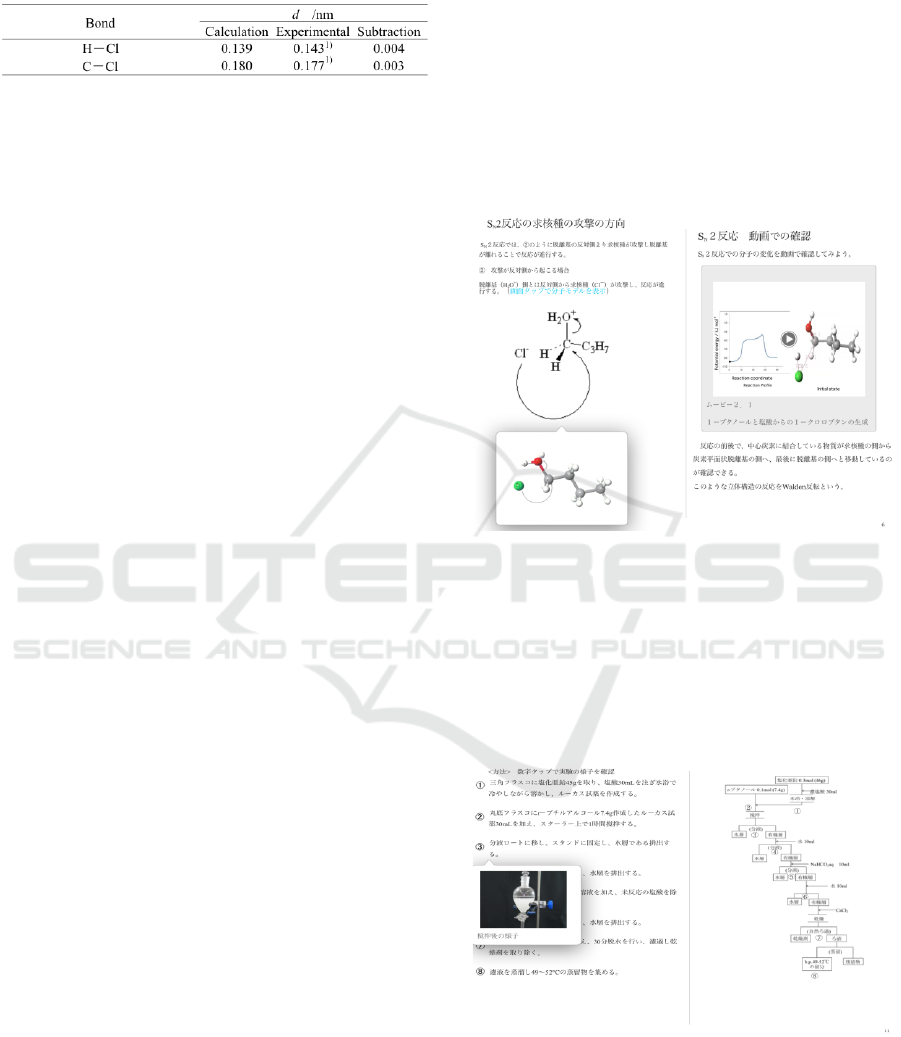
Table 2: Interatomic Distances of Selected Atoms for
Chlorination of 1-Butanol, S
N
2 Reaction.
1) Nihonkagakukai (CSJ) Ed., 1984. Kagaku
binran kisohen (Handbook of chemistry Basic)
3rd ed., Maruzen, 717.
Energy changes during the reactions and the
structures of the reactants and products were
confirmed. Therefore, it was concluded that the
reaction path and the molecular geometry obtained
by the calculation were appropriate for creating the
CG-TM.
2.3 CG-TM and Electronic Lab-book
The AVI file for the reaction path was produced by
SCIGRESS such that which changes in the structure
of the reacting molecules can be clearly seen and
bond formation or bond cleavage is displayed by
changes in diameter of the stick in the ball-and-stick
model; this diameter is related to the calculated bond
order. The AVI file was first converted to a MOV
file and then to the image-sequence file by Quick
Time PRO (ver. 7.66, Apple, Inc.) (lower part of
Scheme 5). The image-sequence file of the ball-and-
stick models was combined with the reaction profile
(which is the potential energy change during the
reaction progress) of the corresponding reaction
stage by Director (ver. 12.0, Adobe, Inc.). It was
confirmed that the drawn CG of the molecular
models of the reactants moved smoothly in the CG
movie. The ball, which indicates the progress of the
reaction, was arranged on the reaction profile, and
simultaneous movements of the ball and the
reactants were also confirmed. An electronic
textbook was produced with iBooks Author (ver.
2.5, Apple, Inc.) and saved to the tablet (iPad Air 2,
Apple, Inc.) by using iTunes (ver. 12.5, Apple, Inc.).
3 RESULTS AND DISCUSSION
3.1 Features of Electronic Lab-book
Teaching materials such as CG or CG movie of the
S
N
1 and S
N
2 reactions were combined with the
chemical experiments from students’ laboratory for
making the electronic lab-book of basic chemistry to
provide observations though experiment, molecular-
level CG, and symbolic-level chemical equation.
CG-TM such as pop-up CG of the molecule and
CG movie of molecular rearrangement in the ball-
and-stick model is also inserted (Figure 3). The pop-
up CG provides a 3D image of the molecule being
described by chemical equations. The CG movie
shows the reaction profile, which demonstrates the
eaction progress by the ball indicating the potential
energy vs. reaction coordinate. When student
touches the CG-TM in the tablet computer, the
image of the structural change during the reaction is
displayed. If student touches the material again, the
Figure 3: Pop-up CG and CG Movie of S
N
2 Reaction in
STEP 1.
Quick Time control bar appears and the red ball on
the profile can move as per the student’s choice.
student can manipulate the reaction back and forth
until he/she obtains the image of the reaction. A
student is expected to obtain a dynamic image of
molecular rearrangement.
Figure 4: Flow Chart and Pop-up Photo of Apparatus for
Experimental Procedure in STEP 2.
A flow chart of the experimental procedure of the
(Figure 4) and pop-up photographs of the apparatus
were inserted into the electronic textbook for
Development of Electronic Lab-book for College Chemistry-Experiment - SN1 & SN2 Reactions -
559
providing a realistic image of the experimental set
up.
In STEP3, the learner is expected to integrate
knowledge about S
N
1 and S
N
2 reactions by
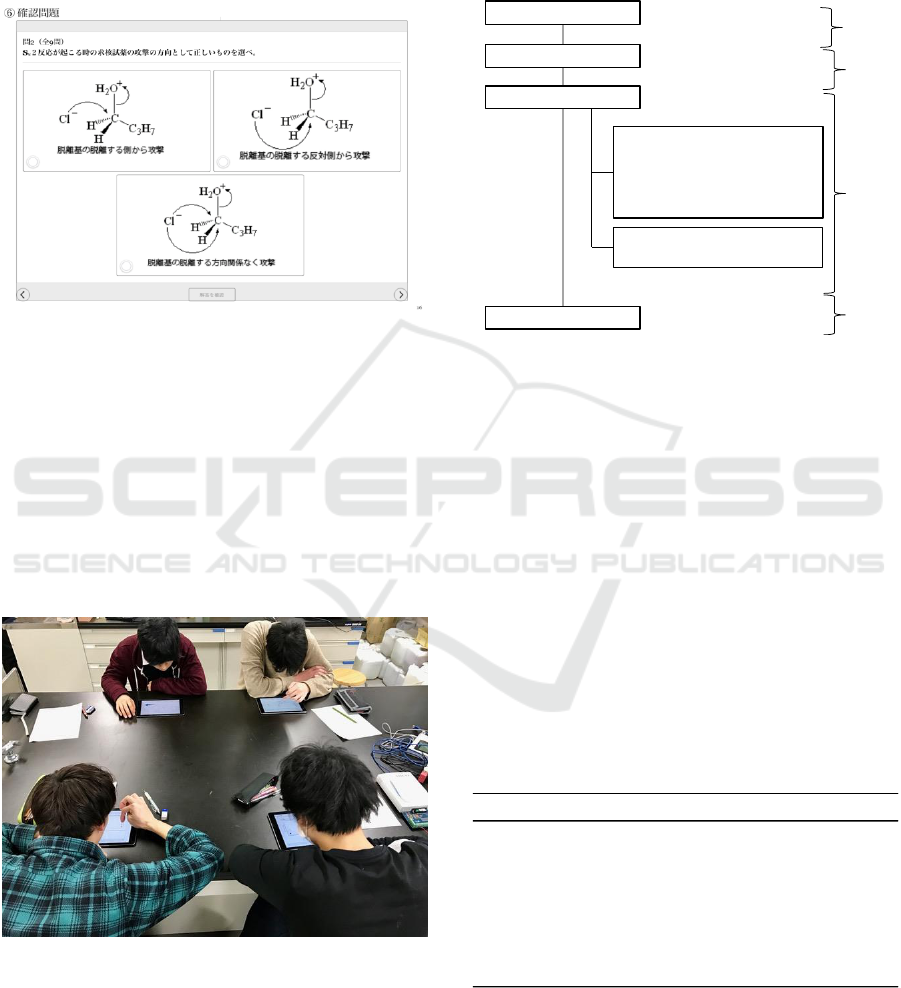
participating in a quiz (Figure 5).
Figure 5: Quiz, Approaching Direction of Nucleophile to
Central Carbon for S
N
2 Reaction in STEP 3.
3.2 Practicing the Use of the Electronic
Lab-book
Four third-year chemistry students of the teachers’
training course at Tokyo Gakugei University, who
took basic organic chemistry in the first year, were
asked to practice STEP1 (grasp the image of S
N
1
and S
N
2 reactions) and STEP3 (quiz part of the
electronic lab-book), as illustrated in Figure 6.
Figure 6: Practice with Electronic Lab-book.
The procedure adopted in the practice session is
shown in Scheme 6. First, the usage of the tablet was
explained, and then, a pre-test was conducted. After
the pre-test, tablets were distributed to each student,
and the students were asked to study STEP 1 and try
to answer the questions in STEP 3. Finally, a post-
test was conducted. Students were confirmed to
concentrate on the subject, as they studied STEP 1 in
detail and attempted to answer the questions in
STEP 3.
Scheme 6: Procedure Adopted for the Lab-book Practice.
The results of the survey are summarized in
Table 3. The average number of correct answers was
2.00 out of 6.00 in the pre-test, and it was increased
to 5.25 in the post-test. After using the electronic
lab-book, students’ knowledge in terms of
“reactivity,” “attacking direction,” and “energy
change” in the S
N
1 and S
N
2 reactions was improved.
The students added their comments in the free
description section of the questionnaire, for
example, “After using iPad, image of the reaction
became certain” and “the movie helped
understanding the reaction mechanism.” These
comments suggested that the electronic lab-book
could provide an image of the S
N
1 and S
N
2
reactions.
Table 3: Results of Survey.
Although a more detailed study must be carried out
on the effectiveness of the electronic lab-book, we
can state that the students could obtain an image of
the S
N
1 and S
N
2 reactions.
Explanation
Pre test
Trial of lab book
Post test
STEP1
・ Introduce reaction mechanism
・ Watch CG movie
・Study stereochemistry
・ Understand reactivity
STEP3
・Quiz
5min
10min
20min
5min
Question
Contents
Pretest
Posttest
1 Reactivity in SN2
1
3
2 Reactivity in SN1
1
4
3
Attacking direction and product in SN1
1
4
4
Attacking direction and product in SN2
3
3
5
Stereochemistry inSN1 , SN2
2
3
6
Reaction energy in SN1 , SN2
0 4
CSEDU 2017 - 9th International Conference on Computer Supported Education
560

4 CONCLUSIONS
We developed CG-TM for university students,
concerning reactions involving a drastic change in
the structures of the reactants in the following
chlorination reactions: S
N
1, formation of tert-butyl
chloride from tert-butanol; S
N
2, formation of 1-
chlorobutane from 1-butanol. The CG-TM could
clearly demonstrate the changes in the structures
during the reaction by the ball-and-stick model, in
addition to the image of the energy change by the
reaction profile. An electronic lab-book for chemical
experiments in the students’ laboratory at the
university was produced. The lab-book could display
pictures of the apparatus, flow chart of the
experimental procedure, and reaction mechanism
with the CG-TM. A preliminary study on the
effectiveness of the CG-TM suggested that students
were able to obtain the image of the S
N
1 and S
N
2
reactions.
ACKNOWLEDGEMENTS
This work was supported by JSPS KAKENHI Grant
Numbers 25350188, 26350227.
REFERENCES
Fukui, K., 1970. A Formulation of the Reaction
Coordinate, J. Phys. Chem., 74, 4161-4163.
Gilbert, J. K., Treagust, D. F., 2009. in Gilbert, J. K.,
Treagust, D. (eds.), “Models and Modelling in Science
Education Vol. 4 Multiple Representations in
Chemical Education”, Springer, 333-350.
Ikuo, A., Nieda, H., Nishitani, N., Yoshinaga, Y., Ogawa,
H., 2016. An Approach to Developing Electronic
Textbook for Chemical Experiment - Taking Walden’s
Inversion as an Example -, Proc. CSEDU 2016, Vol.2,
416-420.
Ikuo, A., Yoshinaga, Y., Ogawa, H., 2015. Development
of Electronic Textbook for Chemical Experiment -
Taking esterification as an example -, Proc. CSEDU
2015, Vol.2, 553-557.
Ikuo, A., Ikarashi, Y., Shishido, T. and Ogawa, H., 2006.
User-friendly CG visualization with animation of
chemical reaction: esterification of acetic acid and
ethyl alcohol and survey of textbooks of high school
chemistry, Journal of Science Education in Japan, 30
(4), 210-215.
Ikuo, A., Nagashima H., Yoshinaga Y., and Ogawa H.,
2009. Calculation of potential energy in the reaction of
“I + H
2
→ HI + H” and its visualization, The
Chemical Education Journal (CEJ), Registration #13-
2.
Ikuo, A., Nishitani, N., Yoshinaga, Y., and Ogawa, H.
2012. Development of teaching material in tablet PC
based on computer graphics by quantum chemistry
calculation – Walden’s inversion -, Proc. The 20th
Intern. Conf. on Computers in Education (ICCE), pp.
418-423.
Kleinman, R. W., Griffin, H. C., Kerner, N. K., 1987. J.
Chem. Edu., 64, 766-770.
McMurry, J., 2001. “Organic Chemistry”5
th
ed., Tokyo
Kagaku Dojin, 367-381.
Morvant, C. M, Halterman, R.L., 2013. “Organic
Chemistry Laboratory Manual”, iBooks Store.
Nihonkagakukai (CSJ) Ed., 1984. Kagaku binran kisohen
(Handbook of chemistry Basic) 3rd ed., Maruzen, 305
and 717.
Stewart, J. J. P., 1989. Optimization of parameters for
semi empirical methods I. Method, J Comp. Chem., 10
(2), 209–220.
Tasker, R., Dalton, R., 2010. in Gilbert, J. K., Reiner, M.,
Nakhleh, M. (Eds.), “Models and Modelling in
Science Education Vol. 3 Visualization: Theory and
Practice in Science Education”, Springer, 103-131.
Tuvi-Arad, I. and Blonder, R., 2006. Continuous
symmetry and chemistry teachers: learning advanced
chemistry content through novel visualization tools,
Chem. Educ. Res. and Pract., 11(1), 48-58.
Velazquez-Marcano, A., Williamson, V. M., Ashkenazi,
G., Tasker, R. F., and Williamson, K. C., 2004. The
use of video demonstrations and particulate animation
in general chemistry, J. Sci. Educ. and Tech., 13(3),
315-323.
Development of Electronic Lab-book for College Chemistry-Experiment - SN1 & SN2 Reactions -
561
