Computational Study of Mechanical Support to the Failing Total
Cavopulmonary Connection
Mauro Grigioni
1
, Giuseppe D’Avenio
1
, Salvatore Donatiello
2
and Antonio Amodeo
2
1
Technology and Health Department, Biomechanics and Rehabilitative Technologies Unit, Istituto Superiore di Sanità,
Viale Regina Elena 299, 00161 Rome, Italy
2
Department of Cardiac Surgery, Bambino Gesù Children’s Hospital, Rome, Italy
Keywords: Cardiovascular Surgery, Fontan Circulation, Bioengineering, Computational Fluid Dynamics (CFD).
Abstract: The performance of an axial flow blood pump in an idealized total cavopulmonary connection (TCPC)
model was intravascularly evaluated. This blood pump was inserted within a modified Fontan surgery using
a reinforced Gore-Tex conduit, to be connected to the caval veins with the pulmonary arteries. Two
different computational models were examined (i) the new geometric model without a pump and (ii) with
the pump. Computational fluid dynamics analyses of these models were performed to assess the hydraulic
performance under varying pump’s operating conditions. Numerical simulations indicate that the pump
generates a pressure distribution which could prove to be beneficial for the univentricular patient with
failing Fontan circulation, allowing to provide a possible intervention, at least as bridge to heart
transplantation or as end-stage pump implant.
1 INTRODUCTION
Patients with single ventricle anomaly, even though
this term groups very different pathologies, must
deal with the condition of having only one of the
two ventricles of adequate functional size. Some of
the anomalies described as single ventricle defects
include tricuspid atresia, hypoplastic left heart
syndrome, double inlet left ventricle and other
cardiac defects. The incidence of this heart defect
constitutes about 1-2 % of all congenital heart
defects (Samanek et al., 1999). Whenever there is
only one ventricle capable to pump blood efficiently,
the circulation must be reconfigured to maximize the
efficiency of this single ventricle without
overloading it.
The total cavopulmonary connection (TCPC)
(Giannico et al., 2006) represents one of the most
successful clinical options to obtain a sufficient lung
perfusion in single-ventricle patients. It consists of
the direct connection of the venae cavae to the
pulmonary arteries, avoiding the usual pathway of
blood through the right heart and then to the lungs,
on account of the dysfunctional ventricle. Even
though the survival to 15-20 years after surgery is
superior to 82% (Gersony, 2008), statistics are
nevertheless indicating that the risk of long-term
failure of TCPCs is remarkable, ultimately requiring
transplantation (Cromme-Dijkhuis et al., 1993).
Actually, the absence of the subpulmonary
ventricle in the Fontan patient induces an elevation
of pressure in the systemic venous circulation. The
central venous pressure (CVP) rises to a mean
pressure of about 12 mmHg, or even more in the
most unfavourable cases (it can reach as high as 20
mmHg).
Clearly, an unphysiologically high CVP is poorly
tolerated with time by the patients. In particular, it
has deleterious effects on the liver and the
splanchnic circulation, possibly resulting in protein-
losing enteropathy and plastic bronchitis (Feldt et
al., 1996), in the worst cases. At the liver level, the
elevated CVP may induce complex liver
dysfunction; consequently, the release of
angiogenesis factors is expected, favouring the
occurrence of venovenous anastomosis, pulmonary
venous fistulas, and aortopulmonary collateral
anastomoses (APCA).
Considering that a single ventricle must work
against both systemic and pulmonary compartments,
the ventricle itself faces a significant increase in
total systemic resistance. Hence, the systemic
ventricle undergoes hypertrophy, with elevated end-
diastolic pressure, which diminishes its diastolic
Grigioni, M., D’Avenio, G., Donatiello, S. and Amodeo, A..
Computational Study of Mechanical Support to the Failing Total Cavopulmonary Connection.
In Proceedings of the 3rd International Congress on Cardiovascular Technologies (CARDIOTECHNIX 2015), pages 43-48
ISBN: 978-989-758-160-1
Copyright
c
2015 by SCITEPRESS – Science and Technology Publications, Lda. All rights reserved
43
performance (Cheung et al., 2000; Gewillig, 2005).
Also as a consequence of the elevated vascular
resistance, the ventricular preload is limited, which
also impairs diastolic performance.
As de Leval (1998) pointed out, the condition of
the Fontan patient is paradoxical, in that there is
systemic venous hypertension and simultaneously
pulmonary arterial hypotension.
Many attempts have been made to optimize the
Fontan connection, in order to make energy losses as
negligible as possible (e.g., Amodeo et al., 2002).
This notwithstanding, the failure of the Fontan
circulation is an occurrence that must always be
considered, with an increasing probability as a
function of the time elapsed since the operation
(Khairy et al., 2008).
Owing to the chronic shortage of heart donors, in
recent years the possibility of using mechanical
assistance for failing TCPC, either as bridge to
transplantation or destination therapy, has been
addressed in several studies.
In order to prevent ventricle hypertrophy induced
by elevated vascular resistance and limited
ventricular preload, it is natural to consider
leveraging on the VAD technology already available
to design therapeutic solutions for the unloading of
the only functional ventricle in the Fontan patient.
In this viewpoint, different connections and
assist devices have been proposed. Lacour-Gayet
(2009) and others suggested the inclusion of an
axial pump model used for circulatory support in an
extracardiac tube that connects caval veins to
pulmonary arteries, avoiding backflow in the
superior vena cava (SVC).
An assistance device positioned in the SVC was
found to increase the pressure in the PAs
(Santhanakrishnan et al., 2013).
A pump installed in the inferior vena cava (IVC)
is in principle capable of generating a strong
pressure decrease, upstream of the device itself,
possibly causing a collapse of the venous vessel. For
this reasons, this study considers the insertion of a
miniaturized axial pump inside a reinforced
GoreTex conduit which connects the caval veins
district to the pulmonary arteries district, in a
different fashion with respect to the classical Fontan
(TCPC) surgery. This connection, together with
properly set pump operating conditions, was thought
to improve the balance of arterial and venous
pressures, preventing also vessel collapse, thanks to
the surgical geometry and the use of a GoreTex
prosthesis.
The herein selected device is supposed to solve
the principal obstacles for long-term implantation.
Various studies showed how the Jarvik 2000 pump
might be among the major candidate for destination
therapy due to its biomechanical characteristics,
particularly for its very low hemolysis rate (Gibber
et al., 2010).
The goal of the collaboration between the
Biomechanics and Rehabilitative Technologies Unit
of the Technology and Health Dept. of ISS and the
Bambino Gesù Children’s Hospital (BGCH) is to
create a permanent solution to the failing Fontan
connection as both bridge to transplant and
destination therapy, avoiding the necessity of a
subsequent transplant for the patient. Then, we
selected an assist device whose long-term in vivo
performance is well documented by the literature,
representing a good reference model for a realistic
therapeutic intervention. In this paper we study by
CFD the feasibility of a surgical approach based on
both an innovative surgical connection and the use
of an axial pump model similar to the child-size
Jarvik 2000, as a permanent solution to sustain the
failing Fontan circulation.
The final aim of the investigation thus was to
investigate the fluid dynamics of TCPC innovative
connection making use of the mechanical support,
defining the axial pump’s condition for safe
performance (range of flow rates, pump speeds,
pulmonary artery pressures) to be related to the
clinical setting to provide indication for a safe
procedure in mechanical support of failing TCPC.
2 METHODS
This study of circulatory support device in the
Fontan circulation uses a model of TCPC
circulation, designed with an innovative connection
with respect to traditional Fontan, and a model of
cardiac pump resembling a pump already on the
market, widely used as a Ventricular Assist Device
(VAD), the child-size Jarvik 2000. The latter has the
essential characteristics (function, size and form
compatible with the insertion in a cylindrical tube)
that are required for our research, among those most
widely used in Europe.
The assistance device was positioned between
the two caval veins and the pulmonary arteries, as
shown in Fig 1. A second ideal geometric model
characterized by cylindrical ducts of intersection
between the caval veins and pulmonary arteries with
no pump was also created for CFD study of the
connection without mechanical support, in order to
evaluate pressure and flow fields characterizing the
connection used, with and without the pump.
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
44
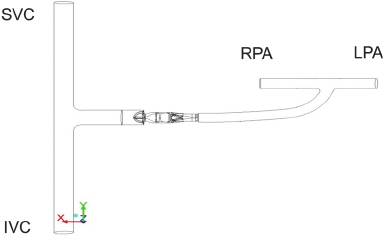
Thus, two 3D clinical Fontan compartment models
were created, using the computer aided design
(CAD) software SolidWorks (SolidWorks, Concord,
MA, USA), to study the properties of the flow inside
the proposed model:
- a first model with an axial pump, whose design
was inspired by the child-size Jarvik 2000 pump,
- a second model without the pump, in order to
have a basis for comparison.
Figure 1: Model of the TCPC with assist device. At the
left side the anastomosis between SVC and IVC is shown.
At the upper right side the two pulmonary arteries are
shown. A GoreTex conduit connects the caval veins and
the PAs, with the axial pump inserted in the conduit.
In order to study the Fontan fluid dynamics, a
vertically oriented tubular venous compartment of
about 20-cm length was chosen to represent both
IVC and SVC joined to the connected right-left
pulmonary arteries by an extracardiac conduit. The
pump model was positioned in the extracardiac
conduit. The total volume of fluid in the model was
approximately 83 cm
3
, vs. the 2.1-cm
3
internal
volume of the pump (priming volume), excluding
the head input. The model was meshed using Gambit
software, with almost 1,800,000 elementary volume
elements, one million of which covered the 2.1-cm
3
internal volume of the pump.
Ansys Fluent 12.1 software was used for fluid
dynamics simulations. To simulate the rotating part
of the pump and analyze the behavior of the blood
fluid during the stationary phase (when the pump is
operating at full capacity), we used the RNG k-ɛ
model, which has a better capability of studying
rotating flows with respect to the standard k-ɛ model
(Yakhot et al., 1992). This feature of the RNG k-ɛ
model stems from more accurate transport equations
for the turbulent kinetic energy (k) and rate of
dissipation of turbulent kinetic energy (ɛ), with
respect to the standard k-ɛ model.
The following performance conditions were
imposed: pump angular velocities were within the
child-size Jarvik 2000 range (4,000 - 18,000
revolutions per minute [rpm]) and 3 flow rate values
(2, 3, 4 l/min) were considered. The pulmonary
arterial mean pressures was set at 10 mmHg, and
with a constant 40%-60% SVC–IVC flow ratio was
considered, according to the physiological flow
partition seen in children (Fogel et al., 1999).
Vessel walls were modelled as rigid tubes. A
constant viscosity value of 0.0035 kg/m*s and fluid
density of 1,060 kg/m3 were used (Cutnell and
Johnson, 1998).
Animal Study – A preliminary investigation about
feasibility of the presented approach in vivo was
carried out on an animal model. All animals received
humane care in compliance with the “Guide for Care
and Use of Laboratory Animals”. The Bambino
Gesù Children’s Hospital Ethical Committee
approved all conditions for animal surgery and care.
A total of 8 sheep (Western breed, 42-48 Kg) were
considered: 2 for preliminary studies, 4 supported
and 2 non supported. Anesthetic drugs included
ketamine (3 mg/kg), diazepam (0.2 mg/kg) and
atropine (0.02 mg/Kg) and induction was obtained
with propofol 1% (2mg/Kg). Animals were
intubated and ventilated using a Servo 900C
volume-cycled respirator (Siemens®, Danvers, MA)
with 100% oxygen and 1% to 2% isoflurane.
Ventilation parameters were: 10 to 15 breaths/min
with tidal volumes of 10 ml to 15 mL/Kg and 4
cmH2O positive end expiratory pressure.
A 16 gauge femoral arterial line (Intracath®,
Becton Dickinson, Sandy, UT) was placed for
systemic blood pressure monitoring. A 16 gauge
femoral venous line was inserted in a jugular vein.
The heart was exposed trough a median
sternotomy. A fiber optic pulmonary catheter
(Opticath®, Abbott Laboratories, North Chicago,
IL) was placed in the main pulmonary artery. All
pressures and flows were continuously monitored
and recorded. CO, PVR, CI were calculated with
Picco® (Pulsion Medical System) using thermo-
dilution technique.
The SVC and IVC were sequentially divided
from the right atrium and TCPC was performed by
interposing a 16 mm polytetrafluoroethylene
vascular graft (Gore-Tex®) between the two cavae
and connected to the pulmonary artery through a
second conduit in a T- junction geometry (2 non
supported TCPC).
In the pump-supported group (4 animals), the
child-size Jarvik 2000 axial pump was inserted in
the conduit to the pulmonary artery. Flow rates were
maintained between 2 to 3 L/m in a range of 5.000,
7.000 and 9.000 rpm, at 1st , 2nd and 3rd hour of
support, respectively. The axial child flow pump was
Computational Study of Mechanical Support to the Failing Total Cavopulmonary Connection
45
positioned as distal as possible from venae cavae to
avoid their collapse. The graft diameters were
chosen to match the sheep’s SVC and IVC
dimensions. The main pulmonary trunk was left in
place to vent the blood from coronary sinus.
Hemodynamic variables were recorded over a period
of 3 hours.
Hourly arterial blood gas measurements were
obtained. In addition, oxygen saturations in PA and
IVC blood, serum lactate levels were measured
before and during operation
.
3 RESULTS
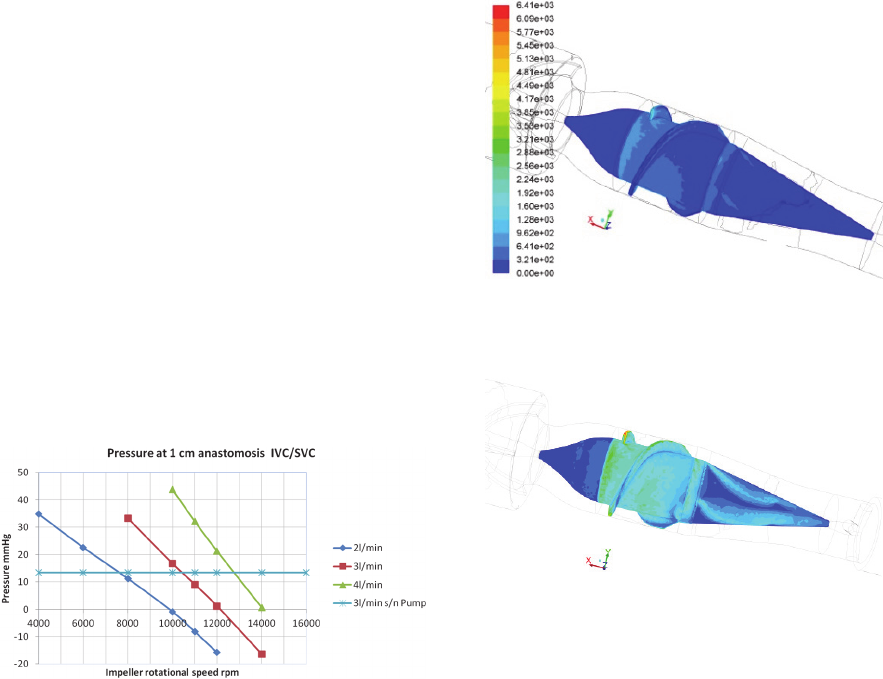
Fig. 2 reports the pressure values obtained in the
conduit, upstream of the device, at 1 cm downstream
of the IVC-SVC anastomosis. Comparing the curves
relative to the geometry with the pump with the line
representing the connection without assistance, it
can be seen that, for each imposed flow rate (2, 3
and 4 L/min), the pump does not constitute a
resistance to the flow, at a sufficiently high angular
velocity (e.g., from 10000 rpm upwards, for 3
l/min). Moreover, the pump can sustain flows of less
than 2 l/min at speeds below 10000 rpm.
Figure 2: Average intravascular pressure, at 1 cm
downstream of the IVC/SVC connection. The horizontal
line indicates the value of the calculated pressure that
would occur in the geometry without the pump, in the case
of a flow rate of 3 l / min (similar values for other flow
rates). Above the horizontal line the rotational speed is not
usable for blood perfusion, whilst the pump represents an
obstacle for the blood; thus the usable region for the pump
speed in relation to the available venous pressure is
comprised between the horizontal line and a pressure level
close to zero.
These results are encouraging, since the axial
pump is meant to be used in patients with low levels
of physiological flow rate (children at the age of a
few years).
The CFD study enabled also an assessment of the
Wall Shear Stress (WSS) values on the surface of
the different parts of the connection. As apparent
from Figures 3 and 4, a positive correlation was
found between regime conditions (pump angular
velocity and blood flow rate) and magnitude of
WSS.
Figure 3: WSS [dyn/cm
2
] distribution over the impeller’s
surface (pump angular velocity: 4000 rpm; blood flow
rate: 1 l/min).
Figure 4: WSS [dyn/cm
2
] distribution over the impeller’s
surface (pump angular velocity: 10000 rpm; blood flow
rate:3 l/min). The color code is the same as in Fig. 3.
It must be underlined, though, that at the highest
regime investigated the maximum WSS value was
6410 dy n/cm
2
, which is not an excessively high
value for shear stresses in physiological flows with
prosthetic devices. As an example, in Grigioni et al.
(2001) a value of around 5500 dyn/cm
2
was found
for the maximum Reynolds shear stress associated to
a bileaflet aortic valve available on the EU market.
Animal Study - The group without pump support had
a sudden deterioration of hemodynamic parameters
and they died within one hour. In the pump-
supported (PS) group all animals survived and
cardiopulmonary function was stable. No hemolysis
at different run speeds, neither thrombotic events nor
venous collapse was observed. In the PS group the
central venous pressure values did not increase
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
46
during the mechanical assistance period, and were
found to be similar to those in baseline condition.
As for the gas exchange values, arterial pH remained
in the normal range, with slightly alkalosis after 3
hours, as an effect of moderate hypercapnia. A trend
in lactates stability was observed under mechanical
assist, which can be related to optimal hemodynamic
function.
4 DISCUSSION
Heart pumps are available to assist the cardiac
function in case of a pathological state. These
devices are mostly implanted to assist ventricular
function, hence they are called Ventricular Assist
Devices (VAD). They aspirate blood from the
ventricle and inject it into the aorta.
Apart from ventricle support, other uses of
circulatory assistance have been proposed. The use
of a pump for circulatory support proposed in this
paper aims to solve the serious circulatory problems
that quite often occur in patients previously operated
on with Fontan surgery. The simulations herein
presented show that it is possible to adapt an already
available commercial VAD for extracardiac
circulatory assistance. The original VAD connection
has been modified to insert the device in the
proposed connection. The CFD analysis allowed us
to determine the range of rotational speed that
should be imposed to avoid veins collapse. Fig. 2
can be used to gauge the upper limits of impeller
rotational velocity, in order to prevent venous
collapse. Considering an output pressure of 10
mmHg in the pulmonary arteries, the upper limits for
2 l/min, 3 l/min and 4 l/min are 10000, 12000 and
14000 rpm, respectively. When the flow is 2 l/min,
Fig. 2 shows that at 10000-rpm rotational speed the
pressure is -0.87 mmHg. This value is very close to
the limit value of -0.5 mmHg reported by Riemer et
al (2005) as a trigger for vein instability/collapse
upstream of the pump. The problem in this case can
be solved in two ways. The first one consists of
reducing the rotational speed of the impeller; in the
second, the length of the pipe connection can be
increased, hence, at equal flow, a greater power loss
will be obtained and the problem of excessive
negative pressure can be minimized.
In the present study, we considered a constant
rotational velocity of the pump. This was done for
two main reasons: 1) the axial pump we referred to,
the child-size Jarvik 2000, functions most of the
time at constant angular velocity. Actually, the pump
is restarted periodically, to mitigate the risk of
stationary flow zones inside the pump, but the
device is essentially a constant-speed pump. 2)
Information about pulsatility in assist devices is still
too scarce to implement a rational strategy of time-
varying pump velocity for the failing Fontan. This
notwithstanding, pulsatile assist devices in the future
might be certainly an interesting option, taking also
into account that pulsatility could reduce the risk of
venous collapse, upstream of the device.
In order to minimize the possibility of venous
collapse related to pump functionment, we tested the
insertion of the device inside a reinforced tube (a
GoreTex reinforced prosthesis). This solution avoids
the collapse of the vein (very likely in extreme
conditions) and prevents physiological or electronic
random changes that could lead to a temporary low
pressure with consequent collapse of the walls. The
CFD simulations made on the model without the
pump allowed us to calculate the pressures generated
by the new connection, using the same boundary
conditions for the model with the pump. To analyze
the data provided by simulation with the pump
inserted, we considered the pressure calculated in
the model without the pump as the reference
pressure, at a flow rate of 3 l/min (Fig. 2). A first
comparison between the pathlines (data not shown)
of the model without the pump and the one with the
pump showed how the device provides an
improvement in blood flow in the immediate vicinity
of the anastomosis. The presence of the pump
caused a reduction of recirculation region on the
wall of the cava that is opposite to the junction
between caval veins and conduit; this effect was due
to the presence of a suction force generated by the
pump which linearizes the flow. In the vessel
downstream of the pump, spiral flow trajectories
could be seen, caused by the torque generated by the
impeller on blood flowing through the device.
Before reaching the pulmonary arteries, the pathlines
showed fairly linear trajectories, which demonstrate
the effectiveness of the flow straighteners of the
pump. Wall Shear Stresses are relevant if an end-
stage implant is thought to be provided, thus WSS
calculated values allowed us to verify that,
predictably, the maximum values were correlated to
pump speed and blood flow rate. In any case, the
WSS values did not reach excessively high levels,
confirming that the proposed study is a feasible
approach to the treatment of the failing Fontan
circulation as destination therapy.
The favourable role of the pump-assisted Fontan
circulation, besides the results of the in-silico study,
has been also confirmed by a preliminary animal
study. Hence, we are confident that the growing
Computational Study of Mechanical Support to the Failing Total Cavopulmonary Connection
47
number of the Fontan patients with impaired
function of the single ventricle will be offered in the
future the possibility to avoid or defer as much as
possible heart transplantation, by means of suitably
designed mechanical assistance to circulation.
REFERENCES
Amodeo A., Grigioni M., Oppido G., Daniele C.,
D'Avenio G., Pedrizzetti G., Giannico S., Filippelli S.,
Di Donato R. M., 2002. The beneficial vortex and best
spatial arrangement in total extracardiac
cavopulmonary connection. J Thorac Cardiovasc Surg;
124(3):471-8.
Cheung Y. F., Penny D. J., Redington A. N., 2000. Serial
assessment of left ventricular diastolic function after
Fontan procedure. Heart;83:420–4.
Cromme-Dijkhuis A. H., Hess J., Hahlen K., et al., 1993.
Specific sequelae after Fontan operation at mid and
long-term follow-up J Thorac Cardiovasc Surg.
106:1126-1132.
Cutnell, John & Johnson, Kenneth, 1998. Physics, Fourth
Edition. Wiley: 308.
de Leval M. R., 1998. The Fontan circulation: what have
we learned? What to expect? Pediatr Cardiol; 19:316 –
20.
Feldt R. H., Driscoll D. J., Offord K. P. et al., 1996.
Protein-losing enteropathy after the Fontan operation.
J Thorac Cardiovasc Surg; 112:672– 80.
Fogel, M. A., P. M. Weinberg, J. Rychik, A. Hubbard, M.
Jacobs, T. L. Spray, and J. Haselgrove, 1999. Caval
contribution to flow in the branch pulmonary arteries
of Fontan patients with a novel application of
magnetic resonance presaturation pulse. Circulation
99:1215–1221.
Gersony, W. M., 2008, Fontan Operation After 3 Decades:
What We Have Learned. Circulation.; 117:13-15.
Gewillig, M., 2005. The Fontan circulation. Heart;
91:839–46.
Giannico, S., Hammad F, Amodeo A, Michielon G,
Drago F, Turchetta A, Di Donato R, Sanders SP, 2006.
Clinical outcome of 193 extracardiac Fontan patients:
the first 15 years. J Am Coll Cardiol. 47:2065-73.
Gibber, M., Wu Z. J., Chang, W., Bianchi G., Hu, J.,
Garcia J., Jarvik R., Griffith, B. P., 2010. In Vivo
Experience of the Child-Size Pediatric Jarvik 2000
Heart: Update. ASAIO Journal; 56(4):369-376.
Grigioni, M.1., Daniele C., D'Avenio G., Barbaro V.,
2001. The influence of the leaflets' curvature on the
flow field in two bileaflet prosthetic heart valves. J
Biomech. 34(5):613-21.
Khairy, P., Fernandes S. M., Mayer J. E. Jr. et al., 2008.
Long-term survival, modes of death, and predictors of
mortality in patients with Fontan surgery. Circulation;
117:85–92.
Lacour-Gayet F. G., Lanning C. J., Stoica S., Wang R.,
Rech B. A., Goldberg S. and Shandas R., 2009. An
Artificial Right Ventricle for Failing Fontan: In Vitro
and Computational Study. The Annals of Thoracic
Surgery. 88:170-176.
Riemer R. K., Amir G., Reichenbach S. H., Reinhartz O.,
2005. Mechanical support of total cavopulmonary
connection with an axial flow pump. J. Thorac
Cardiovasc Surg 130:351-4.
Samanek M., Voriskova M., 1999. Congenital heart
disease among 815,569 children born between 1980
and 1990 and their 15-year survival: a prospective
Bohemia survival study. Pediatr Cardiol 20:411–417.
Santhanakrishnan A., Maher K. O., Tang E., Khiabani R.
H., Johnson J., Yoganathan A. P., 2013.
Hemodynamic effects of implanting a unidirectional
valve in the inferior vena cava of the Fontan
circulation pathway: an in vitro investigation. Am J
Physiol Heart Circ Physiol. Nov 15;305(10):H1538-
47.
Yakhot et al., 1992. Development of turbulence models
for shear flows by a double expansion technique,
Physics of Fluids A; 4(7):1510-1520.
CARDIOTECHNIX 2015 - International Congress on Cardiovascular Technologies
48
