
Research and Engineering Roadmap for Development and Deployment
of Smart Medical Devices
Jan Sliwa and Emmanuel Benoist
RISIS, Bern University of Applied Sciences - TI, Quellgasse 21, CH-2501 Biel, Switzerland
Keywords:
Smart Medical Devices, Interoperability, Translational Medicine, Evidence Based Medicine, Cyber-Physical
Systems, Research Roadmap.
Abstract:
The actual clinical use of smart wireless, software-based, mobile medical devices does not meet the recently
raised expectations. First, current low level of interoperability calls for setting and enforcing open standards
from the device level to the national/global collaboration structure. Second, heterogeneous and frequently
changing devices, operating in various natural, technical and human environments, do not match the classical
approval model. In addition to a time-limited set of clinical trials, they need a system of continuous quality
monitoring. Third, ad-hoc deployment, without dedicated staff with well defined, novel skill sets is not scal-
able. A proper organizational structure is necessary. In this paper we present a modular software structure
and a framework of a system supporting both the direct health care and the continuous quality evaluation. We
expose the location of interfaces crucial for assuring multivendor interoperability. We then define a roadmap
giving structure to the necessary development effort. The structure we propose should permit to coordinate
the actions of independent teams tackling the immense number of multifaceted and interrelated tasks.
1 INTRODUCTION
In spite of brilliant visions of the wireless future of
the medicine
1
the actual adoption of smart medical
devices into the clinical practice lags far behind ex-
pectations. In this paper we discuss the obstacles im-
peding this adoption and propose ways to overcome
them. We focus our attention on the chosen basic sce-
nario: patients with wearable and implantable med-
ical devices, connected via an aggregator (typically
a smartphone) into a larger infrastructure. We dis-
cuss the challenges to the development of a versatile
ecosystem of interoperable devices and support sys-
tems and to their deployment in the actual clinical
practice:
• Interoperability at all levels
• Configuration and deployment to the patients
• Servicing the deployed systems
• Ongoing quality control
Then we propose an organizational framework
that provides a viable reference structure that enables
to decompose the overall problem into manageable
tasks. The solution is not only technical but to a
1
http://www.ted.com/talks/eric topol the wireless future
of medicine, accessed May 7, 2014
great extent organizational and requires a coopera-
tion of many partners, willing to open they systems or
to share their data. Their interests are legitimate and
their concerns have to be treated with respect. There-
fore we discuss the driving forces that should motivate
them to enter into such collaboration.
On the basis of this framework we set a research
and development roadmap that identifies main prob-
lem areas to be solved. The definitions of inter-
faces and data structures deserve a particular atten-
tion, since they are decisive for the current and future
interoperability and flexibility. If well designed, they
will permit a development of solution elements by in-
dependent teams that eventually will match into an
encompassing superstructure.
In the conclusion we invite fellow researchers to
discuss the proposed roadmap and to collaborate in
using it as a scaffold for the actual project plan.
2 RELATED WORK
Mobile medical devices are currently an important re-
search area. We present here only a selection of pa-
pers relevant for our subject. A good starting point
221
Sliwa J. and Benoist E..
Research and Engineering Roadmap for Development and Deployment of Smart Medical Devices.
DOI: 10.5220/0005291002210232
In Proceedings of the International Conference on Biomedical Electronics and Devices (SmartMedDev-2015), pages 221-232
ISBN: 978-989-758-071-0
Copyright
c
2015 SCITEPRESS (Science and Technology Publications, Lda.)

is the roadmap
2
of the Healthcare Information and
Management Systems Society. A broad picture of
future possibilities is given in (Kumar et al., 2013a)
and (Alemdar and Ersoy, 2010). The paper (Bergs-
land et al., 2014) presents various barriers faced by
medical innovation - related to economy, mentality
differences, healthcare organization, inadequate regu-
latory process, intellectual property and ethical issues.
A deeper analysis in the context of Ambient Assisted
Living (AAL) is presented in (Memon et al., 2014).
This paper discusses important issues like interoper-
ability and gives a sobering overview of actually im-
plemented systems.
The problem of technology evaluation is ad-
dressed in (Kumar et al., 2013b), (Tomlinson et al.,
2013) and (Mohr et al., 2013). A novel approach is
crowdtesting (Speidel and Sridharan, 2014). One of
the main topics of this paper, medical registries, is
treated in the paper (Amit et al., 2014) that presents
the Israeli implantable cardioverter-defibrillators reg-
istry, and (Wolpert et al., 2011) that exposes the ne-
cessity of a cardiac registry and the barriers that hin-
der their implementation. An approach to protect the
data privacy in a registry
3
, based on a physical sep-
aration of personal and medical data is presented in
(Sliwa and Benoist, 2012).
At the side of the integration of the patient’s sen-
sors with a smartphone, we witness two major ini-
tiatives: Apple’s Health app
4
and Samsung’s wear-
able sensor device Simband
5
with corresponding soft-
ware on the smartphone. They should define ulti-
mate (competing) personal health monitoring plat-
forms. As for now, it was impossible for us to verify
the claims regarding their validity as open systems.
In any case, they position themselves in the consumer
market segment.
Various approaches to create integration frame-
works for system operation and device comparison
are proposed in (Wagner et al., 2013), (Pawar et al.,
2012), (Seeger et al., 2013), (Viswanathan et al.,
2012) and (Franke et al., 2013). Open mHealth Ar-
chitecture, similar to our goal, is proposed in (Estrin
and Sim, 2010).
The European project “Renewing Health” (RE-
gioNs of Europe WorkINg toGether for HEALTH)
6
2
http://www.himss.org/mobilehealthit/roadmap, access-
ed May 7, 2014
3
http://memdoc.org/, accessed May 7, 2014
4
http://www.apple.com/ios/whats-new/health/, access-
ed Oct. 20, 2014
5
http://www.samsung.com/us/globalinnovation/innova
tion areas/,accessed Oct. 20, 2014
6
http://www.renewinghealth.eu/en/, accessed Oct.20,
2014
has delivered several pilot projects related to the man-
agement of chronic diseases with telemedicine. In
particular, the Telemonitoring for Chronic Heart Fail-
ure project in the Veneto Region
7
shows an organi-
zational structure including explicitly various human
participants.
3 REFERENCE SETTING
In this paper we concentrate on systems of im-
plantable and wearable devices, connected via a Body
Area Network to a data aggregator, currently typically
a smartphone (Fig. 1). This set is to be considered as
a virtual multi-element device, performing a common
task in a coordinated way.
Figure 1: Basic configuration.
They have a specific medical purpose, like mon-
itoring of vital health signals in a chronic disease,
possibly expanded with direct life saving functions.
The latter may be performed by the device itself, like
a pacemaker, defibrillator or an implantable insulin
pump, or may involve alarming qualified medical per-
sonnel. Therefore they do not work in isolation, but
rather are integrated into a well defined infrastructure.
They are selected and prescribed by medical special-
ists as an integral part of a therapy. In the face of the
severity of the medical condition treated, of the im-
portance of the provided function and of the degree of
risk in case of a malfunction, they are subject to the
regulatory oversight of the relevant authority.
We deliberately leave aside the thriving field
of consumer devices used for fitness, promoting a
healthy lifestyle and preventive health monitoring.
Those devices do not have to meet stringent require-
ments and are not in the focus of the approval authori-
ties. In this field the market forces and the consumer’s
satisfaction will play the decisive role.
7
http://www.renewinghealth.eu/documents/28946/9c9db
1d1-8ace-4b07-bc08-8a7a68f55d05, accessed Oct.20,2014
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
222

4 CHALLENGES
4.1 Interoperability
Technically, one of the major problems is interoper-
ability. It is necessary if we want to flexibly config-
ure the systems, selecting the best elements for ev-
ery function from any producer. It has an economic
background, as many producers prefer to protect their
ecosystems with proprietary, undisclosed data for-
mats and protocols. In consequence, the hospital and
the patient may be forced not to include necessary de-
vices that are not supported by a chosen supplier. In
the same way, if we want our devices to communicate
directly with the hospital Electronic Health record
(EHR) system, the EHR system chosen will limit us
to only certain suppliers. Such connection exist or can
be developed on demand
89
, the effort is however sub-
stantial and provides only a 1:1 communication path.
This does not scale into an vendor independent, open
solution.
Figure 2: Complex event.
We also may want the devices to operate in a co-
ordinated way. An important case is the detection of
complex events (Fig. 2). In this figure the device de-
vice D1 detects a peak of the measured value (point
event), D2 assesses a prolonged waveform as an ab-
normal state, and the parameter measured by D3 ex-
ceeds the limit value. The combination of those mea-
surements establishes a medical condition (e.g. an
emergency) with a much better precision then each
of them alone, reducing in that way significantly the
frequency of false alarms. In the case of cardiac mon-
itoring, D1 could be an accelerometer, D2 - an ECG
monitor and D3 - pulse meter.
From the position of the system designer the ne-
cessity of an open, interoperable system is evident.
If the best sensors of the classes D1, D2 and D3 are
available from different producers, we want to be able
8
http://nanthealth.com/, accessed May 7, 2014
9
http://www.isirona.com/, accessed May 7, 2014
to connect them into a closely coupled system - with
one communication device executing the event detec-
tion algorihm and with well synchronized clocks.
As far as possible, existing standards have to be
respected. They currently form a multicolor patch-
work of partly overlapping initiatives backed by vari-
ous organizations (St Cyr, 2013). The list of ECG for-
mats alone is overwhelming (Bond et al., 2011). Over
the time, the technology and market forces will deter-
mine the optimal solutions, whereas open standards
will still coexist with proprietary standards enforced
by major players. In the area of data exchange with
medical devices, the predominant standard on the se-
mantic level is HL7
10
. At the lower level the major
standards are ISO/IEEE 11073
11
(Yao and Warren,
2005), (Trigo et al., 2014) and ASTM F2671
12
. It is
to be examined which competing standards will even-
tually predominate and to what extent they are appli-
cable to low power micro/nano sensors.
4.2 Configuring and Distributing the
Devices
Distributing smart medical devices to the patients is a
complex task. It is difficult from the medical point of
view, especially when the device has to be implanted
(Kramme, 2007), (Lemke et al., 2013). Configuring
a device like a defibrillator requires a special blend
of medical and technical knowledge. This knowledge
is today available in good hospitals. However, if the
composite device has to be configured from individual
sensors into a working Body Area Network together
with an application on a commercial smartphone, we
enter a new domain. In order to set a wireless net-
work, a good network supporter is necessary. Just
trying to assign the tasks of installation and support
to the clinical staff is unfeasible (Wagner et al., 2013).
On the other hand, working with frail and elderly pa-
tients is not a common skill among the network sup-
porters.
4.3 Support
Similarly, establishing a support service is not triv-
ial. We have to remember that not only the patients’
calls have to be handled. The data coming from the
devices may also indicate problems that are not vis-
ible to the patients but require an intervention. For
example, missing or wrong values (outliers) suggest a
10
http://http://www.hl7.org/, accessed Oct.15,2014
11
http://www.continuaalliance.org/accessed Oct.15,
2014
12
http://www.astm.org/Standards/F2761, accessed
Oct.15, 2014
ResearchandEngineeringRoadmapforDevelopmentandDeploymentofSmartMedicalDevices
223

Figure 3: Medical device and its environment.
wrong fixation of the device, empty battery or a sim-
ilar cause. In this case the medical personnel has to
contact the patient, and depending of the severity of
the disease it may be urgent. With a limited number
of patients, normal personnel is sufficient, but above
a certain limit a special organizational unit is neces-
sary, especially that such cases will require not only
medical knowledge but also profound technical skills.
4.4 Quality Control
4.4.1 Smart Medical Devices as Complex
Cyber-physical Systems
A device does not work on its own, but is a part of
an environment that has to be included in the quality
assessment (Fig. 3). It consists of humans, nature and
other technical systems.
The patients are biologically different and may re-
spond differently to the same therapy. In general, the
device may be adequate for their medical condition or
not. If they have to perform some operations them-
selves (attach the device, input settings, read and in-
terpret the messages), the result will depend on their
motoric and cognitive skills.
Among other participating humans we have sev-
eral groups of helpers, whose services will be of value
if they master the necessary novel mixture of ap-
proaches and skills that are currently provided by var-
ious isolated communities (Fig. 4).
As the software based, networked medical devices
communicate electronically with the external world,
they are susceptible to attacks from hackers. They
store and transmit valuable patient data which are in-
teresting to various sorts of data thieves. Aside from
the electronic attacks, the devices can be physically
stolen or destroyed.
The forces of nature play an important destruc-
tive role - the devices break, they corrode inside the
body, fixations loosen, sensors get dirty and nozzles
get clogged. Flesh, bones and cloth attenuate the sig-
Figure 4: Merging approaches and skills.
nal propagation in the Body Area Networks. Land-
scape and buildings obstruct the phone signal.
Networked devices depend on other technical sys-
tems. They need electrical power, phone signal and
Internet connection, possibly also GPS positioning.
We cannot take their existence for granted. On the
other hand, nearby systems may introduce noise and
jam the useful signal. Other wireless systems may use
the same bandwidth and compete for channel capac-
ity.
4.4.2 Formal Approval and Reimbursement
The approval process of medical devices is relatively
new, compared with the process for the drugs (Yin,
2012). In every market the respective regulatory
agency defines precise rules of approval for the med-
ical devices (Abdel-Aleem, 2009). They are divided
in several classes, depending on the possible risk to
the patient. Usually only for the high risk (Life-
Saving and Life-Sustaining) devices the results of a
well-controlled clinical investigation have to be pro-
vided. FDA has recently issued the guidance re-
garding the mobile medical applications
13
that de-
fines which apps are the focus of regulatory over-
sight, which may meet the definition of medical de-
vice but are exempt from enforcement and which are
not considered medical devices. Similarly, the Euro-
pean Commission is working on the update of its reg-
ulatory framework
14
. A good analysis from technical
and legal poin of view can be found in (Sorenson and
Drummond, 2014). As the process is highly dynamic,
we will not comment it here in detail.
In the design phase a risk analysis has to be per-
formed. The main standard used in this context are
ISO 14971:2007 (Application of risk management to
medical devices) and IEC 80001-1:2010 (Applica-
13
http://www.fda.gov/downloads/MedicalDevices/Device
RegulationandGuidance/GuidanceDocuments/UCM263366
.pdf, accessed Mar. 26, 2014
14
http://ec.europa.eu/health/medical-devices/regulatory-
framework/, accessed Oct. 10, 2014
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
224

tion of risk management for IT-networks incorporat-
ing medical devices). The paper (Alemzadeh et al.,
2013) provides an in-depth analysis of this process.
A complex example of its application for hemodial-
ysis devices is shown in (Lodi et al., 2010). The re-
sults depend however on the actual assiduity of the
people who perform the assessment, on their techni-
cal knowledge and capacity to imagine rare and novel
risks.
The most trusted method is a randomized control
trial. A double-blind experiment with medical de-
vices is difficult to implement (Potapov et al., 2011),
(Lipton et al., 2010) and (Castro et al., 2010) as it
is not easy to construct such a fake device that it is
not noticed by the patient. The paper (Zannad et al.,
2014) presents an extensive analysis of the challenges
facing the trials using the example of cardiovascular
medical devices. The approval process is however far
from perfect (Teow and Siegel, 2013) and risky de-
vices may reach the market (Hauser, 2012).
Another important factor is the reimbursement of
the therapies by insurances or government authorities.
There is a large market for consumer devices that are
useful in prevention, e.g. supporting a healthy life
style. The professional devices are however more ex-
pensive and their large scale use is only possible if
they are reimbursed. Therefore the respective payers
require a proof of their medical efficacy as well as of
economic efficiency (what therapy improvement for
what price). The new devices not only induce costs
- extensive use of medical devices may increase the
possibilities of ambulant treatment and hence have a
positive influence on the general costs of the system.
4.4.3 Continuous Monitoring
For many reasons a one-time clinical study is not suf-
ficient for a thorough evaluation of smart medical de-
vices. The devices operate in a variable, undefined
environment consisting of natural and technical con-
ditions, as well humans that use and support them. A
patient can be allergic to the used material, another
can misunderstand the instructions. During travel
phone signal can be missing or bad roaming contract
may inhibit the data transfer.
All detected problems may lead to an update of the
device - its mechanical part (e.g. fixing) or software.
The operating procedures may be changed or the per-
sonnel and the patients may be better instructed. In
all such cases the compound (device + environment)
is not the same, what formally invalidates the previous
tests.
Assessing the quality of updated software is a hard
statistical problem. Let us assume that the basic med-
ical algorithm is correct, but due to bugs the software
Figure 5: Software versioning.
crashes from time to time (Fig. 5). As said before,
a device with a changed software is not the same de-
vice. We have however already collected a valuable
set of data, and resetting the measurements would
be wasteful. Only some well known negative effects
were caused by the bug, the rest is unchanged.
Another factor is time. The device may wear out
with time. The quality of unchanged software, e.g.
without security patches, deteriorates with time
15
.
We want to know the long term effects of the ther-
apy but the necessity of a long clinical trial collides
with the pace of technical progress. Especially if we
require - correctly - that during all conditions remain
exactly the same, the result will be of no value. More-
over, we need to notice any change in the infrastruc-
ture, the deployment of new features (software and/or
hardware) to be able to statistically assess the effect
of the changes.
We therefore advocate to collect data during the
operation of the devices for continuous surveillance
of installed devices in order to detect hidden flaws,
rare defects and results of material wearout and other
forms of degradation. On one hand, we will obtain
problem notifications regarding specific patients that
require contacting them, i.e. knowing their identities.
On the other hand, we will have general results con-
cerning the efficacy of therapy methods and quality
of device classes and models. This information (with
identities removed) will be analyzed by good statisti-
cians and forwarded to the producers and to the ap-
proval authorities.
Those issues are well understood by the med-
ical community. (Sedrakyan et al., 2013) present
the rationale for an international registry of cardio-
vascular devices, (Kesselheim et al., 2014) even ar-
gue for compulsory postmarket research. Several
organizations and initiatives are active in this field.
In the USA we can name the Institute of Medicine
15
http://www.wired.com/2014/01/theres-no-good-way-
to-patch-the-internet-of-
things-and-thats-a-huge-problem/, accessed Oct. 20, 2014
ResearchandEngineeringRoadmapforDevelopmentandDeploymentofSmartMedicalDevices
225

(IOM), Patient-Centered Outcomes Research Insti-
tute (PCORI), FDA’s Sentinel Initiative, Medical De-
vice Epidemiology Network Initiative (MDEpiNet)
or MedWatch, The FDA Safety Information and Ad-
verse Event Reporting Program. Even if their num-
ber suggests overlapping competences, it shows also
a growing importance of the issue.
5 ORGANIZATIONAL
FRAMEWORK
5.1 Basic Scenario
Having discussed the challenges we face, we pass
now to the presentation of the outline of a solution we
propose in this paper. The proposed organizational
units cover the tasks described before. The informa-
tion flow ensures a smooth functioning of the system
and permits to all participants to produce and receive
necessary data.
We consider here a health support system regard-
ing a single medical problem, or a group of related
problems, e.g. cardiovascular diseases. It can be sub-
divided in following parts (Fig. 6):
• Patient’s System: the set of devices delivered to
the patient, the local network and installed software
• Infrastructure System: hospitals and other sup-
porting entities, communicating with the patients via
Wide Area Network
◦ Direct Health Support
◦ Quality Control and Evaluation
Figure 6: Subsystems: patient (PS) and infrastructure (IS);
actors and data flows.
It has to provide following functionality:
• deployment and support
◦ configuring and delivering to patients
◦ network operation (connection with devices)
◦ contacting patients
• notifying producers, distributing updates
• quality assurance and effectiveness evaluation
◦ monitoring technical state
◦ detecting failures
◦ measuring medical effectiveness
• extracting medical knowledge for better therapies
5.2 Actors and Roles
The partners that cooperate in the structure outlined
above are: • Patients
• Hospitals
• Technical support
◦ Configurators
◦ Operators
◦ Call centers
• Producers
• Approval authorities
• Research institutes
We will further discuss the motivation of the part-
ners driving them towards the cooperation outlined
here.
5.2.1 Patients
The patients are the ones that should profit from the
novel therapies. Their feedback will be very useful
in finding and eventually fixing the problems not de-
tected in the laboratory setting. In cases when the sup-
porters see surprising data or detect data losses, the
help of the patients will permit to connect the IT prob-
lems to the events from real life and to identify their
actual causes. Moreover, sharing anonymous data for
research will enable a better understanding of the pro-
cess, provide evidence based medical knowledge and
help to evaluate and improve the deployed devices.
5.2.2 Hospitals
Every patient is related to a hospital, handling his/her
case. We will use the term “home hospital”. This
hospital keeps the patient’s record - on paper or in
electronic form.
5.2.3 Technical Support
In order to assure the operation of the system, a
number of functions has to be performed (Fig. 7).
These functions require special technical equipment
and a novel set of skills that may exceed the current
capacities of a typical hospital. They may be de-
livered by one or several organizations, depending
on the necessary skills and equipment, and whether
a direct contact to the patients and hospitals is needed.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
226

Figure 7: Technical support - subunits.
Configurators A configurator is a unit that con-
figures the complete set of devices received by a pa-
tient. It cares that all hardware components can effec-
tively communicate and that all software packages run
on the data aggregator used by the patient. This ag-
gregator may be a device (e.g. a smartphone) owned
by the patient or a special device preconfigured for
this task. The configurator performs also the final de-
livery to the patient and gives him/her the necessary
instruction. Having a supply of physical devices, the
configurator will be contacted if a service or replace-
ment is needed. The configurator acts on behalf of
the hospital and provides the necessary technical ex-
pertise, missing at the hospital. As the configurators
interact with patients, they need to maintain local of-
fices. In case of implantable devices, the operation is
performed at the hospital, this however seems not to
require the presence of the configurator. Actual con-
nection into a functioning Body Area Network (BAN)
will rather be done later, after the scar is healed. This
geographical distribution permits various forms of or-
ganization - as separate, local companies or connected
in one enterprise.
Operators An operator is a unit that supports the
continuous function of the system of distributed med-
ical devices. We think here mostly of assuring the
network connection between the patients’ aggregator
devices and the hospital and other data destinations.
The operator has to perform all transformations on
data received from the patients and distribute them
according to the predefined scheme. The operator has
to know all communications partners. It also has to
know the types and versions of all devices (sensors,
actuators) used in the system and their producers in
order to generate the status and malfunction notifica-
tions. Likewise, the information about the actual con-
figuration of the device sets delivered to the patients
is necessary for specific service functions, e.q. status
inquiries or software updates.
Call Centers The first function of a call center
is receiving remarks, complaints and service requests
from the patients. Equally important are contacts with
the patient initiated by the service providers (Fig. 8).
The device set delivered to the patient may automat-
ically inform about a problem of which the patient is
not aware. Sensor values systematically out of range
suggest a wrong position or weak contact with the
body. This again can be caused by dirt, humidity or a
loosened fixation. Signal from a device will be miss-
ing if its battery is drained or if the device was un-
comfortable and has been removed. It also could be
broken, lost or stolen. The effective cause is unknown
to the service providers. Therefore the patient has to
be personally contacted, otherwise his/her system will
not be working. The patient can correct the installa-
tion of the devices, but if the problem is caused by a
property of the installed devices, like weight, size or
noise, it rather gives a valuable input to the producers
who should improve the design.
Figure 8: Call center - active contact.
Evidently, if the contact has to be efficient, the pa-
tient has to be available and be in the position to con-
centrate on the problem. Therefore a second call at
another time of the day may be needed, or a follow-
up call if the problem persists or reappears after a cer-
tain time. We see that the tasks of a call center are
very similar to a Customer Relationship Management
(CRM) System. A 24 hours / 7 days operation is rec-
ommended. In addition to the technical and medi-
cal fundamentals, the personnel needs local language
skills and the ability to interact with ill, elderly, un-
certain or angry people.
Producers
The individual devices (sensors, actuators) have
various producers. Typically, they will provide their
own means for reading the status and data recorded
in the devices or for updating the software. In our
case, it is important that the patient’s set of devices is
treated as a system. The operator should know about
the deployed software versions, in our design it is also
the main distributor of the information. This does not
ResearchandEngineeringRoadmapforDevelopmentandDeploymentofSmartMedicalDevices
227

exclude sending data directly to the producer who has
own established programs for data analysis and qual-
ity control. It is also possible that in the initial phase
the procedures deployed in the system proposed here
are not equally mature. It is understandable that the
producer is interested in keeping its trade secrets and
in treating the faults in its products discreetly. On
the other hand, we promote here openness, and this
finally serves all. It is to be decided if the produc-
ers should have a direct commercial relationship with
the end users (and consequently know them) or not.
We endorse the solution where they are isolated from
them by the interface of the configurators and opera-
tors of our system.
They should be informed about the performance
of the devices, mechanical, electrical and networking
issues. The software update process has to be defined:
in what cases the producer is obliged to deliver an up-
date (e.g. software crash, new security leak) and what
are the test and approval procedures.
Approval Authorities
The proposed system should be integrated in the
postmarked surveillance program of the approval au-
thorities. It can generate automatic warnings about
the malfunction of the devices. One goal is ensuring
the patients’ safety. Therefore emergency cases that
put their health in danger have to be reported imme-
diately. Another goal is to evaluate the effectiveness
of the methods and the quality of specific devices. In
the proposed system, long term progress reports can
be generated. They should not be treated as the sole
basis for decisions, they can however be useful in pro-
viding sound quantitative foundation. If the therapies
are refunded, their cost-effectiveness should also be
evaluated. The exact contents and frequency of re-
ports are subject to an individual agreement. In the
case of internationally marketed products, many au-
thorities may be involved.
Research Institute
The main task of the research institute is to sup-
port the registry. The goal of the registry has been
presented in the section 4.4.3. The researchers have
to define the logical structure of registry items that is
a reasonable compromise between diverging require-
ments: heterogeneity, variability and stable structure
for comparison and research. The actual implemen-
tation and hosting may be provided by a partner with
stronger IT skills. The researchers use this data col-
lection to answer questions leading to better medical
and economical decisions. They publish scientific pa-
pers and produce reports for cooperating medical so-
cieties, government offices or for general public.
The researchers have to bring skills in relevant
medical fields and in statistics. The environment is
very far from a clean randomized trial. The patients
represent a known, but neither predefined nor bal-
anced assortment of age, gender, life-style and other
properties. The devices are applied as decided by the
hospital, their elements are replaced, software is fixed
and updated. This makes producing truthful statistics
extremely challenging.
5.3 Driving Forces
The proposed scheme is based on the cooperation of
many partners. It will never function in real life if
they are not sufficiently motivated to participate.
The patients want to receive the best treatment
possible and the hospitals want to provide it. Both
of them want to have freedom of choice of therapy
and devices and to avoid a vendor lock. With the reg-
istry, we propose a secondary use of patients’ data,
not aimed directly to support their own health. Ul-
timately the goal of collecting those data is to serve
them better in optimizing the therapy and eliminating
device faults. This approach corresponds to the trend
towards the predictive, personalized, preventive, par-
ticipative (P4) medicine (Hood, 2013).
Strong, established device producers often prefer
to protect their market share with proprietary stan-
dards. This is a winning strategy only for dominant
players, as for the less strong ones it would limit the
possible options to own products, what may reduce
their chances for new customers. For small, innova-
tive producers, participating in open standards is the
only way to reach the market.
The producers may be reluctant to participate in
the quality monitoring scheme, as it discloses the de-
fects of their devices. On the other hand, not hiding
the issues makes the impression of playing a fair game
and should eventually increase the trust of the patients
towards such producers.
In many countries there is a growing pressure
from the side of the official bodies, like approval au-
thorities to cooperate in the evaluation of medical
effectiveness and financial efficiency. This pressure
may be a decisive argument for participation.
The framework proposed here intends to provide
solution for several basic cases, each of them having
slightly different participants with different interests
and motivations. The following diagrams show those
participants and the respective information flows for
the considered cases:
• Health support (Fig. 9)
• Quality assurance (Fig. 10)
• Medical research (Fig. 11)
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
228
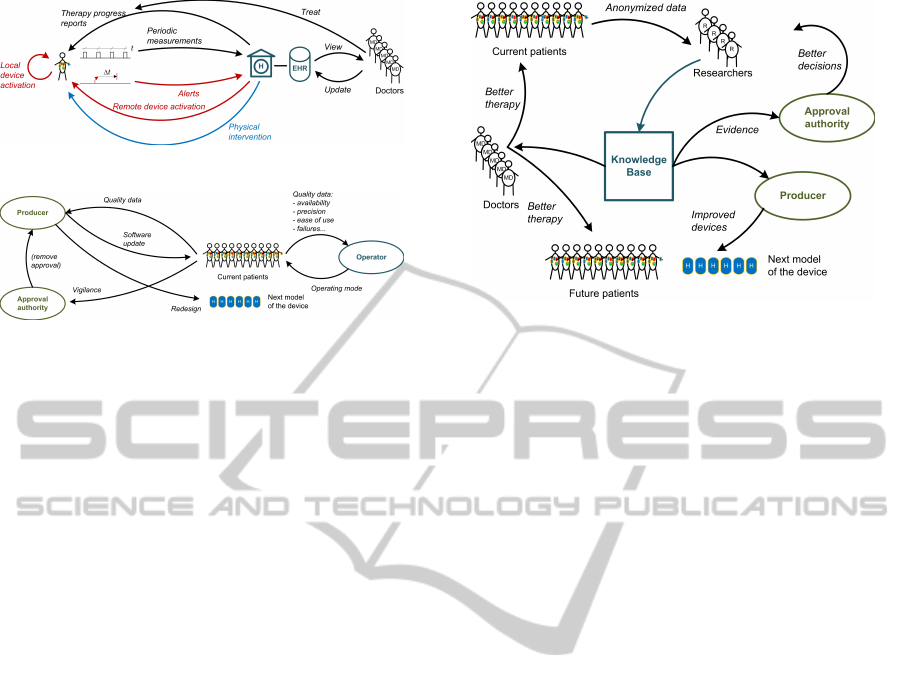
Figure 9: Data flow for health support.
Figure 10: Data flow for quality assurance.
6 DEVELOPMENT ROADMAP
In the preceding sections we have presented the issues
hindering the widespread adoption of smart medical
devices and shown an organizational structure that
should facilitate this adoption. Now we want to out-
line a roadmap that sets the overall structure of a so-
lution permitting to construct flexible healthcare sys-
tems based on smart medical devices. We aim at open
systems where all producers can concentrate on their
core capacities and deliver elements of best quality
that can be selected by the configurators without be-
ing locked by vendors’ proprietary, undisclosed stan-
dards. Our goal will be achieved if these ideas will
help to focus the future development or serve as a
basis for discussion. The terminology used in the
following text relates to the proposed organizational
structure, as shown in Fig. 6 and Fig. 7.
• Building Composite Devices
– Combining personal device clusters into com-
posed multi-element devices
– Selecting / expanding current interoperability
standards
– Creating a generic structure for smartphone ap-
plications for sensor/actuator networks
A modular smartphone application should be able
to integrate various devices from different vendors.
The devices have common features on various levels.
They send or receive several data types: events, sin-
gle values, waveforms. They can send data sponta-
neously (asynchronously or in a time raster) or when
requested. The semantics can be categorized into
a limited (but extensible) number of classes: blood
pressure, oxygen saturation, electrocardiogram. On
all those levels modular software structure has to be
Figure 11: Data flow for medical research.
defined. It is also to be analyzed to what extent the
emerging de facto standards (e.g. from Apple and
Samsung) are really applicable, flexible and extensi-
ble in the open environment.
• Integrating devices in the healthcare system
– Defining data extracts for various recipients
– Implementing data transformations and split-
ting data flow
– Solving related security and privacy issues
Data sent from the composite medical device to
the network operator module consist of a sequence of
items: events and measurements. The operator soft-
ware distributes them to the registered recipients (hos-
pital, producer, quality control, approval authority or
other). The recipients may register for certain types of
items related to various sources (patients). Depend-
ing on the recipient, the forwarded items may be pro-
cessed, e.g. anonymized. A recipient may also submit
an algorithm for processing the sequence of items to
detect interesting events. In such a case the sequence
would be processed locally at the operator on behalf
of the recipient that would be informed only if a de-
fined event occurs. For security and privacy protec-
tion, the data flow has to be verified and approved by
qualified (human) administrators. If basic access limi-
tations for a recipient are defined, he/she can also sub-
mit an algorithm that will be automatically attached to
the system if those limitations are respected. Such a
modular structure will permit to construct the opera-
tor software with no knowledge about the underlying
semantics.
• Monitoring quality
– Defining a flexible and expandable ontology
permitting to compare heterogeneous, evolving
devices
ResearchandEngineeringRoadmapforDevelopmentandDeploymentofSmartMedicalDevices
229

– Establishing a vendor-independent infrastruc-
ture for continuous assessment of devices qual-
ity and therapy methods effectiveness
The devices we want to integrate into our scheme
are heterogeneous, they come from various vendors
and their hardware and software may be frequently
updated. They show however certain similarities. For
example, implantable devices face similar problems:
short battery life, erosion by body fluids, mechani-
cal/chemical/electrical harm to the body, etc. Wire-
less nodes can lose data packets, their signal can be
attenuated by body tissue or clothing. They can also
be subject to malicious human actions like eavesdrop-
ping or impersonation. We see that a device, because
of its properties may belong to many classes. The goal
of the design of the registry is to capture these simi-
larities and to generate standardized data structures in
the database that will permit to make some founded
statements about the (absolute and relative) quality of
the devices and the efficacy of the therapy methods.
• Organization
– Organizing device configuration and distribu-
tion
– Establishing efficient data exchange between
deployed devices and healthcare institutions
– Setting an organization for handling special
cases initiated by patient or by hospital
– Solving international approval issues
– Ensuring a correct reimbursement for specific
medical cases
The tasks described above are necessary if we
want to pass from a technically possible solution to
a solution that is actually applied. Only if we succeed
here, the patients will effectively benefit from our ef-
forts. As the experience shows, overcoming organi-
zational hurdles and convincing potential partners to
enter into a cooperation may be more difficult than
providing the technology. However, a working tech-
nical solution is a condition for
• Statistics
Until now we have discussed the methods to en-
sure the data flow for the system operation and quality
assurance. The next step is to process the collected
data in order to obtain meaningful insights, helping
to choose the best devices and to evaluate the medi-
cal efficacy of the therapies, also with respect to the
costs. The setting is far from a clean clinical trial. The
composition of the patients’ cohort is statistically bal-
anced. The condition tested is not a simple yes or no
- during a treatment the device can be replaced wit a
new model, some elements can be updated, software
bugs can be fixed. Also the result is not reducible to
a single parameter. The algorithm of an insulin pump
may be correct, but the software may crash from time
to time. After a patch, this problem is solved. On
the other hand, the composition of the substance used
may be not optimal for some patients. The device may
be rejected by some patients as too obtrusive. The tip
of an implanted sensor may break. How to determine
which parts of the result are conserved when system
elements are modified? All this is an immense chal-
lenge to the statisticians.
7 CONCLUSION AND FUTURE
WORK
In this paper we have presented the technical and or-
ganizational obstacles that curb the widespread adop-
tion of mobile medical systems. We have then pro-
posed a modular framework of a solution. In the fol-
lowing we have outlined a roadmap that will permit to
channel the activities of the developers ant other part-
ners into a well coordinated effort. It defines the main
tasks and pinpoints the elements critical for the inter-
operability. In our opinion, it can be used as a draft of
an actual project plan. The list of tasks is long, there-
fore they have to be executed by many teams over a
longer period of time. We intend to be a part of it.
Achieving interoperability of smart medical de-
vices and reliable functioning of systems built upon
them is a tremendous challenge. However, without
engaging in this endeavor we will remain at the stage
of isolated solutions and the promising technologies
will not meet our justified expectations.
REFERENCES
Abdel-Aleem, S. (2009). Design, execution, and manage-
ment of medical device clinical trials. John Wiley &
Sons.
Alemdar, H. and Ersoy, C. (2010). Wireless sensor net-
works for healthcare: A survey. Computer Networks,
54(15):2688–2710.
Alemzadeh, H., Iyer, R. K., Kalbarczyk, Z., and Raman, J.
(2013). Analysis of safety-critical computer failures in
medical devices. Security & Privacy, IEEE, 11(4):14–
26.
Amit, G., Suleiman, M., Konstantino, Y., Luria, D.,
Kazatsker, M., Chetboun, I., Haim, M., Gavrielov-
Yusim, N., Goldenberg, I., Glikson, M., et al.
(2014). Sex differences in implantable cardioverter-
defibrillator implantation indications and outcomes:
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
230

lessons from the Nationwide Israeli-ICD Registry. Eu-
ropace, page euu015.
Bergsland, J., Elle, O. J., and Fosse, E. (2014). Barriers
to medical device innovation. Medical devices (Auck-
land, NZ), 7:205.
Bond, R. R., Finlay, D. D., Nugent, C. D., and Moore, G.
(2011). A review of ecg storage formats. International
journal of medical informatics, 80(10):681–697.
Castro, M., Rubin, A. S., Laviolette, M., Fiterman, J., Lima,
M. D. A., Shah, P. L., Fiss, E., Olivenstein, R., Thom-
son, N. C., Niven, R. M., et al. (2010). Effectiveness
and safety of bronchial thermoplasty in the treatment
of severe asthma: a multicenter, randomized, double-
blind, sham-controlled clinical trial. American journal
of respiratory and critical care medicine, 181(2):116.
Estrin, D. and Sim, I. (2010). Open mHealth architec-
ture: an engine for health care innovation. Sci-
ence(Washington), 330(6005):759–760.
Franke, M., Seidl, C., and Schlegel, T. (2013). A seamless
integration, semantic middleware for cyber-physical
systems. In Networking, Sensing and Control (IC-
NSC), 2013 10th IEEE International Conference on,
pages 627–632. IEEE.
Hauser, R. G. (2012). Here we go againanother failure
of postmarketing device surveillance. New England
Journal of Medicine, 366(10):873–875.
Hood, L. (2013). Systems Biology and P4 Medicine: Past,
Present, and Future. Rambam Maimonides medical
journal, 4(2).
Kesselheim, A. S., Rajan, P. V., et al. (2014). Regulat-
ing incremental innovation in medical devices. BMJ,
349:g5303.
Kramme, R. (2007). Medizintechnik: Verfahren, Sys-
teme, Informationsverarbeitung; mit 170 Tabellen.
Springer.
Kumar, S., Nilsen, W., Pavel, M., and Srivastava, M.
(2013a). Mobile health: Revolutionizing health-
care through trans-disciplinary research. Computer,
46(1):28–35.
Kumar, S., Nilsen, W. J., Abernethy, A., Atienza, A.,
Patrick, K., Pavel, M., Riley, W. T., Shar, A., Spring,
B., Spruijt-Metz, D., et al. (2013b). Mobile health
technology evaluation: the mhealth evidence work-
shop. American journal of preventive medicine,
45(2):228–236.
Lemke, B., Fr
¨
ohlig, G., Jung, J., Carlsson, J., and Koglek,
W. (2013). Herzschrittmacher-und Defibrillator-
Therapie: Indikation-Programmierung-Nachsorge.
Georg Thieme Verlag.
Lipton, R. B., Dodick, D. W., Silberstein, S. D., Saper, J. R.,
Aurora, S. K., Pearlman, S. H., Fischell, R. E., Rup-
pel, P. L., and Goadsby, P. J. (2010). Single-pulse
transcranial magnetic stimulation for acute treatment
of migraine with aura: a randomised, double-blind,
parallel-group, sham-controlled trial. The Lancet Neu-
rology, 9(4):373–380.
Lodi, C. A., Vasta, A., Hegbrant, M. A., Bosch, J. P.,
Paolini, F., Garzotto, F., and Ronco, C. (2010). Mul-
tidisciplinary evaluation for severity of hazards ap-
plied to hemodialysis devices: an original risk analy-
sis method. Clinical Journal of the American Society
of Nephrology, pages CJN–01740210.
Memon, M., Wagner, S. R., Pedersen, C. F., Beevi, F.
H. A., and Hansen, F. O. (2014). Ambient assisted liv-
ing healthcare frameworks, platforms, standards, and
quality attributes. Sensors, 14(3):4312–4341.
Mohr, D. C., Cheung, K., Schueller, S. M., Hen-
dricks Brown, C., and Duan, N. (2013). Continuous
evaluation of evolving behavioral intervention tech-
nologies. American journal of preventive medicine,
45(4):517–523.
Pawar, P., Jones, V., Van Beijnum, B.-J. F., and Hermens,
H. (2012). A framework for the comparison of mobile
patient monitoring systems. Journal of biomedical in-
formatics, 45(3):544–556.
Potapov, E., Meyer, D., Swaminathan, M., Ramsay, M.,
El Banayosy, A., Diehl, C., Veynovich, B., Gregoric,
I. D., Kukucka, M., Gromann, T. W., et al. (2011).
Inhaled nitric oxide after left ventricular assist de-
vice implantation: a prospective, randomized, double-
blind, multicenter, placebo-controlled trial. The Jour-
nal of Heart and Lung Transplantation, 30(8):870–
878.
Sedrakyan, A., Marinac-Dabic, D., and Holmes, D. R.
(2013). The international registry infrastructure for
cardiovascular device evaluation and surveillance.
JAMA, 310(3):257–259.
Seeger, C., Laerhoven, K. V., Sauer, J., and Buchmann, A.
(2013). A Publish/Subscribe Middleware for Body
and Ambient Sensor Networks that Mediates between
Sensors and Applications. In Healthcare Informat-
ics (ICHI), 2013 IEEE International Conference on,
pages 199–208. IEEE.
Sliwa, J. and Benoist, E. (2012). A web architecture based
on physical data separation supporting privacy protec-
tion in medical research. International Journal of Re-
liable and Quality E-Healthcare, 1(4).
Sorenson, C. and Drummond, M. (2014). Improving medi-
cal device regulation: The united states and europe in
perspective. Milbank Quarterly, 92(1):114–150.
Speidel, D. and Sridharan, M. (2014). Quality Assurance
in the Age of Mobile Healthcare. The Journal of
mHealth, 1(2):42–46.
St Cyr, T. J. (2013). An overview of healthcare standards. In
Southeastcon, 2013 Proceedings of IEEE, pages 1–5.
IEEE.
Teow, N. and Siegel, S. (2013). Fda regulation of medi-
cal devices and medical device reporting. Pharmaceut
Reg Affairs, 2(110):2.
Tomlinson, M., Rotheram-Borus, M. J., Swartz, L., and
Tsai, A. C. (2013). Scaling up mHealth: where is the
evidence? PLoS medicine, 10(2):e1001382.
Trigo, J. D., Kohl, C., Eguzkiza, A., Mart
´
ınez-Espronceda,
M., Alesanco, A., Serrano, L., Garc
´
ıa, J., and Knaup,
ResearchandEngineeringRoadmapforDevelopmentandDeploymentofSmartMedicalDevices
231

P. (2014). On the seamless, harmonized use of
iso/ieee11073 and openehr. Biomedical and Health
Informatics, IEEE Journal of, 18(3):872–884.
Viswanathan, H., Chen, B., and Pompili, D. (2012). Re-
search challenges in computation, communication,
and context awareness for ubiquitous healthcare.
Communications Magazine, IEEE, 50(5):92–99.
Wagner, S., Hansen, F. O., Pedersen, C. F., Memon, M.,
Aysha, F., Mathissen, M., Nielsen, C., and Wesby,
O. (2013). Carestore platform for seamless deploy-
ment of ambient assisted living applications and de-
vices. In Proceedings of the 7th International Con-
ference on Pervasive Computing Technologies for
Healthcare, pages 240–243. ICST (Institute for Com-
puter Sciences, Social-Informatics and Telecommuni-
cations Engineering).
Wolpert, C., Lubinski, A., Bissinger, A., Merkely, B., Pri-
ori, S., and Brugada, J. (2011). Barriers to imple-
mentation of evidence-based electrical therapies and
the need for outcome research: role of European reg-
istries. Europace, 13(suppl 2):ii18–ii20.
Yao, J. and Warren, S. (2005). Applying the ISO/IEEE
11073 standards to wearable home health monitoring
systems. Journal of clinical monitoring and comput-
ing, 19(6):427–436.
Yin, G. (2012). Clinical Trial Design: Bayesian and Fre-
quentist Adaptive Methods, volume 876. John Wiley
& Sons.
Zannad, F., Stough, W. G., Pi
˜
na, I. L., Mehran, R., Abra-
ham, W. T., Anker, S. D., De Ferrari, G. M., Farb,
A., Geller, N. L., Kieval, R. S., et al. (2014). Current
challenges for clinical trials of cardiovascular medical
devices. International journal of cardiology.
BIODEVICES2015-InternationalConferenceonBiomedicalElectronicsandDevices
232
