
On the Cryopreservation of Individual Cells in Volumes Less than
Nano Liter
B. Galmidi
1
, Y. Shafran
1
, R. Orvieto
2
, N. Zurgil
1
and M. Deutsch
1
1
The Biophysical Interdisciplinary Schottenstein Center for the Research and Technology of the Cellome,
Physics Department, Bar Ilan University, Ramat Gan, Israel
2
Infertility & IVF Unit, Dept. Obstetrics & Gynecology, The Chaim Sheba Medical Center,
Tel Hashomer, Ramat Gan, Israel
1 STAGE OF THE RESEARCH
Micro-arrayed donut-shaped chambers (DSCs) are
miniature vessels which were developed in the frame
of this work. Each chamber is designed to act as an
individual isolated reaction compartment, which
creates an in-vitro assay, mimicking biology
environments. Such a device enables individual live
cell treatment and analysis, with the assistance of a
designated image processing algorithm. In this work,
we use DSCs for cryopreservation of individual
sperm cells. Preliminary experiments show that fluid
does not exit the donut during the freezing-thawing
cycle. Figure 1 shows fluorescent images of the
donut structure with fluorescein (5uM) drop, and
following the freezing-thawing cycle after partial
removal with blotting paper.
Figure 1: Fluorescein based preliminary experiment:
freezing-thawing cycle. A - Fluorescent image of donut
structure with fluorescein (5M) drop, B – following the
freezing-thawing cycle after partial removal with blotting
paper.
Next, Molt 4 cells were used for viability test after
freezing-thawing cycle. FDA and PI fluorescence
dyes were added to the cell suspension. Then,
suspension was loaded onto the donut array and into
a standard cryo-tube for the control experiment.
Donut array was carefully washed using a pipette
with a washing medium, and a bright field image of
the same region was taken before (Figure 2A) and
after (Figure 2B) washing. It is clearly seen that cells
in between donut structures can be easily washed
out, while cells within donuts retain their position. A
fluorescent image (Figure 2C), which was taken
after thawing, demonstrate that most cells inside the
donut structures are alive. The green fluorescing
spots are FDA positive cells (live cells), and the red
spots are PI positive cells (dead cells). Similar
experiments were performed using donuts of
differing diameters, with volumes between nl and l,
all showing similar results.
Figure 2: Cryo-preservation of individual Molt 4 cells
within donut structures. A - a bright field image was taken
after suspension was loaded onto the donut array B- the
same region after donut array was carefully washed. C-
Viability test of the cells following the freezing-thawing
cycle. The green spots are FDA positive cells (live cells)
and the red spots are PI positive cells (dead cells).
Finally, cryo-preservation of individual sperm
cells within donut structures was examined. Sperm
cells were collected using a micro-manipulator
pipette. The pipette, via the micromanipulator, was
brought into the proximity of the sperm cells
(suspended in a drop of medium, covered by oil
layer, (Figure
3
A) after which cells were collected
by gentle pumping. Then, the collected sperm cells
were released into cryo-preservation medium inside
donuts with varying diameters, covered by oil
(Figure 3B,C). Sperm cell suspension was also
loaded into a standard cryo-tube for the control
experiment. Results with sperm cells show that (a)
following freezing-thawing procedure most of the
cells (>90%) remained in the original donuts; (b) the
percentage of motile cells in the μl volume donuts
was about 80%, which is even greater than that of
the control experiment and (c) opposed to (b), sperm
cells in the lnL volume donuts, did not survive at
all.
88
Galmidi B., Shafran Y., Orvieto R., Zurgil N. and Deutsch M..
On the Cryopreservation of Individual Cells in Volumes Less than Nano Liter.
Copyright
c
2014 SCITEPRESS (Science and Technology Publications, Lda.)

Figure 3: Cryo-preservation of individual sperm cells
within donut structures – micro-manipulation technology.
(A) Sperm cell collection using a pipette which reaches
the sperm washing medium drop through a covering oil
drop. (B) Release of the collected sperm cell into cryo-
preservation medium inside the donut covered by oil (with
air bubble trapped inside). (C) Single sperm cell inside a
single donut in cryo-preservation medium.
The fact that vitality of sperm cells following
freezing-thawing cycle in relatively large volumes
was found to be extremely larger than that obtained
within the nL volumes, urges us to more directly
examine the dependency of vitality of sperm cells
upon the freezing-thawing volume. Cryo-
preservation medium drops containing sperm cells,
was injected to oil inside a petri dish with varying
diameter (volume) between 50-800μm, (Figure 4).
Figure 4: 4x microscopic image of cryopreservation
medium drops containing sperm cells, placed on a Petri
dish under oil.
Before freezing, about 80% of the sperm cells were
motile. Following the freezing-thawing cycle, more
than 99% of the sperm cells lost their motility. Only
the 800µm drop contained about 10% of the motile
cells. As a control experiment, a drop of 5μl with
sperm cells was frozen. Following thawing, about
50-60% of the cells remained motile.
To date, we have promising results with sperm
cells that have undergone the freezing-thawing cycle
in suspension volume of about 1-2 μl. Under the
same freezing-thawing procedure, the motility of
thawed sperm cells in a nl volume was
unsatisfactory. Results suggest that freezing sperm
cells in suspension volume less than 1 nl damages
the motility of thawed sperm cells.
2 OUTLINE OF OBJECTIVES
Design and production of cellular chip array of
nl containers of chosen size.
Examination of spermatozoa freezing-thawing
protocols on a nl volume scale.
Investigating the chemo-physical aspect of
freezing-thawing cycle of suspension in
nanoliter volume.
Finding an ideal method for sealing the small
volume container in order to avoid evaporation
(e.g. oil layer or hard cover).
Connecting a micromanipulator and a micro-
pump to the microscope system which will
enable control of minute (nl) volumes required
to fill individual DSCs.
Development of an efficient protocol for sperm
cryopreservation within a cellular chip array at a
single cell resolution.
3 RESEARCH PROBLEM
Initial attempts to cryopreserve and thaw
spermatozoa in nano liter containers yielded
exceptionally high mortality of cells, in comparison
with that obtained when
cryopreservation and
thawing of sperm cells was performed within
microliters containers. Furthermore,
with Molt4
cells, cryopreservation and thawing in nano liter
containers was quite successful. The conjecture that
the overcrowding of the nl containers may damages
the tennis racket like shape sperm cells, was
immediately rebutted when spermatozoa incubated
both in nl containers and in a standard cryotube,
yielded the same cell motility. Furthermore,
OntheCryopreservationofIndividualCellsinVolumesLessthanNanoLiter
89

spermatozoa have shown satisfactory motility after
thawing when fluid within the donuts was physically
connected with the fluid in between donuts.
These findings, i.e. the dependency of a
successful freezing-thawing cycle of sperm cells
upon freezing-thawing volume, shape of cell and
upon fluid contact lead us to suspect that a unique
physical mechanism might be the cause for the
explored phenomena.
Therefore, an analytical-physical research is
under way, to understand the microscopic changes in
the freezing-thawing process of water, solution and
cell suspension (with different shapes), at very small
volumes, together with a biology research aimed to
understand the effect of these changes on
spermatozoa. These will be followed by medical
research for the development of applied clinical
device and protocol.
4 STATE OF THE ART
Sperm freezing is one of the most explored fields of
cryobiology. The first freezing experiments in the
1930s were done with frogs. Cryopreservation of
human spermatozoa was introduced in the 1960s and
has been recognized as an efficient procedure for
management of male fertility. A great number of
medical conditions, as well as many biological and
environmental factors can cause low sperm count
temporarily or permanently, and require the freezing
and retrieval of very few sperm cells. However, the
conventional methods for sperm cryopreservation
are not suitable for cryopreservation of small
numbers of sperm cells, such as epididymal and
testicular spermatozoa. When individual cells are
cryopreserved, they are virtually undetectable after
thawing, in the large volume of a standard cryo-tube.
Efficient cryopreservation of surgically retrieved
spermatozoa reduces the number of surgical
interventions and evades the logistic problems
associated with coordinating oocyte retrieval with
spermatozoa retrieval. Although novel
cryopreservation approaches have been designed for
limited numbers of motile sperm in very small
volumes (Table 1), all with limitations and
disadvantages, to date, no trials have been conducted
to demonstrate that any single carrier is superior to
another. No fully established technique for
cryopreservation of a single human spermatozoon is
being used today by the majority of IVF
laboratories.
Furthermore, to date there is limited use of these
technologies in the majority of IVF programs. This
suggests that novel cryopreservation technology
designed to handle small numbers of sperm needs to
be further explored.
Table 1: List of biological and non-biological carriers
proposed for the cryopreservation of small numbers of
spermatozoa.
Cryopreservation
techniques
Main disadvantages
Microdroplets
(M. Gil-Salom,
2000)(C. Quintans,
2003)(E. Sereni, 2008)
(N. Bouamama, 2003)
Risk of cross-
contamination; shape and
size of dishes make it
difficult to handle and
store in conventional
freezers and liquid
nitrogen tanks
ICSI pipette
(Adamson, 2001)
(J. O. Sohn, 2003)
Not practical for long-
term storage; fragility of
ICSI pipettes; risk of
cross-contamination
Empty zona pellucida
(M. Montag, 1999)
(Y. Y. Hsieh, 2000)
(J. Liu, 2000)
(P. E. Levi-Setti, 2003)
(A. Cesana, 2003)
Risk of biological
contamination
Volvox globator
spheres
(A. Just, 2004)
Exposure to genetic
material from the algae;
constant source of algae
Alginate beads
(A. Herrler, 2006)
Decrease sperm motility
with encapsulation
Agarose microspheres
(D. A. Isaev, 2007)
Clinical value of this
approach not evaluated
Cryoloop
(F. Nawroth, 2002)
(T. G. Schuster, 2003)
(N. Desai C. C., 2004)
Open system: risk of
cross-contamination
Straws
(N. Desai D. G., 1998)
(V. Isachenko, 2005)
(I. Koscinski, 2007)
Not ideal for severely
impaired specimens;
sperm loss due to
adherence to the vessel
5 METHODOLOGY
5.1 Measurement System
Images are acquired using a motorized Olympus
inverted IX81 microscope (Tokyo, Japan). The
microscope is equipped with a sub-micron
Marzhauser-Wetzlar motorized stage type SCAN-
IM, with an Lstep controller; (Wetzlar-Steindorf,
Germany) and a filter wheel including fluorescence
cube (excitation filters, dichroic mirrors, and
emission filters, respectively) for fluorescein: 470-
BIOSTEC2014-DoctoralConsortium
90

490 nm, 505 nm long pass and 510-530 nm, for DSC
auto-fluorescence: 355-405 nm, 410 nm long pass
and 420-450 nm. All filters were obtained from
Chroma Technology Corporation (Brattleboro, VT,
USA). Objectives of X4/X10, X20 and X60 were
used for the nL, pL and fL DSCs, respectively. A
cooled, highly sensitive 14-bit, ORCA II C4742-98
camera (Hamamatsu, Japan) was used for imaging.
Olympus Cell^P software was used for image
analysis (Tokyo, Japan). TransferMan®NK2
Eppendorf micromanipulator and P625 Peristaltic
Pump are used to accurately control the minute nl
and smaller volumes, required for this work.
5.2 Original Developed Device
DSCs are micro-arrayed, miniature vessels, in which
each chamber acts as an individual isolated reaction
compartment (Figure 5). Individual live cells can
settle in the pL and nL DSCs, share the same space
and be monitored under the microscope in a
noninvasive, time-resolved manner. theDSCs was
designed and constructed to accommodatethe
requirements of cryopreservation, namely
thefreezing and thawing conditions. It is made of
materials having appropriate durability for
cryopreservation conditions and to adjust to
cryopreservationequipment generally and to cryo-
microscopyin particular.
Figure 5: Micro-arrayed donut-shaped chambers (DSCs).
The DSC arrays were fabricated using a Photo
Lithographic Patterning technique. A 175 µm thick
BSG glass type D263 was spin coated at 3500 rpm
with SU8-5 photoresist, to a thickness of 2-2.5µm.
The DSC array was patterned on the photoresist by
illuminating it through a prefabricated Chromium-
mask. This was followed by thermal annealing at
175˚C for 60 min, resulting in stiff and smoothed
surfaces of the structured SU8-5 donuts. Finally, the
arrayed glass was sawed into 5x5 mm
2
chips,
cleaned (mainly from glass debris) by water jetting,
dried with clean compressed air and kept in
antistatic bags until fabrication. Then, DSC arrays
were glued onto a standard microscope slide with a
small droplet of NOA81 cured by UV light for 25
sec. Donut structure device was suited for
conventional cryo-preservation by gluing it to a
spoon-like carrier (
Figure 6
) and storing in a
Standard cryo-tube.
Figure 6: A spoon-like carrier and a standard cryo- tube
for device storing.
5.3 Analytical-physical Research
For a theoretical understanding of the microscopic
processes involved in freezing and thawing of
minute volumes particularly, it is necessary to
develop the heat transfer equation for our specific
case of nL scale of cylindrical shape suspension. The
flow rate of heat energy (Q) through a surface at
distance d from the object core, is given by Fourier's
law and is proportional to the temperature gradient
across the surface:
(1)
where K is the thermal conductivity, A is the surface
area from where the heat energy is transferred, T is
the time-dependent temperature within the object,
andT
is the time-dependent object edge
temperature. Heat flows from a body to the liquid or
gaseous environment around it by convection,
following Newton's law of cooling:
(2)
where T
is the environment temperature (e), and h
is the heat transfer coefficient. When dividing
Equation 2 by Equation 1, we get:
(3)
The ratio
⁄
is known as "the Biot number"
(Bi). It is clearly seen that when Bi is very small,
one can overlook the temperature difference along
the cooling body, hence the conductivity. (accepted
criterion is 0.1
⁄
(DeWitt, 2007)).
Loss of heat through radiation is negligible due to
low temperature, and loss of heat through
conduction is negligible either due to the minuscule
OntheCryopreservationofIndividualCellsinVolumesLessthanNanoLiter
91
dimensions of the body. As the dimensions of a
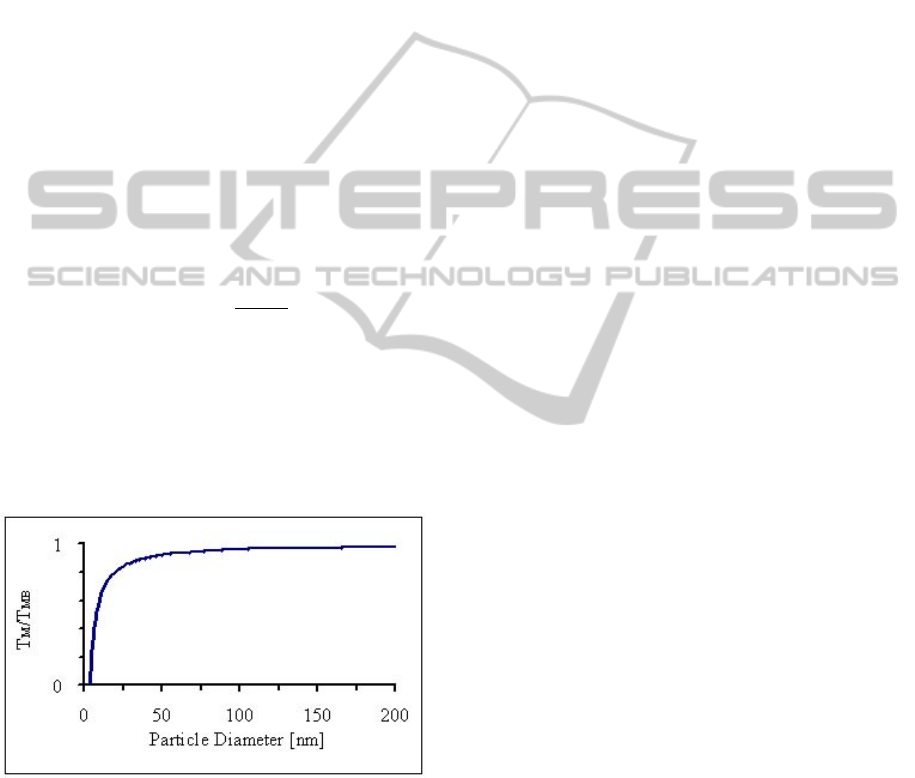
material decrease, the melting temperature scales
with the material dimensions (Figure 7).
Nanoparticles have a much greater surface to
volume ratio than bulk materials. The increased
surface to volume ratio means surface atoms have a
much greater effect on chemical and physical
properties of a nanoparticle. Surface atoms bind in
the solid phase with less cohesive energy because
they have fewer neighboring atoms in close
proximity compared to atoms in the bulk of the
solid. Each chemical bond an atom shares with a
neighboring atom provides cohesive energy, so
atoms with fewer bonds and neighboring atoms have
lower cohesive energy. The decrease in melting
temperature can be on the order of tens to hundreds
of degrees compared to the standard melting
temperature of a bulk (T
. The theoretical size-
dependent melting point of a material (T
d
can
be calculated through classical thermodynamic
analysis. The result is the Gibbs-Thomson equation
(Haas, 1936) below:
1
4
(4)
Where σ
is the solid-liquid interface energy, H
is
the bulk heat of fusion, ρ
is density of solid and d is
the particle diameter. Although suspension volume
in this work is larger than nanometer scale, pre-
melting is initiated at the corners and edges of the
crystals (Pan D, 2011), and must be considered in
this work.
Figure 7: A normalized melting curve for gold as a
function of nanoparticle diameter. Experimental melting
curves for near spherical metal nanoparticles exhibit a
similarly shaped curve.
6 EXPECTED OUTCOME
The aim of this work is to understand the unique
physical phenomenon of freezing in very small
volumes, and its effect on lodged live sperm cells.
This knowledge will make it possible to develop a
device, and protocols for freezing and retrieving
small numbers of sperm cells at a pre-selected
location, on a novel cryo-preservation chip. Such an
innovation will improve treatment of male infertility
for those who suffer from Oligo-Terato-
Asthenozoospermia (OTA) syndrome, as well as
those with azoospermia after Testicular Sperm
Extraction (TESE) procedure. Worldwide, there are
about 20,000 IVF labs. At the average, each
laboratory treats about 50 cases of low sperm count
related diseases a year, with unsatisfactory,
unsuitable cryopreservation procedures and means.
In this respects, we strongly believe that overcoming
the above mentioned temporary problems of
cryopreservation of individual sperm cells within
volumes less than nano liter, using our novel device
for cryo-preservation, will ensure there is no loss of
spermatozoa during the freezing-thawing process.
REFERENCES
A. Cesana, P. N. (2003). Sperm cryopreservation in oligo-
asthenospermic patients in Proceedings of
spermatozoa in yolk-filled human zonae pellucidae.
75(no. 4).
A. Herrler, S. E. (2006). Cryopreservation of spermatozoa
in alginic acid capsules. 85(no. 1).
A. Just, I. G. (2004). Novel method for the
cryopreservation of testicular sperm and ejaculated
spermatozoa from patients with severe oligospermia: a
pilot study. Fertility and Sterility, 445-447.
Adamson, M. G. (2001). A method of successful
cryopreservation of small numbers of human
spermatozoa. 76.
C. Quintans, M. D. (2003). Development of a novel
approach for cryopreservation of very small numbers
of spermatozoa. 15.
Cohn, j. R. (2012). Gibbs Thomson Equation . book on
Demand.
D. A. Isaev, S. Y. (2007). Artificial microcontainers for
cryopreservation of solitary spermatozoa.
DeWitt, B. L. (2007). Fundamentals of Heat and Mass
Transfer. John Wiley & Sons.
E. Isachenko, V. I. (2004). DNA integrity and motility of
human spermatozoa after standard slow freezing
versus cryoprotectant-free vitrification. 19(no. 4).
E. Sereni, M. A. (2008). Freezing spermatozoa obtained
by testicular fine needle aspiration: a new technique.
16(no. 1).
F. Nawroth, V. I. (2002). Vitrification of human
spermatozoa without cryoprotectants. 23(no. 2).
Haas, F. G. (1936). A Commentary on the Scientific
Writings of J. Willard Gibbs. New Haven,
Connecticut: Yale University Press, 544.
BIOSTEC2014-DoctoralConsortium
92

I. Koscinski, C. W.-K. (2007). Optimal management of
extreme oligozoospermia by an appropriate
cryopreservation programme. 22(no. 10).
J. Liu, X. Z. (2000). Cryopreservation of a small number
of fresh human testicular spermatozoa and testicular
spermatozoa cultured in vitro for 3 days in an empty
zona pellucida. 21(no. 3).
J. O. Sohn, S. H. (2003). Comparison of recovery and
viability of sperm in ICSI pipette after ultra rapid
freezing or slow freezing. 80.
M. Gil-Salom, J. R. (2000). Intracytoplasmic sperm
injection with cryopreserved testicular spermatozoa.
169(no. 1-2).
M. Montag, K. R. (1999). Laser-assisted cryopreservation
of single human spermatozoa in cell- free zona
pellucida. 31(no. 1).
N. Bouamama, P. B. (2003). Comparison of two methods
of cryoconservation of sperm when in very small
numbers. 31(no. 2).
N. Desai, C. C. (2004). Cryopreservation of single sperm
from epidydimal and testicular samples on cryoloops:
preliminary case report. 82.
N. Desai, D. G. (1998). A convenient technique for
cryopreservation of micro quantities of sperm. 70.
N. N. Desai, H. B. (2004). Single sperm cryopreservation
on cryoloops: an alternative to hamster zona for
freezing individual spermatozoa. 9(no. 1).
P. E. Levi-Setti, E. A. (2003). Cryopreservation of a small
number of spermatozoa in yolk-filled human zonae
pellucidae. 75(no. 4).
Pan D, L. L. (2011). Melting the ice: on the relation
between melting temperature and size for nanoscale
ice crystals. ACS Nano, 4562-9.
T. G. Schuster, L. M. (2003). Ultra-rapid freezing of very
low numbers of sperm using cryoloops. 18(no. 4).
V. Isachenko, E. I. (2005). Clean technique for
cryoprotectant-free vitrification of human
spermatozoa. 10(no. 3).
Y. Y. Hsieh, H. D. (2000). Cryopreservation of human
spermatozoa within human or mouse empty zona
pellucidae. 73(no. 4).
OntheCryopreservationofIndividualCellsinVolumesLessthanNanoLiter
93
