
The Value of a New Cancer Biomarker fHER-2
Proto-oncogene in the Diagnosis of Feline Mammary
Carcinoma
Maria Soares
1
, Jorge Correia
1
, José Cabeçadas
2
, Conceição Peleteiro
1
and
Fernando Ferreira
1
1
CIISA, Faculty of Veterinary Medicine, Technical University of Lisbon
1300-477, Lisbon, Portugal
2
Anatomical Pathology Service, IPOFG-EPE, 1099-023, Lisbon, Portugal
Abstract. The overexpression of the H
uman Epidermal growth factor Receptor-
2 (HER-2) oncogene in human breast cancer is associated with a poor prognosis
and a specific treatment. Because of its importance and as a first line option for
diagnosis, well established guidelines for its detection are based in
immunohistochemical techniques, Still, in Veterinary Medicine there is little
and inconsistent information about this subject. The aim of our study was to
achieve an optimal immunohistochemical protocol for detection of fHER-2 in
F
eline Mammary Carcinoma (FMC). Five commercial anti-HER-2 antibodies
were tested using three different protocols. The fHER-2 protein overexpression
was detected in 10 of the 30 FMC cases (33.3%), when the optimized protocol
was performed (associating the A0485 antibody with a longer antigen retrieval
method). These results suggest that fHER-2 may play an important role in
Feline Oncology and that the Cat can be a suitable animal model for human
breast cancer research.
1 Introduction
The HER-2/neu proto-oncogene encodes a 185kD transmembrane glycosylated
protein that belongs to the human epidermal growth factor receptor’s family [10]. In
humans, this gene is located on chromosome 17 and its amplification, identified in 20
to 30% of breast cancers, is an important diagnostic and prognostic marker [9]. In
most of the cases, HER-2 gene amplification leads to an increase in protein
expression levels which results in an increase number of HER-2 receptors in the cell
membrane. Because it is clinical relevance, evaluations of HER-2 status by
immunohistochemical (IHC) and by in situ hybridization assays were recently
validated by the A
merican Society of Clinical Oncology (ASCO). Also in last years,
Gentech/Roche companies engineered a humanized monoclonal antibody that inhibits
the receptor’s dimerization providing a longer survival period in breast cancer patients
[12].
In Feline Oncology, the mammary tumors are very common. Indeed, they are the
third most common tumor in clinical practice and represent 17% of the tumors in
female cats. F
eline Mammary Carcinomas (FMC) have display some particularities
that distinguish them from the dog mammary tumors. They are very aggressive (85%
Soares M., Correia J., Cabeçadas J., Peleteiro C. and Ferreira F..
The Value of a New Cancer Biomarker fHER-2 Proto-oncogene in the Diagnosis of Feline Mammary Carcinoma.
DOI: 10.5220/0003879300090017
In Proceedings of the International Workshop on Veterinary Biosignals and Biodevices (VBB-2012), pages 9-17
ISBN: 978-989-8425-94-2
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)

are malignant), showing a poor prognosis and a short survival period [15]; [3]; [4].
Recently, some studies had revealed a wide frequency range of mammary tumors
fHER-2+ (5%-90%) using one or three commercial antibodies [1]; [14]; [10]. Also,
the role of fHER-2 in oncogenic mechanisms remains unknown.
In this study we aim to improve the immunodetection of fHER-2 in order to obtain
a better evaluation of HER-2 status on formalin-fixed, paraffin wax-embedded tissue
sections of FMC. For this we used 5 different commercial antibodies (two of them
never used in feline samples) and three different antigen retrieval (AR) methods. In
the end, we intend to contribute for the characterization of the frequency of FMC-
fHER-2 and compare our results with others authors, for a better understanding
Beyond the potential applications in Veterinary Medicine, the study of the status of
this oncogene could have clinical relevance, while cats can be a suitable natural
model for studying human HER-2 positive breast cancer.
Finally, we also point out the future contributions that Engineering Sciences can
bring to improve the fHER-2 immunodetection and the immunotherapy of FMC’s.
2 Material and Methods
2.1 Sample Collection and Histology
The 30 mammary gland samples used in this study were obtained from the
Anatomical Pathology Diagnostic Service archives, Faculty of Veterinary Medicine,
Lisbon, Portugal and complemented with clinical information provided for each case.
These mammary specimens were fixed in formaldehyde and embedded in paraffin
blocks. Only samples of carcinomas fixed for less than 72 hours were considered for
the study (Table 1). For histologic examination, sections of 4μm thickness were
stained with haematoxylin-eosin (HE) and tumors were classified according to the
World Health Organization (WHO) criteria [7].
Table 1. Histologic classification according to the WHO and grading of the samples submitted
to immunohistochemical evaluation.
Histologic classification
Malignant
Grade
Samples (%) Total
Cribiform carcinoma
II 1/30 (3.3%)
11/30 (36.7%)
III 10/30 (33.4%)
Tubulopapillary
carcinoma
II 4/30 (13.3%)
9/30 (30%)
III 5/30 (16.6%)
Tubular
adenocarcinoma
II 2/30 (6.6%)
6/30 (20%)
III 4/30 (13.3%)
Mucinous carcinoma
III 1/30 (3.33%)
4/30 (13.3%)
Simple Carcinoma
I 1/30 (3.33%)
Solid carcinoma
III 1/30 (3.33%)
Squamous cell
carcinoma
III 1/30 (3.33%)
10

2.2 Immunohistochemical Study
HER-2/neu Antibodies. Five commercial antibodies were tested for fHER-2/neu
immunostaining on paraffin tissue sections: a rabbit polyclonal anti-human HER-2
(A0485 from DAKO, Glostrup, Denmark), two rabbit monoclonal anti-human HER-2
antibodies (4B5 from Ventana, Tucson, Arizona and SP3 from Zytomed, Berlin,
Germany) and two mouse monoclonal anti-human HER-2 antibodies (CB11 from
Zytomed and TAB250 from Invitrogen, Carlsbad, California). Each one of these
antibodies has literature showing that they recognize the HER-2 protein in human
tissues by binding to extracellular domain of the HER-2 receptor (TAB250 and SP3)
or to recognize the intracellular domain (CB11, 4B5 and A0485). We also note that
SP3 and TAB250 antibodies were used for the first time in order to detect fHER-2 in
this work.
Immunohistochemical Technique. Sections were mounted on Starfrost
®
microscope
slides and dried at 60ºC for one hour. Each slide were deparaffinized and rehydrated
in distilled water through a series of graded alcohols and then submitted to antigen
retrieval with buffer citrate solution (NaCH
3
COO, pH=6) in a water bath at 95ºC for
30 minutes or for 60 minutes as resumed in Table 1I. In parallel, to improve antigen
recognition of TAB250 antibody, we performed an enzymatic digestion of tissue
samples with Protease K (Zymed) for 10 minutes following manufacturer’s
recommendations. To exhaust endogenous peroxidase activity, a Peroxide-Block
solution (Zytomed) was applied for 10 minutes and each primary antibody was
incubated during 1h at room temperature. After several PBS washes, primary
antibodies were detected with a secondary antibody for 30 minutes (HER2easy kit
IHC from Zytomed) and 3,3’-diaminobenzidin-tetrahydrochlorid (DAB) was used as
the chromogen prior to counterstain with Mayer’s haematoxylin.
Positive and negative controls were obtained from human breast carcinomas
known to overexpress HER-2 receptor and previously classified as 3+ or, classified as
0 without HER-2 expression (see Table 3).
Table 2. Resume of the immunohistochemical protocols used for fHER-2 detection.
Primary antibody
Antigen retrieval
Clone
Dilution Incubation time
CB11
RTU 60’
Buffer citrate solution 95º C for
30’ and 60’
4B5
RTU 60’
A0485
1:250 60’
SP3
1:100 60’
TAB250
1:50 60’
Buffer citrate solution 95º C for
30’ and 60’
Proteinase K for 10’
RTU = Ready to use
Interpretation Criteria. Overexpression of fHER-2 was defined as a membranous
staining in more than 10% of neoplastic cells and staining was examined over the
maximum area of staining intensity according to the DAKO guidelines (Table 3).
11

Samples classified as 0 or 1+ were considered negative, whereas scores of 2+ or 3+
were considered positive. Cytoplasmic staining was considered nonspecific staining.
All slides were submitted to blind scoring by two independent DVM pathologists and
one DVM clinician. Any discordant interpretation was debated and settled using a
multiviewer microscope.
Table 3. Interpretation Criteria (HercepTest Interpretation Manual from DAKO).
Grade Interpretation
0
No staining.
1+
Weak, incomplete membranous staining in any proportion of tumor cells.
2+
Complete membrane staining that is either no uniform or weak in intensity but with
obvious circumferential distribution in at least 10% of cells.
3+
Uniform intense membrane staining of at least 10% of invasive tumor cells.
2.3 Statistical Study
The association between fHER-2 overexpression and grade of malignancy or
histological classification were assessed by Fisher’s Exact Test. Values of p < 0.05
were considered to reflect statistical significance.
3 Results
The mean age of the 30 queens at the time of mastectomy was 10.4 years. Cribiform
carcinoma (36.7%) was the most common type of malignant mammary tumor,
followed by the tubulopapillary (30%) and tubular carcinomas (20%). The histologic
grading reveals that almost all of these tumors (73.3%) were poorly differentiated
carcinomas, showing a grade III.
The immunodetection of fHER-2 by some commercial antibodies (CB11, 4B5 and
A0485) was revealed by a cellular membrane labeling in several FMC showing a
species cross reactivity. Positive (3+) and negative (0) controls show the label
intensity expected in all protocols (Figure 1).
Fig. 1. Images of positive and negative controls using a CB11 antibody after 60’ of antigen
retrieval. (A) Human positive control scored 3+ showing an intensive and continuous label of
cellular membrane (x400); (B) Human negative control scored 0, with no staining (x400).
A
B
12
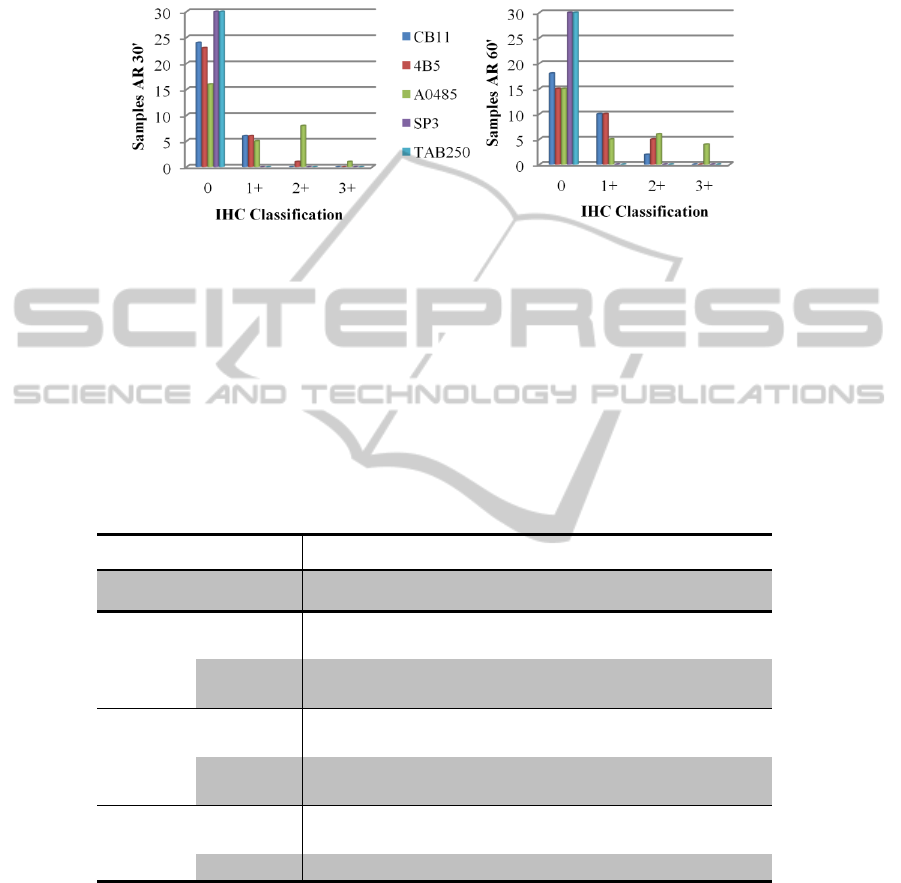
Results obtained from the employment of TAB250 and SP3 antibodies showed no
staining in all samples even after use a longer antigen retrieval protocol and in regards
of the others, the best results were achieve with A0485, as its shown in Figure 2.
Fig. 2. Results of IHC protocols using two antigen retrieval durations (30’ or 60’). Note that
regardless the protocol used, there are no staining for SP3 and TAB250. It’s also clear the
improvement in the results with 60’ of antigen retrieval in all the remaining three antibodies.
(A) Scores of IHC with the lower antigen retrieval (30 minutes); (B) Scores of IHC with the
longer antigen retrieval (60 minutes).
Table 4 summarizes our results, in which we observe a fHER-2 overexpression in
6.7% of the samples using CB11 antibody, 16.7% with 4B5 antibody and 33.3% with
polyclonal antibody A0485 from DAKO, when we use a longer antigen retrieval
method (Figure 3). When compared, all samples that demonstrated overexpression
with CB11 or 4B5 had the same or a better score with A0485.
Table 4. IHC results using the CB11, 4B5 and A0485 as primary antibodies.
IHC Classification 0 1+ 2+ 3+ TOTAL
Antibody / AR method
CB11
AR 30’ 24 (80%) 6 (20%) 0 (0%) 0 (0%) 30 (100%)
AR 60’ 18 (60%) 10 (33.3%) 2 (6.7%) 0 (0%) 30 (100%)
4B5
AR 30’ 23 (76.7%) 6 (20%) 1 (3.3%) 0 (0%) 30 (100%)
AR 60’ 15 (50%) 10 (33.3%) 5 (16.7%) 0 (0%) 30 (100%)
A0485
AR 30’ 16 (53.3%) 5 (16.7%) 8 (26.7%) 1 (3.3%) 30 (100%)
AR 60’ 15 (50%) 5 (16.7%) 6 (20%) 4 (13.3%) 30 (100%)
13
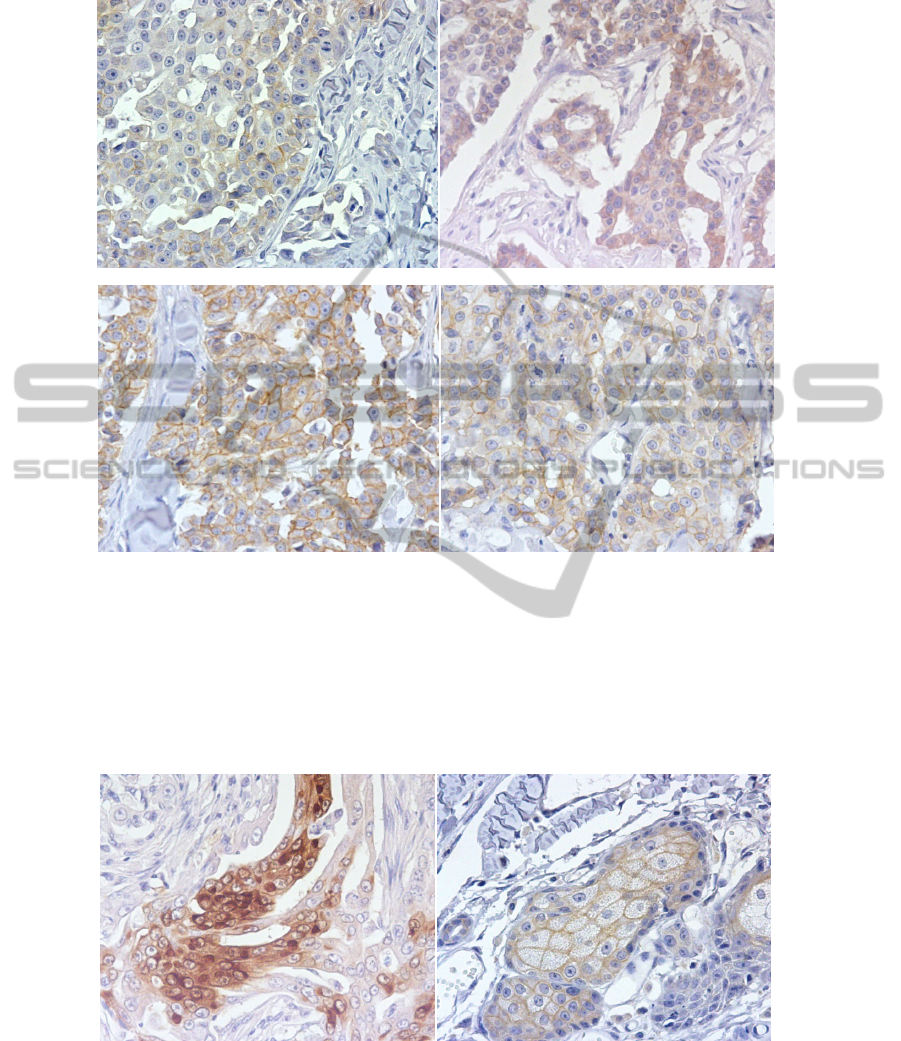
Fig. 3. Expression of fHER-2 in the same sample, classified as Cribiform Carcinoma (A) Score
classification 1+ using CB11 and 60’AR (x400). (B) Score classification 1+ using 4B5 and
60’AR (x400). (C) Score classification 3+ using A0485 and 60’AR (x400). (D) Score
classification 2+ using A0485 and 30’AR (x400).
The 4B5 antibody was the one that showed more cytoplasmic staining, with
samples with diffuse homogeneous staining in the cytoplasm and sometimes with dot
artifacts. Also a weak to moderate non-specific cytoplasmic labeling was seen in the
dermal adnexal structures (Figure 4).
Fig. 4. (A) Cribiform carcinoma scored 1+ with 4B5 and 30’ AR showing homogeneous no
specific cytoplasmic staining (400x); (B) Strong staining of a dermal adnexal structure, a
sebaceous gland (x400).
D
C
B A
A
B
14

When analyzed, fHER-2 overexpression in FMC did not evidenced significant
correlation with histological classification (p-value = 0.28) neither with malignance
grade (p-value = 0.47).
4 Discussion
In the past decades we have seen an increased attention and development of
companion animals health services, which create new needs that led to obvious
improvements and findings in Veterinary Medicine. If for one hand this made our pets
more resistant to diseases and with larger/bigger life-expectancy, on the other hand it
urged the rise of pathologies usually confined to the geriatric population, where the
neoplasms fit. Our animals share the same lifestyle as humans and start to be seen as
potential models to research, especially for Cancer Research.
In Cancer Research, the Molecular Biology has a fundamental role, and research
in this area continues to rise, with new information and discoveries published every
day. Unfortunately, in Veterinary Medicine there is little information available on
molecular alterations and the biological behavior of tumors in our companion
animals. In FMC, it is usual to request the histological and grading classification, but
the proteomic status of some receptors isn’t routinely performed.
In the present study we compared five different antibodies anti-HER-2, and we
have found different results, with the most promising one being the A0485, mostly
when combined with a longer antigen retrieval method than the time suggested by the
manufacturer (preferably 60 minutes AR duration), where we obtained an
overexpression of 33.3%, a result similar to those published for Human, with ranges
between 10 to 40% [5], [16].
For human patients, ASCO guidelines are very specific and with well defined
exclusion criteria. Among these recommendations the fixation is imperative and all
the samples fixed in fixatives other than neutral buffered formalin should be excluded.
Beyond this, the samples must be fixated for longer than 6h and less than 48hours
[16]. As our samples did not fulfilled all these requirements we can not exclude that
this may have influenced the results, especially for the SP3 and TAB250 antibodies,
that recognize the extracellular domain, which could be more affected by the
inadequate fixation and as consequence did not show any staining; nor can we
disregard the influence of fixation for the discrepant results obtained among the three
antibodies recognizing the intracellular domain.
The CB11 and the 4B5 antibodies are monoclonal while the A0485, with best
results, is a polyclonal antibody. If we associate the fact that the first two antibodies
that recognize a human epitope of the HER-2 protein, with a aminoacid sequence
homology of 93% (when compared to cat) and, the evidence that the latest antibody
recognizes several human epitopes of the same protein we can suspect that the wide
range of results (6.6% to 33.3%) that were obtained can be due, besides the fixation
problems, to the not total homology between the HER-2 and fHER-2, which makes
A0485 more suitable to recognize the protein.
We can also easily conclude that the antigen retrieval method is critical in the
immunohistochemical assessment of HER-2 in feline tissues and that when this step is
shorter (30’ instead of the 60’), it may significantly lower the threshold of positivity.
15

These findings are contrary to those of a recent study, where they found a decreased
positivity with most prolonged antigen retrieval [10].
If we compare our findings with the results present by other authors, the incidence
of fHER-2 overexpression is similar to some of the studies [1]; [7]; [10] but markedly
lower than in other reports [6]; [14]. Besides the interpretation criteria in one of these
studies being different to the one we have used [14] the other have respected the
tissue proceeding guidelines [6], which reinforces the importance of this step, so often
neglected. Indeed, in the majority of Diagnostic Services of Anatomical Pathology, it
is usual to receive samples that do not fulfill the requisites for a correct
immunohistochemical analysis. So, it is important to sensitize the clinicians and the
surgeons for this problem moreover since the histological classification and malignant
grade show to be insufficient to classify the tumors and, because none of them
demonstrated predictive value for determination of fHER-2 status in our studies,
which is concordant with others publications [6], [10].
The possibilities to use and introduce engineering sciences to improve the fHER-2
evaluation and anti-fHER-2 clinical treatments were studied. However, two extra
obstacles would have to be passed to achieve the total optimization of fHER-2
immunodetection in feline mammary tumors, whereas engineering can give an
important contribution. One of them is the automatization absence of the technique
which leads to different results between different laboratories and the other is the
interobserver subjectivity in scoring the HER-2 expression in formalin-fixed,
paraffin-embedded breast cancer tissues, due to a very high cellular heterogeneity and
to an extensive calcification/necrotic tumor areas [13], [2].
In human oncology, the American Society of Clinical Oncology (ASCO)
recommends the use of standardized operating procedures, the validation of
laboratories methodologies and also suggests that two or more expert pathologists
should score independently the same patient’s tissue sample to avoid wrong
classifications. To minimize the variability of the results and enhance the
reproducibility, automated systems were developed recently for human samples,
where all the steps of the technique can be regulated (from the deparaffinization of the
tissues till the mounting of slides). Also very recently, a new Automated Cellular
Imaging System (ACIS®, Clarient ChromaVision Medical Systems) was announced
to standardize the detection, the counting and the classification of tumor cells based
on recognition of cellular bodies with a specified shape, size and color.
In the near future, we think that Engineering Sciences can bring a substantial
contribution by developing/adapting the automatized devices similar to the ones used
in the human tissues processing. Additionally, we see as a very promising tool the
improvement of the adjunctive computer-assisted methodology to feline mammary
carcinomas samples, providing reproducibility in the acquisition and scoring of
immunohistochemical images evaluated by a qualified pathologist, after the
development of new image processing algorithms.
Acknowledgements
The authors thank Dr. Rita Ribeiro for the statistical analysis, Dr. João Matos and Dr.
16

Sandra Carvalho from the Anatomical Pathology Service. This work was supported
by CIISA and FCT (SFRH/BD/70720/2010).
References
1. De Maria R., Olivero M., Iussich S., Nakaichi M., Murata T. Bartolomeo B. and Di Renzo
M.F.. (2005). Spontaneous feline mammary carcinoma is a model of HER2 overexpressing
poor prognosis human breast cancer. Cancer Research, 65, 907-912.
2. Gancberg D., Järvinen T., Leo A., Rouas G., Cardoso F., Paesmans M., Verhest A., Piccart
M., Isola J. and Larsimont D. (2002). Evaluation of HER-2/NEU protein expression in
breast cancer by immunohistochemistry. Breast Cancer Research and Treatment, 74, 113–
120.
3. Martin de las Mulas, J., Van Niel M., Millán, Y., Blankenstein M.A., Van Mil F. and
Misdorp W. (2000). Immunohistochemical analysis of estrogen receptors in feline
mammary gland benign and malignant lesions: comparison with biochemical assay.
Domestic Animal Endocrinology, 18, 111–125
4. Martin de las Mulas, J., Van Niel M., Millán, Y., Ordás J., Blankenstein M.A., Van Mil F.
and Misdorp W. (2002). Progesterone receptors in normal, dysplastic andtumourous feline
mammary glands. Research in Veterinary Science, 72, 153-161.
5. Ménard S., Tagliabue E., Campiglio M. and Pupa S.M. (2000). Role of HER2 Gene
Overexpression in Breast Carcinoma. Journal of Cellular Physiology, 182, 150–162.
6. Millanta F., Calandrella M., Citi S., Della Santa D. and Poli A. (2005). Overexpression of
HER-2 in feline invasive mammary carcinomas. Veterinary Pathology, 42, 30-34.
7. Misdorp W., Else R.W., Hellmen E. and Lipscomb T.P. (1999). Histologic Classification of
Mammary Tumors of the Dog and the Cat. Armed Force Institute of Pathology and World
Health Organization. Washington, DC.
8. Ordás J., Millán Y., Dios R., Reymundo C. and de Las Mulas J.M. (2007). Proto-oncogene
HER-2 in normal, dysplastic and tumorous feline mammary glands. BMC Cancer, 7(179).
9. Press M., Slamon D., Flom K., Park J., Zhou J. and Bernstein L. (2002). Evaluation of
HER-2/neu Gene Amplification and Overexpression. Journal of Clinical Oncology, 20,
3095-3105.
10. Rasotto R., Caliari D., Castagnaro M., Zanetti R. and Zappulli V. (2011). An
Immunohistochemical Study of HER-2 Expression in FMC. Journal Comparative
Pathology, 144, 170-179.
11. Sáez A., Andreu F.J., Seguí M.A., Baré M.L., Fernández S., Dinarés C. and Rey M. (2006).
HER-2 gene amplification by CISH compared FISH. The Breast, 15, 519-527.
12. Stebbing J., Copson E. and O’Reilly S. (2000). Herceptin (trastuzamab) in advanced breast
cancer. Cancer Treatment Reviews, 26, 287-290.
13. Thomson T., Hayes M., Spinelli J., Hilland E., Sawrenko C., Phillips D., Dupuis B. and
Parker R. (2001). HER-2/neu in Breast Cancer. Modern Pathology, 14 (11), 1079-1086.
14. Winston J., Craft D.M., Scase T.J. and Bergman P.J. (2005). Immunohistochemical
detection of HER-2/neu expression in spontaneous feline mammary tumours. Veterinary
and Comparative Oncology, 3, 8-15.
15. Withrow, S. J. and Vail, D. M. (2007). Withrow & MacEwen's Small Animal Clinical
Oncology. (4
th
Ed.). Saunders, Philadelphia: Elsevier Inc.
16. Wolff A.C., Hammond E.H., Schwartz J.N., Hagerty K.L., Allred D.C., et al. (2007).
ASCO/CAP guideline recommendations for human epidermal growth factor receptor 2
testing in breast cancer. Journal of Clinical Oncology, 25(1), 118-45.
17
