
DIABETES COMPLICATIONS
Development of a New Tool for Obliterating Arteriopathy of the Lower Limbs
Detection
Stéphane Roeslin
1
, Nadège Marthouret
1
, Louis Benazet
2
, Christophe Roncato
3
, Gabriel Camelot
3
,
Anca Loppinet
3
, Xavier Racadot
3
, Sylvie Grandperret
4
, Hayet Bourezane
4
, Christian Pieralli
1
,
Lionel Pazart
2
and Bruno Wacogne
1,2
1
FEMTO-ST Institute, UMR 6174, 16 Route de Gray, 25030 Besançon cedex, France
2
INSERM CIT 808, Besançon University Hospital, Place Saint Jacques, 25030 Besançon cedex, France
3
Chirurgie Vasculaire et Médecine Vasculaire, CHU Minjoz, Bd Fleming, 25000 Besançon, France
4
Diabétologie et Endocrinologie, CHU Minjoz, Bd Fleming, 25000 Besançon, France
Keywords: Diabetes complications, Medical device, Obliterating arteriopathy of the lower limbs, Point of care,
Photoplethysmography, Signal processing.
Abstract: Obliterating arteriopathy of the lower limbs (OALL) is a common complication in diabetes. This
vasculopathy, which is associated with mild injury and with the diabetic neuropathy, is the source of
diabetic foot ulcers which precede approximately 85% of amputations. Simple measures may avoid this
dreaded complication if it is identified in time. OALL detection is currently undertaken by measuring ankle
systolic pressure. The latter could be evaluated with microcirculatory technique but these techniques have a
number of limitations: time consumption and cost. OALL detection is therefore limited to a small number of
specialized units. In order to allow detection of OALL in ambulatory medicine, we propose a simple system
based on photoplethysmography. The idea is to apply a pre-set "warning" pressure to the patient's toe and to
optically check if arterial pulsation still exists. If not, the patient is directed to the adequate hospital unit for
full diagnosis. This "warning" system which can easily be used at the general practitioner’s office is meant
to help detecting the OALL at an early stage, hence reducing the number of amputations. In this position
paper, we present the system, some early results and we propose a discussion concerning the screening of
OALL.
1 INTRODUCTION
Obliterating arteriopathy of the lower limbs (OALL)
is a common complication in diabetes (between 17
and 21% of the diabetic population between 50 and
75 years old) (HAS, 2006). This vasculopathy,
which is associated with mild injury and with the
diabetic neuropathy, is the source of diabetic foot
ulcers which precede approximately 85% of
amputations (VALMI, 2008). The prevalence of
amputations would be 1.3% of diabetic patients.
Simple measures may avoid this dreaded
complication if it is identified in time (Boccalon,
2004 – Girach, 2006), and one of the five-year
objectives fixed by the Saint Vincent European
Declaration is to reduce the rate of foot amputations
among diabetic patients by 40%.
Given the extent of this issue and the objectives
fixed, Alfediam, followed by HAS (French Health
High Authority), recommend OALL screening
among patients over 40 years old or having suffered
from diabetes for over 20 years.
This detection is currently made by measuring
ankle Systolic Pressure Index (SPI). This
examination consists in measuring the humeral
systolic pressure and the ankle systolic pressure. The
SPI is simply given by the ratio of these two
measurements.
These ankle examinations, however, are easily
disturbed due to the common existence of
mediacalcosis among diabetic patients (prevalence
93
Roeslin S., Marthouret N., Benazet L., Roncato C., Camelot G., Loppinet A., Racadot X., Grandperret S., Bourezane H., Pieralli C., Pazart L. and
Wacogne B..
DIABETES COMPLICATIONS - Development of a New Tool for Obliterating Arteriopathy of the Lower Limbs Detection.
DOI: 10.5220/0003703700930098
In Proceedings of the International Conference on Biomedical Electronics and Devices (BIODEVICES-2012), pages 93-98
ISBN: 978-989-8425-91-1
Copyright
c
2012 SCITEPRESS (Science and Technology Publications, Lda.)
between 17 and 24% of diabetics). SPI could be
evaluated with microcirculatory techniques (TcPO2
and Laser Doppler) which usually offer a reliable
insight into the quality of distal vascularisation
(Becker, 1989 – De Graff, 2003). But, these
techniques have a number of limitations: time limits
(45 mins) undervalue (15.01 euros) requiring a
considerable initial investment (42000 € for a
PERIMED brand microcirculation unit), precise
interpretation requiring specialist expertise, and
numerous limitations in the process itself (infection,
oedema, general haemodynamic, etc). This situation
has confined its use to rare specialist centres which
are almost entirely based in hospitals, while these
examinations have become essential for classifying
and managing arteriopathy in diabetic patients.
Ankle SPI can be extremely perturbed by the
presence of a mediacalcosis which leads to an over
estimation of the SPI. In this case toe SPI can be
used together with a clinical and Doppler
examination (Moe, 2002 – Kröger, 2003 – Carter,
1996). The PERIMED system can be used for SPI
measurement. However, due to its cost, its use is
limited to a small number of specialized centres.
Recently, the SysToe
®
system has been launched by
Atys Medical. This equipment, based on
photoplethysmography, is much more affordable
(2500 €) but still too expensive for being used on a
routine basis in the physicians office. Indeed, we
recall that the number of amputations could
drastically be reduced with a large scale screening of
OALL.
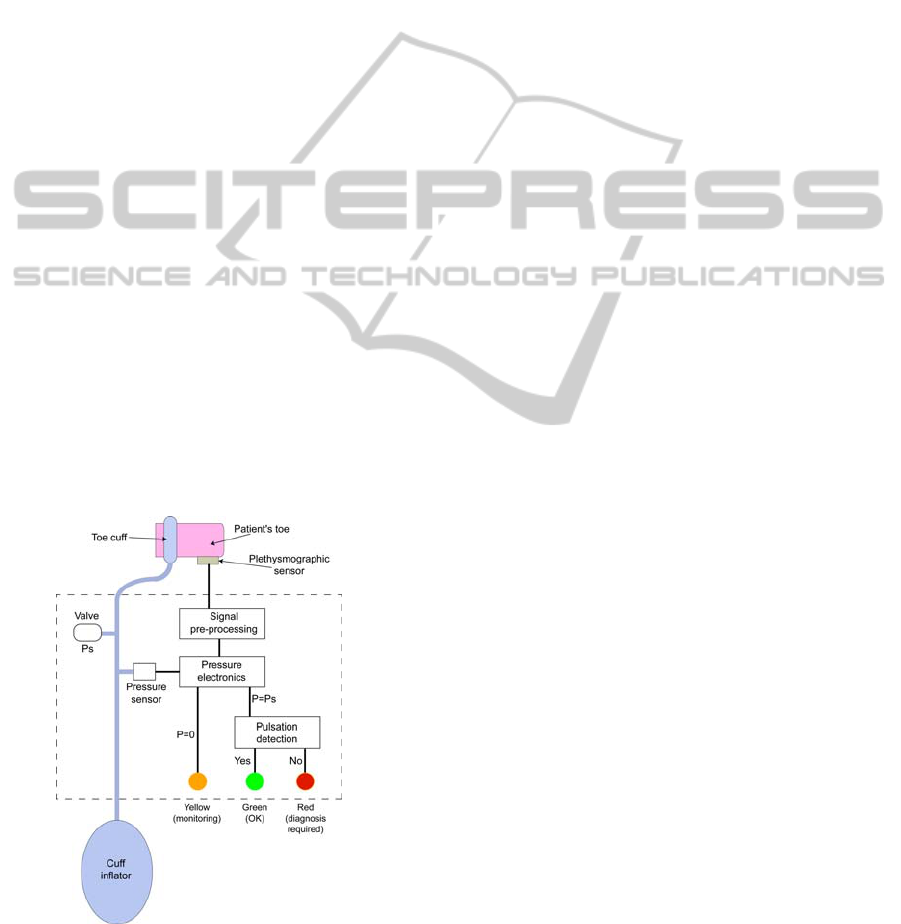
Figure 1: Description of the device.
In order to allow detection of OALL in
ambulatory medicine, we propose a simple system
based on photoplethysmography. The new concept
consists in applying a pre-set "warning" pressure to
the patient's toe and to optically check whether
arterial blood circulation is observed or not. If not,
the patient is directed to the adequate hospital unit in
order to undergo specialized diagnosis. This
"warning" system which can easily be used at the
general practitioner’s office is meant to help
detecting the OALL at an early stage, hence
reducing the number of amputations.
2 DESCRIPTION OF THE
DEVICE
We recall that our system is a "warning" system
used to screen the possible existence of an OALL.
The principle consists in applying a "warning
pressure" to the patient's toe and to check whether or
not an arterial circulation is detected. A schematic
description of the device is shown in figure 1. It
consists of a pneumatic and an electronic part that
are connected together via a pressure sensor. First of
all, the physician places the toe cuff and the
photoplethysmographic sensor onto the patient's toe.
The signal delivered by the optical sensor is pre-
processed in the pre-processing unit (see next
section).
At that moment, the cuff inflator has not yet
been used and the pressure in the pneumatic circuit
is zero. When the pressure is zero, only the driving
of the yellow LED is enabled. It pulsates at the
patient's heart rate and acts as a sensor position
monitor. Indeed, when the yellow LED is pulsating
the physician knows whether the sensor is correctly
installed or not.
Now that the optical sensor is correctly installed,
the physician uses the cuff inflator in order to reach
the "warning pressure" value. Let us call it Ps. A
calibrated valve is used to adjust the pressure to the
"warning" value. When the pressure Ps is reached,
the pressure electronics disables the yellow LED and
enables the pulsation detection unit. (Arterial
pulsation is detected by comparing the signal to a
threshold value that has been determined with
healthy volunteers.) Now, if an arterial pulsation is
detected, the green LED is switched on; everything
is alright with the patient. Conversely, if no
pulsation is detected, the red LED is switched on
and the patient is addressed to a specialized hospital
unit for further diagnosis.
Note that for the moment, and according to the
very little literature, the "warning" pressure is set to
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
94
70 mmHg (Boccalon, 2004 – De Graff, 2003 –
Johanson, 2002). The actual value will have to be
confirmed with clinical trials we are setting up at the
moment.
3 EXPERIMENTAL RESULTS
3.1 Signal Pre-processing and
Pulsation Detection
3.1.1 Signal Preprocessing
While reading the above mentioned description one
could think that everything is trivial in this system.
However, photopletysmographic sensors are used to
measure absorption of light into the tissue. In order
to detect arterial pulsation, we must be able to
measure the small absorption variation due to
arterial blood only. Therefore, the signal to noise
ratio at the direct output of the optical sensor is quite
low. Furthermore, the optical sensor also detects
variations of light that can be due to patient's
movements or changes in the ambient light.
A signal pre-processing is therefore required. In
what follows, we briefly describe the signal
processing we used. The rest of the electronic
circuitry is somehow more conventional and the
description of it may lengthen this paper.
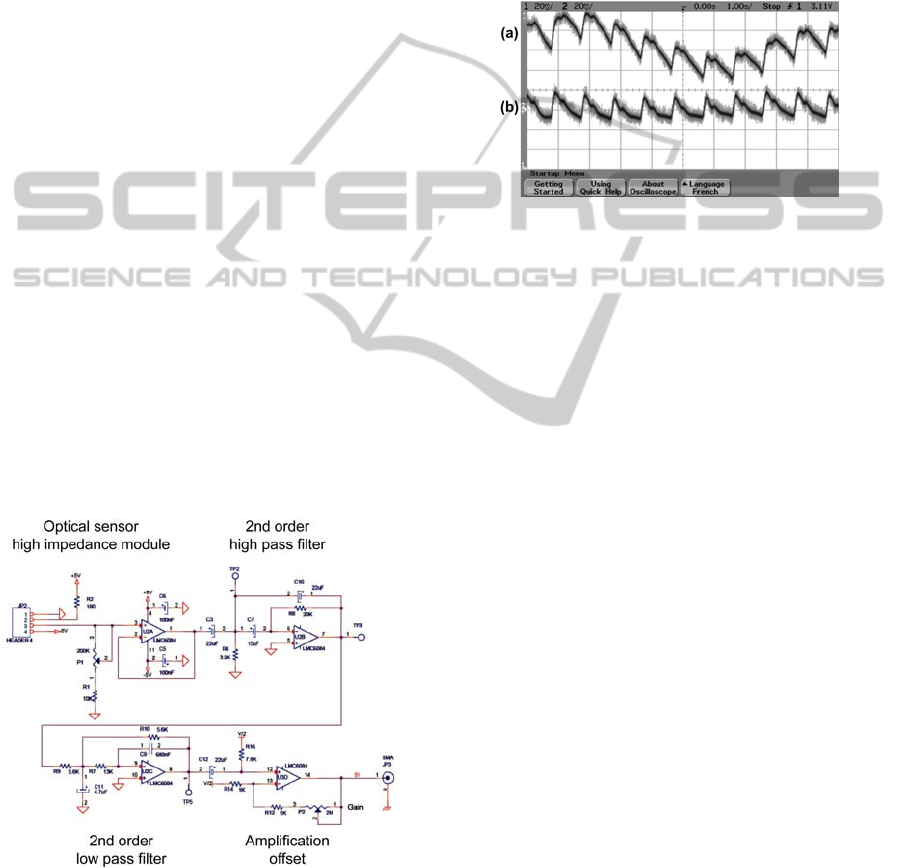
Figure 2 shows the signal pre-processing we
developed.
Figure 2: Signal pre-processing.
Right after the optical sensor head, we use a high
impedance follower in order to reduce the influence
of noise. The signal obtained at the output of this
stage is shown in figure 3(a).
At that stage, the signal exhibits a slowly varying
offset due to patient's movements and variations in
the ambient light. A second order high pass filter
(cut-off @ 1 Hz) is used to cancel this varying
offset. The result is shown in figure 3(b).
Here, the signal is somehow independent of
external perturbations but is still quite noisy. The
noise is now rejected by means of the second order
low pass filter (cut-off @ 10 Hz).
Figure 3: Detail of the signal pre-processing. (a) at the
optical sensor and follower output. (b) at the high pass
filter output.
Finally, a ×100 gain amplifier is used and an
offset is added in order to centre the signal around 6
Volts, a value compatible with the rest of the
electronic circuit. Figure 4 summarizes the signal
pre-processing. Figure 4(a) shows the signal after
the follower while figure 4(b) shows the signal after
pre-processing. It can be noted that the signal issued
from the optical sensor looks different between
figure 3(a) and 4(a). This difference arises because
the sensor is not placed exactly the same way
between the two measurements. This is not a
problem because signal filtering changes the signal
shape (frequency filtering). At the end, the signal
always looks like figure 4(b) after signal processing.
This shows that the device is independent not only
of unwanted movements or light variations, but also
of placement inaccuracies.
A last amplification (not shown in the figure) is
used in order to set the signal to an amplitude
compatible with the rest of the electronic circuit.
3.1.2 Pulsation Detection
Pulsation detection consists in comparing the pre-
processed signal with a reference voltage. This
reference voltage was determined after tests
conducted with healthy volunteers. In figure 5(a), a
pressure of 60 mmHg was applied to the toe. With
this pressure, arterial pulsation still exists. In
particular, maxima of the signal are greater than the
reference voltage. In this case, the green LED is
switched on. Conversely, when the pressure is 100
DIABETES COMPLICATIONS - Development of a New Tool for Obliterating Arteriopathy of the Lower Limbs Detection
95

mmHg, the signal is lower than the reference
voltage. Here, the red LED is switched on.
Figure 4: Comparison between the signal issued from the
sensor (a), and the signal obtained after pre-processing (b).
Figure 5: LEDs driving. (a) Pressure=60 mmHg, signal
crossing the reference voltage, green LED is switched on.
(b) Pressure=100 mmHg, the signal not crossing the
reference voltage, red LED is switched on.
3.2 Experimental Device
The device is shown in figure 6.
Toe cuff, optical sensor and cuff inflator are not
shown in the figure. These three elements were
purchased from Hokanson:
• Infra-red photoplethysmographic sensor:
COPPHO
• Toe cuff: UPC2.5
• Cuff inflator: DS400
All the electronic circuits were made using
surface mounted components in order to reduce the
size of the device.
On figure 6, we can see that we used an external
power supply. Of course, this device is not the final
one. In the last version, batteries will be used instead
of the external power supply. Also, for this
intermediate version, SMA connectors have been
included in order to monitor different signals at
strategic points of the electronic circuit. These
measurements were used to define the reference
voltage mentioned above and to tune the offsets and
the gain of the amplifier to an appropriate value.
The device can be operated in two ways for
clinical trial purposes. We mentioned above that the
monitoring yellow LED is driven only when the
pressure is zero. Conversely, when the "warning"
pressure is applied, only the red and green LEDs are
driven. This configuration corresponds to the normal
used in ambulatory medicine.
Figure 6: Experimental device.
However, clinical trials imply various
experimental configurations. For the first clinical
trial (to be described in the next section) the pressure
sensor must be disconnected. Therefore, we also
design the electronic circuit so that this
configuration can be used. In this case, the three
LEDs are driven simultaneously.
3.3 First Clinical Trials
Clinical trials for this device can be separated in
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
96

three parts.
In a first time, we have to check whether or not
the measurements made with our new device are
consistent with what is observed with the gold
standard technique. In a second time, the intra-
operator and inter-operator stability of our system
will be evaluated. These two first trials are
conducted at the Besançon University Hospital, with
the assistance of the cardio-vascular surgery unit and
the diabetology unit. These trials are performed with
patients suffering of various grade of OALL.
Finally, clinical trials in physician’s office with any
kind of patients will be done and the screening result
will be compared with diagnosis made at the
hospital.
Up to now, only the first step has been passed.
Patients were first tested with conventional Doppler
technique. Their toe systolic pressure was recorded
for both toes.
Then, they were tested with the new device. For
this, discrete pressure values were applied to their
toe according to the systolic pressure measured
before. The pressures ranged from 50 mmHg above
the systolic pressure down to 50 mmHg below with
10 mmHg steps. To do this, the pressure sensor in
our device was disabled as explained above. In order
to control the pressure values, we used the Hokanson
compressor (ref. AG101) and the corresponding
pressure regulator (ref. E20). Some results are
summarized in figure 7.
Figure 7: Some examples of measurements made during
the first clinical trial step.
The colours of the cells correspond to the colour
of the LED lighting during the test. For example, for
patient 12 right toe, the red LED was on from 150 to
110 mmHg and the green was on from 100 to 50
mmHg. If we compare to the systolic pressure
measured by means of the Doppler equipment, we
see that the behaviour of our device is correct
because the LEDs switch from red to green when the
pressure steps from 110 to 100 mmHg. This is
indicated by the “OK” appearing in the cells. The
same behaviour is observed with the left toe when
the pressure steps from 110 to 100 mmHg.
However, discrepancies are sometimes observed.
This is illustrated with patient 16 right toe were the
LED should have been red for 60 mmHg. This
discrepancy is indicated by the “NNNNN” in the
cell. Up to now, we are still analysing the complete
set of data (new device and Doppler records) in
order to fully understand this aspect.
4 SHORT DISCUSSION
We still have to finish the clinical trials before
deciding of the benefit of this method. Questions or
problems to be solved are:
• understanding the discrepancies that occur
sometimes
• investigating the possibility to use digital
electronics instead of analog technique
• defining an actual “warning pressure”
• investigating the intra or inter operability
• conducting the final clinical trials.
In the case of success with these aspects, EC
labelling is foreseen before any commercialization.
However, we recall that up to now, there is no
means of screening OALL. The only devices
commercially available concern the measurement of
the systolic pressure index (SPI) which is more a
diagnosis than a screening technique. Furthermore,
the price of these systems restricts their use in
specialized centres.
We think that detecting the presence or the
absence of arterial pulsation when a “warning
pressure” is applied to the patient is a potential
alternative to the current cost effective and time
consuming techniques. The market study we ordered
shows that a device price of about 250 € would be
accepted by most of the possible users. It is very
likely that the device we propose can be sold at this
low price, as long as it is fabricated at a scale large
enough.
5 CONCLUSIONS
In this position paper, we proposed a new device
DIABETES COMPLICATIONS - Development of a New Tool for Obliterating Arteriopathy of the Lower Limbs Detection
97

that could be used to screen OALL in ambulatory
medicine. It is meant to be used by general
practitioners but also by podiatrists. These
professionals have been identified by a market study
we recently ordered.
The principle is not to measure the systolic
pressure index (SPI), as it is commonly done in
specialized centres that can afford the expensive
equipment required. The new concept simply
consists in applying a “warning pressure” to the
patient’s toe and to check whether an arterial
pulsation is detected or not. If not, the patient is
directed to a specialized centre for complete
diagnosis.
First clinical trials show that our screening
device is consistent with what is observed with
conventional diagnosis techniques. However, full
clinical trial programme should be concluded before
deciding of the benefit of our method.
ACKNOWLEDGEMENTS
The authors would like to acknowledge the financial
support of OSEO, the CNRS and the programme
“maturation de projets innovants”.
REFERENCES
Becker, F. 1989, Classification Clinique et
hémodynamique des artériopathies oblitérantes des
members inférieurs, PhD Thesis, Burgondy University.
Boccalon, H., 2004, Artériopathies des membres inférieurs
chez le diabétique, La revue de médecine interne,
Vol.25, pp. S337-S338, doi:10.1016/j.revmed.2004.
10.2008.
Carter, S. A., Tate, R. B., 1996, Value of toe pulse waves
in addition to systolic pressures in the assessment of
the severity of peripheral disease and critical limb
ischemia, J. Vasc. Surg., Vol. 24, pp. 258-265.
De Graff, J. C., Ubbink, D. T., Legemate, D. A., Tijssen,
J. G. P., Jacobs, M., 2003, Evaluation of toe pressure
and transcutaneous oxygen measurements in
management of chronic critical leg ischemia: a
diagnostic randomized clinical trial, J. of Vasc. Surge.,
Vol. 38, pp. 528-234.
Girach, A., Vignati, L., 2006, Diabetic microvascular
complications – can the presence of one predict the
development of another ?, J. Diabetes and its compl.,
Vol.20, pp. 228-237.
HAS report, 2006, Prise en charge de l'arthériopathie
chronique oblitérante artéroscléreuse des members
inférieurs, http://www.has-sante.fr/portail/upload/docs
/application/pdf/AOMI_fiche.pdf
Johanson, K. E. A., Marklund, B. R. G., Fowelin, J. H. R.,
2002, Evaluation of a new screening method for
detecting peripheral arterial disease in primary health
care population of patients with biabetes mellitus,
Diab. Med., Vol. 19, pp. 307-310.
Kröger, K., Stewen, C., Santosa, F., Rudofsky, G., 2003,
Toe pressure measurement compared to ankle artery
pressure measurements, Angiology, Vol. 54, pp. 39-44.
Moe, S. M., O'Neill, K. D., Duan, D., Ahmed, S., Chen,
Stephen, N. X., Leapman, S. B., Fineberg, N.,
Kopecky, K., 2002, Medial artery calcification in
ESRD patients is associated with deposition of bone
matrix proteins, Kindney Int., Vol. 61, pp. 638–647.
VALMI, 2008, Veines, Artères, Lymphatiques,
Microcirculation, http://cemv.vascular-e-learning.net/
Valmi
BIODEVICES 2012 - International Conference on Biomedical Electronics and Devices
98
