
ON USING SIMULATION AND STOCHASTIC LEARNING FOR
PATTERN RECOGNITION WHEN TRAINING DATA IS
UNAVAILABLE
The Case of Disease Outbreak
Dragos Calitoiu
1
and B. John Oommen
1, 2
1
School of Computer Science, Carleton University, 1125 Colonel By Drive, Ottawa, Canada
2
Department of Information and Communication Technology
University of Agder, 36 Grooseveien, Grimstad, Norway
Keywords:
Disease outbreak, Stochastic point location, Learning automata.
Abstract:
Pattern Recognition (PR) involves two phases, a Training phase and a Testing Phase. The problems associated
with training a classifier when the number of training samples is small are well recorded. Typically, the
matrices involved are ill-conditioned and the estimates of the probability distributions are very inaccurate,
leading to a very poor classification system. In this paper, we report what we believe are the pioneering results
for designing a PR system when there are absolutely no training samples. In such a scenario, we show how
we can use a model of the underlying phenomenon and combine it with the principle of stochastic learning
to design a very good classifier. By way of example, we consider the case of disease outbreak: Learning the
Contagion Parameter in a black box model involving healthy, sick and contagious individuals. The parameter
of interest involves η which is the probability with which an infected person will transmit the disease to a
healthy person. Using the theory of Stochastic Point Location (SPL), the problem is reduced to a PR or
classification problem in which the SPL is first subjected to a training phase, the outcome of which is used for
the testing phase.
1 INTRODUCTION
The Training phase of every PR problem is, in one
sense, the most difficult. It first of all involves un-
derstanding the type of classifier that is to be used.
Once this is determined, the various class-conditional
distributions have to be learned, and this incorpo-
rates all the facets of learning. Indeed, it is so all-
pervasive that almost all of PR considers how training
can be achieved for parametric/non-parametric data,
for different distributions and when one encounters
the “curse of dimensionality”. But in every case, the
basic premise is that the designer is given a reasonable
number of training samples. Of course, every prac-
titioner would like an “infinite” number of training
samples, because then, everything converges beauti-
fully. The problems associated with training a classi-
fier when the number of training samples is small, are
well recorded. In such cases, the matrices involved
are ill-conditioned and the estimates of the probabil-
ity distributions are very inaccurate, implying that the
classification system is very poor.
In this paper, we consider how we can design a PR
system when we have no training samples. Although
such cases are rare, they are extremely important. In-
deed, policy-related decisions concerning the spread
of infectious diseases always involve phenomena that
have almost never been encountered before. In a cer-
tain disease outbreak, we show how we can use a
model of the underlying phenomenon and combine
it with the principles of stochastic learning to design
a very good classifier. As far as we know, the entire
process of designing PR systems and training them in
such situations is open, and thus, we believe that the
results we present are, in one sense, pioneering. To
clarify issues, rather than dealing with a PR problem
in an abstract domain, we shall consider how PR can
be achieved in the so-called “Learning the Contagion
Parameter” (LCP) problem.
As a preface, we first present the environment
against which the LCP problem must be studied and
the proposed solution utilized. We assume that we are
dealing with a geographical area of fixed dimensions
which could represent a certain district of a city. In the
45
Calitoiu D. and John Oommen B. (2010).
ON USING SIMULATION AND STOCHASTIC LEARNING FOR PATTERN RECOGNITION WHEN TRAINING DATA IS UNAVAILABLE - The Case of
Disease Outbreak.
In Proceedings of the 2nd International Conference on Agents and Artificial Intelligence - Artificial Intelligence, pages 45-52
DOI: 10.5220/0002716800450052
Copyright
c
SciTePress

interest of simplicity the population of this area is as-
sumed constant. When an infection starts within this
geographical area it is desirable, first of all, that the
contagion is contained within the area. The second is-
sue which is primarily of concern to our present study
involves understanding how the disease can spread to
healthy people within this geographical area. In other
words, we would like to determine when the outbreak
of the disease is under control, and also to be able to
detect an uncontrollable explosion - which are the re-
spective classes in our PR problem. Once we are able
to detect these scenarios, it will be the task of the pol-
icy makers to propose strategies by which quarantine
decisions are made. This present study and the pro-
posed results, hopefully, submit a small step in this
direction. To present the problem in the right perspec-
tive, we submit a brief explanation of the disease out-
break problem and the epidemiological model used.
1.1 The Disease Outbreak Problem
A disease outbreak is commonly defined as the occur-
rence of an illness in a community or region, where
the number of cases of the illness occur with a fre-
quency clearly in excess of normal expectancy. The
number of cases indicating the presence of an out-
break will vary according to the infectious agent, size
and type of the population exposed, previous experi-
ence or lack of exposure to the disease, and the time
and place of the occurrence(s). Thus, the status of
an outbreak is relative to the usual frequency of the
disease in the same area, among the same population,
and at the same season of the year (Chin, 2000).
In outbreak situations, one often must introduce
preventive interventionsto control pathogen transmis-
sion and adverse outcomes. Control measures that
have proven effective in similar outbreaks in the past
can thus be immediately implemented.
Confirming an outbreak begins with the calcula-
tion of the background rate of infection (or adverse
event) and then comparing the outbreak period rate
with the background rate. Such a comparison can be
performed using the rate ratio (R
r
):
R
r
=
Attack rate during epidemic period
Attack rate during background period
(1)
An outbreak become uncontrollablewhen the pro-
posed control measures are not able to keep the rate
ratio constant or, to decrease it.
The models are used by policymakers, public
health workers, and other researchers who want to an-
alyze and compare the outcomes to better understand
how an outbreak occurs, and how a disease spreads.
They are also useful for understandinghowto respond
to emerging infectious diseases. If a disease outbreak
occurs, simulations can be done by tuning specific
models to aid public officials in their decision-making
processes.
Our method is an alternative approach to the fol-
lowing two classical methods, namely, a Sensitiv-
ity Analysis and Monte Carlo Investigations, respec-
tively.
Sensitivity analysis estimates what the true effect
measure (e.g., the rate ratio) would be in the light of
the observed data and a hypothetical level of bias. It
produces one or more hypothetically adjusted point
estimates for the specific measure of interest (Green-
land, 1998).
Monte Carlo investigations, (i.e., at least in the
present context), involve the simulation of real phe-
nomena, or their idealized models, involving a ran-
dom or probabilistic element in their structure, by the
deliberate use of random numbers. These methods
have already played an important role in many appli-
cations of stochastic models and processes, both by
way of background material (in understanding quali-
tatively some of the properties of such models), and
more quantitatively, in the study of particular prob-
lems that are not amenable to complete mathematical
solution (Robert and Casella, 2005).
We propose a new methodology to investigatehow
an outbreak occurs, which possesses an advantage of
less computational effort compared with the above-
mentioned approaches.
1.2 Principles of Contagion
Epidemiology is the study of the spread of any dis-
ease, with regard to space and time. Its objective is to
trace the factors and parameters that are responsible
for, or contribute to, their occurrence (Diekmann and
Heesterbeek, 2000).
In the process of studying the spread of a disease,
the spatial structure of a population, its density and
the geographical area involved can have a major con-
tribution. As opposed to this, the non-spatial mathe-
matical models can provideonly a simple image about
the dynamics of the transmission. Consequently, this
requires, in many situations, more realistic models
that include the description of the space and the spa-
tial contact between individuals. The basic idea of
studying the dynamics of spreading a disease is to
distinguish individuals from one another according to
their features, their interaction with the environment,
and involves discovering the variation in time of these
features. In the case of an infectious disease, the prin-
cipal characteristic is the computation of the “force”
of infection of a given agent that modifies the state of
ICAART 2010 - 2nd International Conference on Agents and Artificial Intelligence
46

an individual (Diekmann and Heesterbeek, 2000).
The state of the individual is specified with the
minimal “degree of freedom” so that we can ade-
quately predict the future of the individual from an
epidemiologic point of view. We attempt to describe
the state of the population and nature, symbolically
given as S(t + 1) at time ‘t + 1’, be only a function
of S(t). The evolution uses the assumption that the
state S(t) completely captives the history of what hap-
pened prior to the time instant, ‘t’, as per the so-called
Markovian assumption. Also, the simplest epidemic
models assume that any pair of individuals is equally
likely to interact with each other during a given time
interval. This is referred to as the well-mixed assump-
tion.
In our research, we investigate the problem of
learning the Contagion Parameter (CP) in the process
of transmission and perpetuation of a virus in a (hu-
man) population, by using the so-called regular lat-
tice model.
A classical example of such a model for conta-
gion simulates the transmission of a virus in a human
population. The model is initialized with N people,
of which I are infected at time ‘t’. People move ran-
domly about the lattice, being either (i) healthy but
susceptible to infection (S), (ii) sick and infectious (I),
(iii) recovered and immune (R), or (iv) dead (D). We
do not allow for individual to reproduce or enter in the
geographical area, implying that N=S+I+R+D.
The density of the population also affects how
often infected, immune and susceptible individuals
come into contact with each other. To render the
model tractable, we assume that infection leads to ei-
ther death due to the illness or immunity. Thus, no
individual can be infected twice. Additionally, we
model the system with the understanding that contam-
ination can take place when two individuals, either of
whom is sick, make “contact” with each other. This
is characterized by the probability that a contact be-
tween a contagious and a susceptible person actually
leads to transmission. This is symbolized by the Con-
tagion Parameter (CP), η, assumed to be constant
but unknown. Finally, the period of the infection is the
same for all individuals. Thus, the infectivity quanti-
fies the probability that the transmission of the illness
will occur when a contagiousand a susceptible person
occupy the same physical grid location.
1.3 Formal Model of Contagion
Solving the problem in its absolute generality - even
from a modeling perspective - is probably intractable.
To render this problem tractable, obviously, we have
to resort to some simplifying assumptions listed be-
low.
Assumption A1: We assume that we are working
within a square grid of width W units.
Assumption A2: We assume that there are N, con-
stant, individuals moving in this grid in a locally ran-
domized manner. The number of sick people at time
‘t’ is given by n
s
(t).
Assumption A3: We assume that we are working in a
discretized time space, t = 1···t
max
where t
max
is the
total period of observation.
Assumption A4: We consider a scale-independent
model.
Assumption A4.1: First of all, the number of indi-
vidual is specified in term of a density coefficient ρ,
where ρ =
N
W
2
.
Assumption A4.2: The number of the sick people at
any time ‘t’, namely n
s
(t), will be determined by the
ratio σ, where σ(t) =
n
s
(t)
N
.
Assumption A5: We assume that every individual C
i
is characterized by a 4-tuple hx
i
(t), y
i
(t), s
i
, d
i
i where,
at any time instant t,
Assumption A5.1: x
i
, y
i
∈ {1···W} (i.e. the condi-
tions of the location of the individual).
Assumption A5.2: s
i
is an indicator signifying the
state of the individual C
i
. By convention, a healthy
person is assigned the index s
i
= 0. He is sick if s
i
= 1,
immune if s
i
= 2, and dead if s
i
= 3.
Assumption A5.3: d
i
is the duration of time that has
elapsed since the instant when the value s
i
was set to
unity. It represents the time that has elapsed since the
sick individual was infected.
Assumption A6: At every time instant the individual
C
i
is permitted to move to one of his neighboring grid
locations or to stay where it is. We assume that the
individual stays at its current location with the prob-
ability θ (assumed to be fixed and known, and moves
to one of its neighboring locations with the probabil-
ity
1−θ
K
, where K is the number of cells which are
neighboring to hx
i
, y
i
i.
Assumption A7: Every individual can infect another
with a probability η which is the unknown parameter
within the simplified model of contagion.
Assumption A8: Every sick individual can either die
or become immune to the illness after a period, τ, also
referred to as the Period of Infectivity. Only during
this period he is capable of infecting a healthy person.
With the above assumptions, we first formalize
the model. We assume that N individuals are mov-
ing within the grid of width W, and that at every time
instant each individual is allowed to stay or move to
a neighboring cell as per Assumption A6. Whenever
two individuals are on the same grid point, the con-
tagion possibilities are three-fold: (i) If both the in-
dividuals are healthy they remain healthy. (ii) If both
ON USING SIMULATION AND STOCHASTIC LEARNING FOR PATTERN RECOGNITION WHEN TRAINING
DATA IS UNAVAILABLE - The Case of Disease Outbreak
47
the individuals are infected, they continue to be in-
fected. (iii) If exactly one of them is sick, he contami-
nates the other with the probability η. Based on these
assumptions, since ρ is the density of the number of
individuals and σ
t
is the proportion
1
of sick people
(among the entire population) at time t, σ
t+1
has the
following form: σ
t+1
= f(σ
t
, ρ, η), where the func-
tional form of f(·) is unknown. Our aim is, quite sim-
ply, to achieve the PR on the process, which in turn
implies estimating η, and we achieve this by using a
learning methodology applied to solve the Stochastic
Point Location (SPL) problem.
2 STOCHASTIC POINT
LOCATION
The SPL problem (Oommen, 1997; Oommen and
Raghunath, 1998; Oommen et al., 2006) considers a
general learning problem in which the learning mech-
anism (which could be a Learning Automaton (LA),
or in general, an algorithm) attempts to learn a “pa-
rameter”, say η
∗
, within a closed interval. Consider
the problem of a robot moving around on a real line
attempting to locate a particular point. To assist the
mechanism, it communicates with an Environment
which provides it with information regarding the di-
rection in which it should go. If the Environment is
deterministic, the problem is the “Deterministic Point
Location Problem”.
This problem is akin to the field of LA (Laksh-
mivarahan, 1981; Narendra and Thathachar, 1989;
Poznyak and Najim, 1997; Thathachar and Sastry,
2003), in which the learning mechanism attempts to
learn from a stochastic Teacher. More specifically,
unlike the traditional LA model in which the LA at-
tempts to learn the optimal action offered by the En-
vironment, we consider the following learning prob-
lem: the learning mechanism is trying to locate an un-
known point on a real interval by interacting with the
stochastic Environment through a series of informed
guesses.
Unlike the deterministic problem alluded to
above, in the SPL, rather than receive deterministic
responses as to where it should go, the learning mech-
anism is given, at every time step, a stochastic (i.e.,
possibly erroneous) response. Thus, when it should
really be moving to the “right” it may be advised to
move to the “left” and vice versa, as formalized be-
low.
1
In the interest of readability, for all time instants, σ
t
will be used to represent σ(t).
2.1 Formulation of the SPL Problem
We assume that there is a learning mechanism whose
task is to determine the optimal value of some vari-
able (or parameter), η. We assume that there is an op-
timal choice for η - an unknown value, say η
∗
∈ [0, 1].
The question which we study here is the one of learn-
ing η
∗
. Although the mechanism does not know the
value of η
∗
, we assume that it has responses from an
intelligent “Environment” E which is capable of in-
forming it whether the current value of η is too small
or too big. E may tell us to increase η when it should
be decreased, and vice versa. However, to render the
problem tangible we assume that the probability of
receiving an intelligent response is p > 0.5.
Observe that the quantity “p” reflects on the “ef-
fectiveness” of the Environment, E. Thus, whenever
the current η < η
∗
, the Environment correctly sug-
gests that we increase η with probability p. It simulta-
neously could have incorrectly recommended that we
decrease η with probability (1 − p). Similarly, when-
ever η > η
∗
, the Environment tells us to decrease η
with probability p, and to increase it with probability
(1− p).
We shall assume that η is any number in the in-
terval [0, 1]. The question of generalizing thus will be
considered later. The crucial issue that we have to ad-
dress is that of determining how to change our guess
of η
∗
in [0, 1]. We shall attempt to do this in a dis-
cretized manner by subdividing the time unit interval
into R steps {0,
1
R
,
2
R
, ...,
R−1
R
, 1}, where R is the reso-
lution of the learning scheme. A larger value of R will
ultimately imply a more accurate convergence to the
unknown η
∗
.
The scheme which attempts to learn η
∗
is as be-
low. Let η(t) be the value at time step “t”. Then,
η(t + 1) := η(t) +
1
R
, (2)
if E suggests increasing η and 0 ≤ η(t) < 1;
η(t + 1) := η(t) −
1
R
, (3)
if E suggests decreasing η and 0 < η(t) ≤ 1.
At the end states, the scheme obeys:
η(t + 1) := η(t), (4)
if η = 1 and E suggests increasing η;
η(t + 1) := η(t), (5)
if η = 0 and E suggests decreasing η.
The Markov Chain representing these transitions
is given in Figure 1 below.
Notice that although the above rules are determin-
istic, because the “environment” is assumed faulty,
ICAART 2010 - 2nd International Conference on Agents and Artificial Intelligence
48

Figure 1: The Markov Chain used for solving the SPL.
The states are represented here by integers in {0,1,2,..., R},
where state i represents a value of η =
i
R
.
the state transitions are stochastic. The main result
concerning the above scheme is the following:
Theorem. The parameter learning algorithm speci-
fied by Equations (2)-(5) is asymptotical optimal.
The proof of the theorem can be found in (Oom-
men et al., 2008).
3 USING SPL FOR LEARNING
THE CP AND TO ACHIEVE PR
This section describes the strategy by which the so-
lution to the SPL will be used to learn the CP of the
contagion model. In essence, we shall reduce this to a
Pattern Recognition (PR) problem, and thus, this will
consist of two phases: a Training phase and a Testing
phase. The Training phase can be considered to be a
calibration module, which essentially learns how an
arbitrary disease will spread under the constraints of
our model. This can be obtained in a completely non-
real-life setting because we need to understand how
the number of sick individuals at any time is related
to the number of sick individuals at the prior time in-
stant, the density, ρ, and the contagion parameter, η.
The Testing phase would then involve dealing with
the sick individuals in the real life or simulated set-
ting, and attempting to estimate η. We now discuss
both of these phases.
3.1 The Training Phase
We shall first explain the principle used in Training,
and then illustrate the results of the training that we
have achieved.
As mentioned in the modeling phase, since ρ is
the density of the number of individuals and σ
t
is the
proportion of sick people (among the entire popula-
tion) at time t, σ
t+1
has the following form: σ
t+1
=
f(σ
t
, ρ, η). Clearly, the functional form of f is un-
known, and it is our task to first of all learn it, and to
secondly, use the learned form in the inference prob-
lem associated with learning what the true CP, η
∗
is.
The Training is achieved by explicitly invoking
the functional interdependence of σ
t+1
on σ
t
. This
will, in turn, be achieved by allowing the individu-
als, to move and contaminate/recover from the ill-
ness as per the model specified earlier. Thus, the
training phase can be given by the following algo-
rithm, formally described below in Algorithm Train-
ing Phase.
Assumptions: The algorithm is able to move the
location of an individual as per Assumption A6.
This is achieved by invoking the function Move.
Input:
W: The size of the grid, which is assumed to be a
square W ×W.
N: The number of individuals in the grid.
θ: The probability of an individual not moving in
the grid.
n
s
: The number of initial sick individuals.
η: The probability of infection.
τ: The Period of Infectivity.
p
r
: The Probability of Recovery
Output:
The updated n
s
as a function of ρ, the density of the
population and η.
BEGIN Algorithm Training Phase
Initialize every C
i
= hx
i
, y
j
, s
i
, d
i
i to a random posi-
tion x
i
, y
i
.
Set the value of s
i
to be 1 with the proportion deter-
mined by N, ρ, and n
s
(0).
d
i
is initialized to zero for every individual.
repeat forever
/*Compute the position for each individual*/
for i=1 to N do
hx
i
(t + 1), y
i
(t + 1)i := Move[hx
i
(t), y
i
(t)i, θ];
end for
/*Compute the sick state for each individual*/
for i=1 to N do
if (s
i
(t) = 1 and d
i
< τ) then
s
i
(t + 1) = 1; d
i
= d
i
+ 1;
end if
if (s
i
(t) = 1 and d
i
= τ) then
s
i
= 2 with probability p
r
, and s
i
= 3 with with
probability 1 − p
r
.
end if
/*Check if C
i
should get infected depending on
where he is and on his neighbors*/
if ∃ a k s.t hx
k
(t+1), y
k
(t+1)i = hx
i
(t+1), y
i
(t+
1)i
V
s
k
(t + 1) = 1
V
s
i
(t) = 0 then
s
i
(t +1) = 1 with probability η; /*C
i
becomes
infected */
n
s
= n
s
+1; /* Update the number of sick peo-
ple */
end if
end for
ON USING SIMULATION AND STOCHASTIC LEARNING FOR PATTERN RECOGNITION WHEN TRAINING
DATA IS UNAVAILABLE - The Case of Disease Outbreak
49
end repeat
END Algorithm Training Phase
To now, obtain the training curves, we will have
to merely run Algorithm Training Phase for dif-
ferent initialized parameters. Observe that we have
also considered the question of individuals recover-
ing and dying, and are simultaneously able to keep
track of the ratio σ(t + 1) =
n
s
(t+1)
N
, as a function of
σ(t) =
n
s
(t)
N
, since we have available the quantities
n
s
(t) and n
s
(t + 1).
Since the phenomenon of contagion also depends
on the density, ρ, we have to do the training for two
different types of settings listed below:(i) In the first
setting, we assume that ρ is constant, and that η is the
free variable. In other words, if the density of indi-
viduals within the grid is constant, the question now
is that of understanding how σ(t + 1) is a function
of σ(t) as η changes. (ii) In the second setting, we
assume that η is constant, and that ρ is the free vari-
able. In other words, in this setting, if the contagion
parameter is fixed, we would like to observe how the
disease spreads as the density of the population in-
creases. Thus, the question now is one of understand-
ing how σ(t + 1) is a function of σ(t) as ρ changes.
The functional form of n
s
(t + 1) in terms of n
s
(t) and
η
∗
is is merely a “scaled” version of the functional
form of σ(t + 1) in terms of σ(t) and η
∗
, and so, to
avoid confusion, we shall refer to both of them by the
same function f(·).
3.1.1 Results of the Training Phase
The training phase was conducted for numerous set-
tings. However, since the graphs display random vari-
ables, any meaningful representation will have to in-
volve an ensemble of experiments. In our case, we
report the results for only one set of parameters and
for an ensemble of 10 experiments each, as explained
below: N = 500 individuals were placed in the grid
of dimensions W ×W, where W = 30 units. This cor-
responds to a value of ρ = 0.55. For this scale, Al-
gorithm Training Phase was allowed to run, and the
quantities σ
t+1
= f(σ
t
, ρ, η) were recorded for sev-
eral values of n
s
(0).
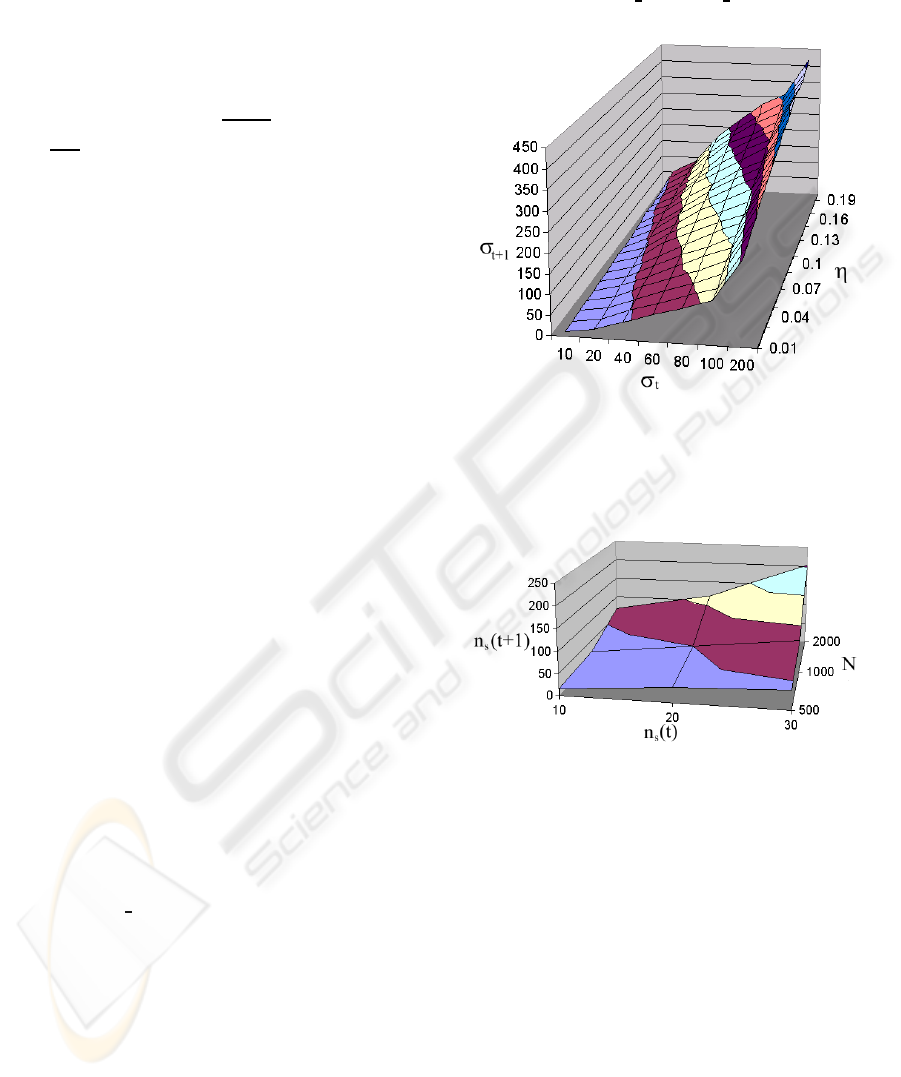
The parametric 3-dimension plots of σ
t+1
=
f(σ
t
, ρ, η) are given in Figure (2). This figure depicts
the case when ρ is constant, and η is the free variable.
Similarly, the 3-dimension plots of σ
t+1
= f(σ
t
, ρ, η)
when η is constant, and when ρ is the free variable are
given in Figure (3). The general observations that can
be made from these two graphs are: (i) The graphs
are always monotonically increasing; (ii) As the den-
sity increases, even if the CP, η, is maintained con-
stant, the disease will spread; (iii) If the density is
kept constant, the disease will spread (as we see it,
more rapidly) as the CP, η increases; (iv) Since we
are computing Present state/Next State maps, the sat-
uration conditions are never encountered.
Figure 2: The evolution of the ensemble average over 10
experiments of σ
t+1
= f (σ
t
, ρ, η) as a function of η when
ρ = 0.55 (i.e., when 500 individuals are placed in the 30×
30 grid). The scale for the X and Y axes should be divided
by N = 500.
Figure 3: The evolution of the ensemble average over 10
experiments of n
s
(t + 1) = f (n
s
(t), ρ, η) as a function of ρ
when η = 0.05. Observe that the plot here is not of σ, but
rather of n
s
(·). The value of σ at any point can be obtained
by dividing the value of n
s
(t+1) by the corresponding value
of N.
3.2 The Testing Phase
The reader will observe that we have reduced our LCP
problem to a Pattern Recognition (PR) or classifica-
tion problem. Indeed, we have transformed the prob-
lem to one of classifying a certain disease outbreak
phenomenon in terms of its contagion parameter, η.
Thus, as in every PR problem, we are first required
to do a training phase which trains the classifier, and
then achieve the testing. The question now is one of
devising an efficient testing module which uses the
training graphs and figures obtained above. We plan
ICAART 2010 - 2nd International Conference on Agents and Artificial Intelligence
50
to accomplish the Testing by using the SPL as an in-
tegral part of the PR process.
Assume that we currently have η(t), that it is
our current estimate of the true but unknown CP, η
∗
.
Based on the value of the density of people and the
proportion of sick people within this area, and our cur-
rent understanding of η(t), we can now use our train-
ing plots to locate the current quiescent point on the
corresponding curve. Now, using this as our estimate,
we can determine the proportion of sick people that
would result on the next day, if η
∗
was indeed η(t). If
the number of sick people estimated by η(t) for this
current quiescent point exceeds the actual number of
sick people that do occur, we have an indication that
our current estimate η(t) is too high. In that case, we
chose to decrease η(t) by one step based on the reso-
lution parameter, to obtain η(t + 1). Similarly, if η(t)
underestimates the number of sick people at the next
time instant, η(t + 1) is increased to the next corre-
sponding curve in the testing graphs. As long as these
step-sizes are relatively small and the parameter η
∗
is
unchanged, the results of Section 3 will guarantee that
η(t) converges to η
∗
. Of course, to assist us in this
updating, it is more beneficial to have functional plots
of σ
t+1
= f(σ
t
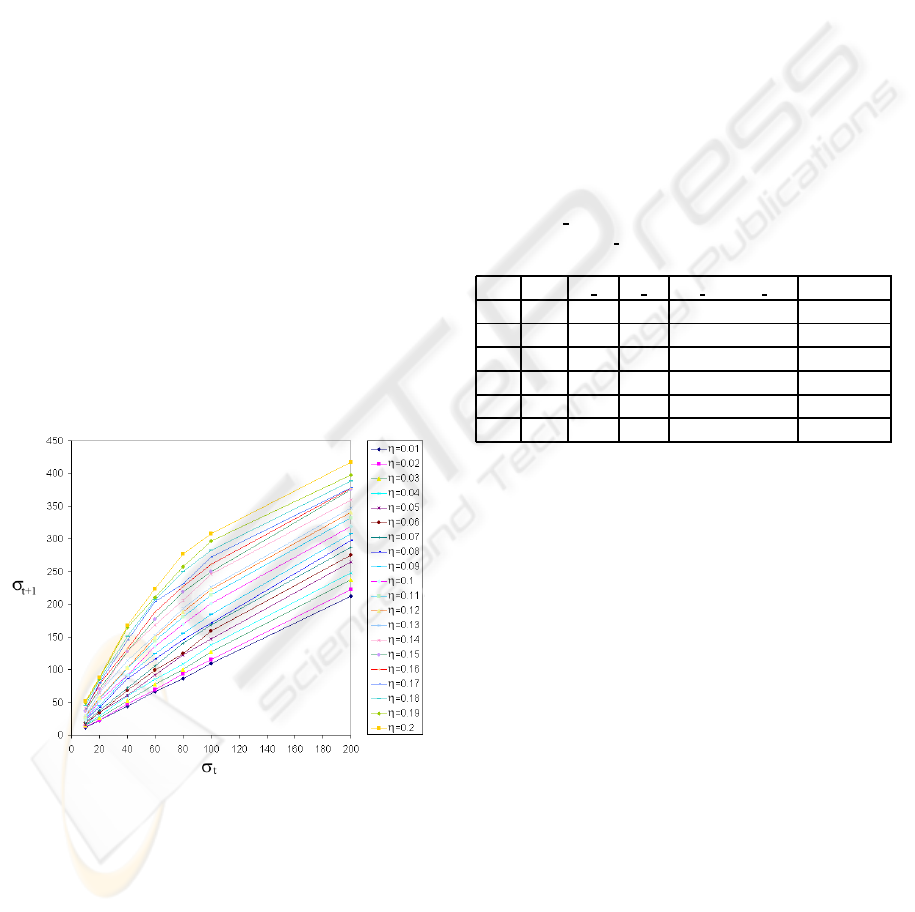
, ρ, η) as 2-dimensional plots. One of
these plots is given in Figure 4. Similar graphs can
be obtained for the other settings but are not included
here.
Figure 4: The 2-dimensional evolution of the ensemble av-
erage over 10 experiments of n
s
(t + 1) = f (n
s
(t), ρ, η) as a
function of η when ρ = 0.55.
A simple example will help clarify matters. Sup-
pose that we are dealing with a value of η
∗
, unknown
to the user, but known to be in the closed interval
[0.01, 0.2]. Also, let us assume that we are deal-
ing with the scenario when N = 500 individuals are
within theW ×W area. We assume that the resolution
parameter R divides [0.0, 0.2] in steps of 0.01. Let
us assume that the current value of η(t) for the test-
ing phase is 0.03. Suppose that the number of sick
individuals at time ‘t’ is n
s
(t) = 20. If, indeed, η
∗
was 0.03, the number of sick people at time ‘t + 1’
would have been (on the average) n
s
(t + 1) = 26.
If, however, it turns out that in the testing scenario
n
s
(t + 1) = 25, we have an indicator that η(t) under-
estimates η
∗
, and so the solution to the SPL would
dictate that we increase η(t) by the resolution (i.e.,
0.01) to set η(t + 1) to 0.04. The alternate scenario
describes the overestimating case and is omitted. By
successively updating η(t), we move along different
curves on the corresponding 3-dimensional plot de-
scribed by σ
t+1
= f(σ
t
, ρ, η), to converge to η
∗
.
Table 1: Results obtained by using the SPL algorithm to
learn the CP when η
∗
= 0.09. The starting value is η(0) =
0.05; the resolution for updating η was set to be 0.01. We
denote by n
s Tr
the number of sick people from the train-
ing phase and by n
s Ts
the number of sick people from the
testing phase.
step η n
s Tr
n
s Ts
n
s Tr
< n
s Ts
η± ∆η
0 0.05 10 10
1 0.05 16 20 16<20 yes 0.05+0.01
2 0.06 33 52 33<52 yes 0.06+0.01
3 0.07 90 92 90<92 yes 0.07+0.01
4 0.08 158 169 158<169 yes 0.08+0.01
5 0.09 268 267 268<267 stop η
∗
= 0.09
The parameter learning mechanism described here
was experimentally evaluated to verify the validity of
our analytic results and to examine its rate of con-
vergence. Based on the assumption that the learning
algorithm was ignorant of η
∗
, at any time instant, the
number of sick people was used to update η as de-
scribed earlier. The results of the algorithm are pre-
sented for two cases listed below: (i) In the first case,
the value of the unknown η
∗
was set to be 0.09. The
starting value of η was initialized to 0.05. The re-
sults are presented in Table 1, from which we observe
that η(t) converges to η
∗
. (ii) In the second case, the
value of the unknown η
∗
was set to be 0.02. The start-
ing value of η was again initialized to 0.05, and η(t)
again converges to η
∗
(Table 2).
4 CONCLUSIONS
In this paper, we have considered how we can de-
sign a PR system when we have no training sam-
ples. Although such cases are rare, they are ex-
tremely important, for example, when we consider
policy-related decisions concerning the spread of in-
ON USING SIMULATION AND STOCHASTIC LEARNING FOR PATTERN RECOGNITION WHEN TRAINING
DATA IS UNAVAILABLE - The Case of Disease Outbreak
51
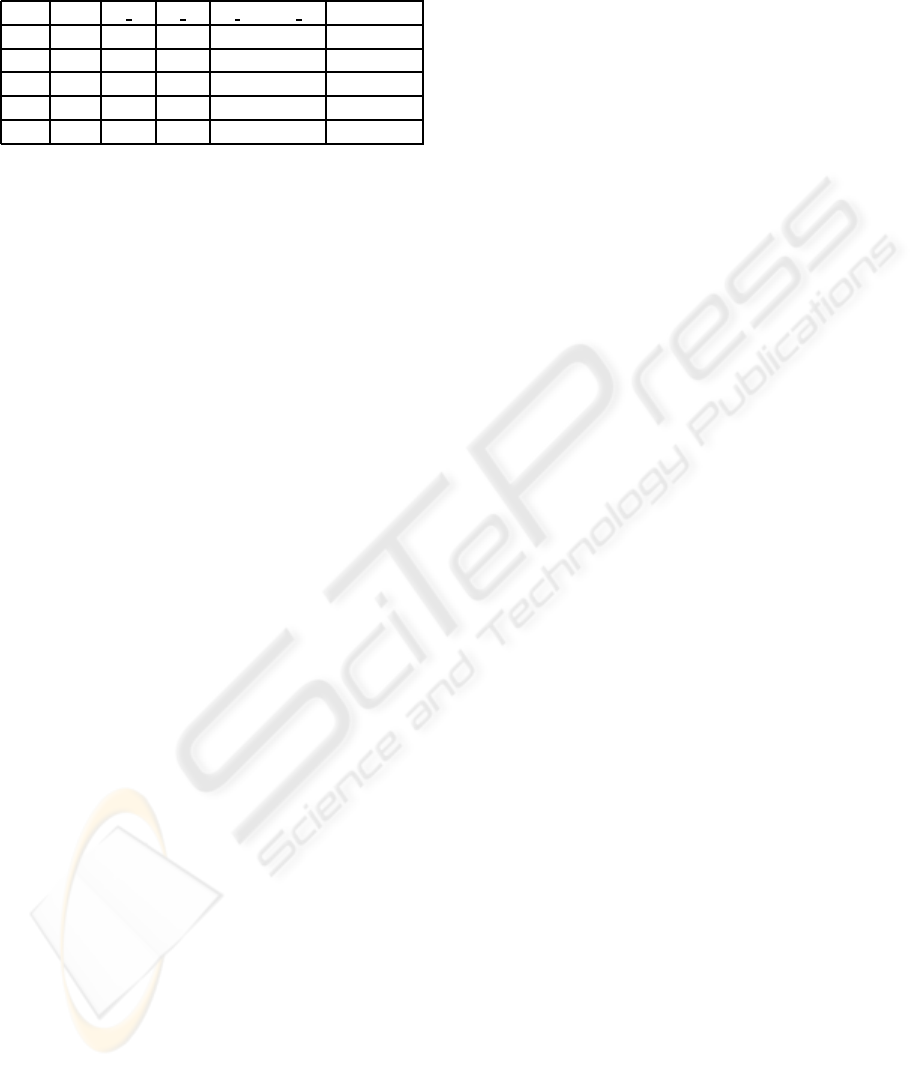
Table 2: Results obtained by using the SPL algorithm to
learn the CP when η
∗
= 0.02. The starting value is η(0) =
0.05; the resolution for updating η was set to be 0.01.
step η n
s Tr
n
s Ts
n
s Tr
< n
s Ts
η± ∆η
0 0.05 10 10
1 0.05 16 12 16<12 no 0.05-0.01
2 0.04 18 13 18<13 no 0.04-0.01
3 0.03 19 18 19<18 no 0.03-0.01
4 0.02 27 27 27<27 stop η
∗
= 0.02
fectious diseases. Thus, the PR solution, which in-
volves stochastic learning, considers the problem of
recognizingthe seriousness of a contagion by learning
the Contagion Parameter (CP) in a black box model
involving healthy, sick and contagious individuals. In
our study, PR involves the parameter of interest, η,
which is the probability with which an infected per-
son will transmit the disease to a healthy person. η
is learnt using the theory of Stochastic Point Location
(SPL) which reduces the issue to a PR problem with
Training and Testing phases.
The following are some of the possible avenues
for future work:
1. As mentioned in (Oommen, 1997; Oommen and
Raghunath, 1998; Oommen et al., 2006), apart
from the problem being of importance in its own
right, the SPL also has potential applications in
solving optimization problems. Indeed, a SPL can
be used as a scheme by which the parameters of
an optimization algorithm can be determined, so
as to prevent it from converging all-too sluggishly
on the one hand, or from converging erroneously
or oscillating, on the other. The use of the solution
to the SPL to optimally converge to η
∗
within our
model of contagion is open.
2. An SPL can also be used to assist in the design of
neural networks. Thus, if we consider the back-
propagation neural network, it is well known that
given a particular input, the network uses its “cur-
rent” set of weights to compute the corresponding
output. The obtained output is compared to the
expected output and the network weights are con-
sequently modified so as to minimize the expected
resultant error. Thus, we could use the solution to
the SPL to devise neural techniques to learn η
∗
.
3. The problem of learning η
∗
when this quantity
changes with time is an extremely interesting
problem. We believe that solutions to this prob-
lem could have profound implications in a real-
life pandemic.
4. It would be very interesting to see if a SPL solu-
tion can be applied to a contagion model which
is more general than the one we have considered
here. We believe that such a strategy is both fea-
sible and expedient.
REFERENCES
Chin, J. (2000). Control of Communicable Disease Manual.
American Public Health Association, Washington.
Diekmann, O. and Heesterbeek, J. A. P. (2000). Mathe-
matical Epidemiology of Infectious Diseases: Model
Building, Analysis and Interpretation. John Wiley.
Greenland, S. (343–357, 1998). Basic methods for sensitiv-
ity analysis and external adjustment. In K.J. Rothman
KJ and S. Greenland - Modern epidemiology, 2nd ed.
Philadelphia, PA: Lippincott-Raven Publishers. Lip-
pinicot Williams & Wilkins.
Lakshmivarahan, S. (1981). Learning Algorithms Theory
and Applications. Springer-Verlag.
Narendra, K. S. and Thathachar, M. A. L. (1989). Learning
Automata. Prentice-Hall.
Oommen, B. J. (SMC-27B:733–739, 1997). Stochastic
searching on the line and its applications to parameter
learning in nonlinear optimization. In IEEE Transac-
tions on Systems, Man and Cybernetics.
Oommen, B. J., Kim, S. W., Samuel, M., and Granmo,
O. C. (SMC-38B:466-476, 2008.). A solution to the
stochastic point location problem in meta-level non-
stationary environments. In IEEE Transactions on
Systems, Man and Cybernetics.
Oommen, B. J. and Raghunath, G. (SMC-28B:947–954,
1998). Automata learning and intelligent tertiary
searching for stochastic point location. In IEEE Trans-
actions on Systems, Man and Cybernetics.
Oommen, B. J., Raghunath, G., and Kuipers, B. (SMC-
36B:820–836, 2006.). Parameter learning from
stochastic teachers and stochastic compulsive liars. In
IEEE Transactions on Systems, Man and Cybernetics.
Poznyak, A. S. and Najim, K. (1997). Learning Automata
and Stochastic Optimization. Springer-Verlag, Berlin.
Robert, C. P. and Casella, G. (2005). Monte Carlo Statisti-
cal Methods (Springer Texts in Statistics). Springer.
Thathachar, M. A. L. T. and Sastry, P. S. (2003). Net-
works of Learning Automata : Techniques for Online
Stochastic Optimization. Kluwer Academic, Boston.
ICAART 2010 - 2nd International Conference on Agents and Artificial Intelligence
52
