
RESONANCES IN THE CARDIOVASCULAR SYSTEM
Investigation and Clinical Applications
Evgeny G. Vaschillo, Bronya Vaschillo, Jennifer F. Buckman, Marsha E. Bates
and Robert J. Pandina
Center of Alcohol Studies, Rutgers,The State University of New Jersey, 607 Allison Road, Piscataway, NJ 08854, U.S.A.
Keywords: Baroreflex, Closed-loop control system, Resonance frequency, HRV biofeedback.
Abstract: The baroreflex, as a control system with negative feedback, is a mechanism that buffers changes in blood
pressure (BP), thereby precluding strong, abrupt shifts in arterial pressure. As a closed-loop control system
with delays, the baroreflex possesses resonance features at frequencies of about 0.1 and 0.03 Hz. These
resonance frequencies correspond to a ~5-s delay in the BP response to changes in heart rate (HR) (HR
baroreflex closed-loop) and a ~15-s delay in the vascular tone (VT) response to changes in BP (VT
baroreflex closed-loop). Thus, whereas a single impact on the cardiovascular system (CVS) elicits a HR,
BP, and VT oscillatory response that fades over time, 0.1 or 0.03 Hz rhythmical stimulation of the CVS
produces steady HR, BP, and VT oscillations with significantly higher amplitudes comparing to stimulation
at other frequencies. Resonances in the baroreflex system are essential for the maintenance of optimal health
by keeping autonomic regulation active via HR, BP, and VT variability, providing adaptive responses to
internal and external stimuli, and buffering stress and emotional reactivity via inhibitory effect in the brain.
This study investigates the phenomenon of resonances in the CVS and the ability to employ these
resonances for clinical applications.
1 INTRODUCTION
The arterial baroreflex is a mechanism that
participates in blood pressure (BP) control. A shift in
BP triggers the baroreflex, which changes heart rate
(HR) and vascular tone (VT) to counteract the BP
shift. Most often, these HR and VT baroreflex
systems are modelled using the classic “control
system theory” approach, which conceptualizes the
baroreflex as a closed-loop control system with
negative feedback. These models are consistent with
the premise that a critical function of the baroreflex
system is to buffer BP oscillation (Just et al., 1994;
Jones, Christou, Jordan, & Seals, 2003; Jordan et al.,
2002). Control system models of the baroreflex,
however, often identify resonance properties at
certain frequencies (Magosso, Biavati, & Ursino,
2001; Julien, 2006; van de Vooren et al., 2007),
which, at first glance, seems inconsistent for a
system that is defined by its ability to buffer BP
variability. By definition, though, a closed-loop
control system with a delay possesses resonance
features and thus the classic control system theory is
consistent with both the baroreflex’s buffering
functions and its resonance properties.
The baroreflex system in humans demonstrates
resonance properties at frequencies of about 0.1 Hz
and 0.03 Hz (van de Vooren et al., 2007; Vaschillo
et al., 2002). In the HR baroreflex closed-loop, a
shift in BP causes a compensatory HR response that
is delayed for approximately 5 seconds. In the VT
baroreflex closed-loop, the compensatory response
of the vasculature is delayed for approximately 10-
15 seconds (Vaschillo et al., 2002; Magosso,
Biavati, & Ursino, 2001). These delays of 5 and 15
seconds coincide with resonance oscillations at 0.1
and 0.03 Hz because the periods of these oscillations
are equal to twice the value of the delay.
A closed-loop system always possesses
resonance properties because all biological or
technical control systems have delays associated
with inertia. When creating a stabilizing technical
system with a closed-loop, the delay is manipulated
so that the resonance frequency falls far outside of
the operating frequency range. In the case of the
baroreflex system, there are two resonance
frequencies within its very narrow (~0.01-0.5 Hz)
21
G. Vaschillo E., Vaschillo B., F. Buckman J., E. Bates M. and J. Pandina R. (2010).
RESONANCES IN THE CARDIOVASCULAR SYSTEM - Investigation and Clinical Applications.
In Proceedings of the Third International Conference on Bio-inspired Systems and Signal Processing, pages 21-28
DOI: 10.5220/0002691200210028
Copyright
c
SciTePress
operating range.
Despite the current view that the main role of the
baroreflex is to buffer BP oscillations and “that
resonance is the price to be paid for effective
buffering at other frequencies” (van de Vooren et al.,
2007), we consider resonance properties of the
baroreflex as essential elements for the regulation of
autonomic and central nervous system functions. We
posit that the resonances in the baroreflex systems
are integral for the vast autonomic variability that
underlies efficient and effective homeostatic
reflexes. This is supported by evidence that
baroreflex resonance properties can act to amplify
adaptive responses to internal and external stimuli
and buffer stress and emotional reactivity through
the initiation of a cascade of neurobiological events
that produces a generalized inhibitory effect on the
brain (Dworkin et al., 1994; Nyklicek et al., 2005;
Yasumasu et al., 2006).
This paper presents the results of our
investigations of baroreflex resonance features using
a classic engineering approach. Based on the
importance of autonomic variability as well as of the
frequency dependence of autonomic reactions to
external and internal influences, our goal is to
develop therapeutic methods based on the resonance
properties of the baroreflex.
2 0.1 HZ RESONANCE IN THE
CVS
Gatchel & Lang (1973) and Lang et al. (1993) found
that HR responses to a single stimulus tended to last
approximately 10 seconds and have a triphasic
waveform, consisting of a small initial HR
deceleration, a larger mid-interval acceleration, and
a final deceleration. Onset of any stimulus - visual
(Lang, Greenwald, & Bradley, 1993) or acoustical
(Bradley & Lang, 2000), long (few second) (Bradley
& Lang, 2000) or brief (few tens ms) (Codispoti,
Bradley, & Lang, 2001) - caused the same HR
waveform response. We suggest that the triphasic
waveform of the instantaneous HR response results
directly from the inherent resonance properties of
the HR baroreflex closed-loop. Further, we
hypothesize that this basic feature of the HR
baroreflex closed-loop can serve as the foundation
for eliciting stable resonance oscillations in HR, BP,
and VT using rhythmical 0.1 Hz stimulation.
2.1 0.1 Hz Resonance in the CVS
Caused by Respiration
Respiratory activity continually perturbs the
cardiovascular system. It is well known that
breathing modulates HR with respiratory
periodicities, a phenomenon known as respiratory
sinus arrhythmia. Clynes (1960) showed that a
single inhalation or exhalation elicits nearly identical
triphasic HR waveform responses. In addition, he
reported that 0.1 Hz breathing caused high
amplitude oscillations in HR. Angelone &
Coulter
(1964) then calculated amplitude and phase transfer
functions with respiration as the input and HR as the
output for one subject who performed paced
breathing exercises at different frequencies (0.01 –
0.5 Hz range). They found that the 0.1 Hz breathing
produced the highest HR oscillation and defined this
phenomenon as resonance in the CVS. These early
studies guided the development of a heart rate
variability (HRV) biofeedback procedure based on
paced resonance frequency breathing as a novel
approach for correcting abnormal autonomic
regulation (Lehrer, Vaschillo, & Vaschillo, 2000).
HRV biofeedback has significant clinical
potential because respiration is a physiological
Respiration Volume (RV)
[ml]
-450
0
450
Beat-to-beat heart intervals (RRI)
[ms]
700
800
900
1000
Pulse Transit Time (PTT)
Time [s]
[ms]
400 500 600 700
230
240
250
260
270
280
RV Spectrum
PSD [10
6
ml
2
/Hz]
0.0
0.5
1.0
1.5
2.0
RRI Spectrum
•PSD [10
6
ms
2
/Hz]
0.00
0.25
0.50
0.75
1.00
PTT Spectrum
Frequency [Hz]
PSD [ms
2
/Hz]
0.0
0.1
0.2 0.3
0
2500
5000
7500
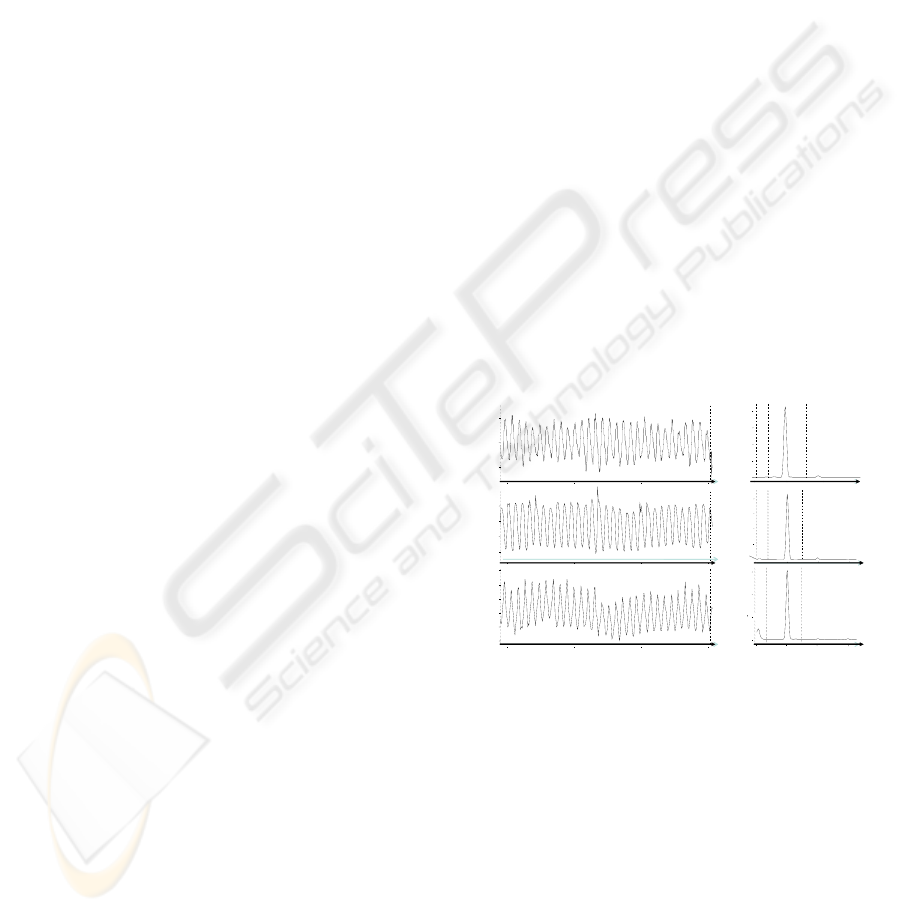
Figure 1: 0.1 Hz paced breathing triggers resonance
oscillations in cardiovascular functions.
function that is under voluntary control and
breathing at one’s resonance frequency produces
high-amplitude oscillations in HR, which, through
the baroreflex, spreads to other functions, such as
BP and VT (Fig. 1).
Further, we recently calculated the amplitude and
phase transfer functions of the HR control system
for eight participants and observed that each
participant demonstrated a unique resonance
frequency between 0.075 – 0.107 Hz, and that the 0º
phase shift between respiration and HR curves
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
22
occurred precisely at the resonance frequency
(Vaschillo & Vaschillo, 2009). Thus, paced
breathing represents an easily manipulated trigger
for inducing maximal oscillations in HR and other
interrelated cardiovascular functions.
In contrast to the usual respiratory rate of 12-18
breaths per minute (0.2-0.3 Hz), HRV biofeedback
teaches participants to breathe easily and naturally
(i.e., slowly but not too deeply, to support normal
minute ventilation) at a rate of ~6 times per minute.
As part of this procedure, participants are instructed
to breathe at their resonance frequency for ~ 40
minutes per day over a 10-week period.
HRV biofeedback successfully normalized
autonomic regulation, as measured by increased
baroreflex gain and peak expiratory flow (Lehrer et
al., 2003). It demonstrated efficacy in the treatment
of asthma (Lehrer et al., 2004), major depression
(Karavidas et al., 2007), fibromyalgia (Hassett et al.,
2007), neurosis (Chernigovskaya et al., 1990), and
hypertension (McCraty et al., 2003). The therapeutic
effects in these studies were achieved through
systematic, everyday use of the HRV biofeedback
procedure and the elicited high amplitude oscillation
in HR, BP, VT, and other autonomic functions
retrained and toned homeostatic reflexes. Activation
of the baroreceptors by these oscillations also
activated inhibitory processes in the brain, thereby
dampening stress. Taken together, these data suggest
that daily “exercise” of autonomic functions
associated with normalized autonomic regulation
can restore sympathetic-vagal balance (Lehrer et al.,
2004) and buffer patients from the negative
influences of stress.
2.2 0.1 Hz Resonance in the CVS
Caused by Rhythmical Muscle
Tension
The respiration is natural rhythmical stimulator of
the CVS; however, there are other effective methods
to stimulate the CVS at its resonance frequency. For
example, the CVS functions adaptively to react to
physical load. This suggests that rhythmical paced
muscle tension (muscle tense-release cycles) at a
frequency of 0.1 Hz may also trigger resonance in
the CVS.
Method: Sixteen young healthy participants (9
female, 7 male) performed four 3.5-minute tasks (30
second inter-task interval), including a paced, 6
breaths/minute (~0.1 Hz) task as well as three paced
muscle tension tasks at frequencies of 0.05, 0.1, and
0.2 Hz in random order. Participants were seated in
a comfortable armchair in front of a computer screen
with their legs extended and supported parallel to the
floor. During the paced muscle tension tasks
participant tensed their skeletal muscles when the
computer screen turned red and relaxed their
muscles when the screen color changed to green.
ECG and finger pulse were recorded during all tasks.
Beat-to-beat HR and pulse transit time (PTT) and
their Fourier spectra were calculated for each task.
PTT was considered as estimation of the vascular
tone (shorter PTT corresponds to higher VT). The
power of the spectra at tested frequencies was used
to estimate HR and VT reactions in each task.
Results: The 0.05, 0.1, and 0.2 Hz muscle tension
manipulations produced HR and VT oscillations at
corresponding frequencies in all participants;
however, only rhythmical 0.1 Hz muscle tension
caused high amplitude HR oscillations like those
observed with 0.1 Hz breathing. Averaged across all
participants, HR and VT reactions to muscle tension
at 0.1 Hz were 4-6 times higher than at 0.2 Hz or
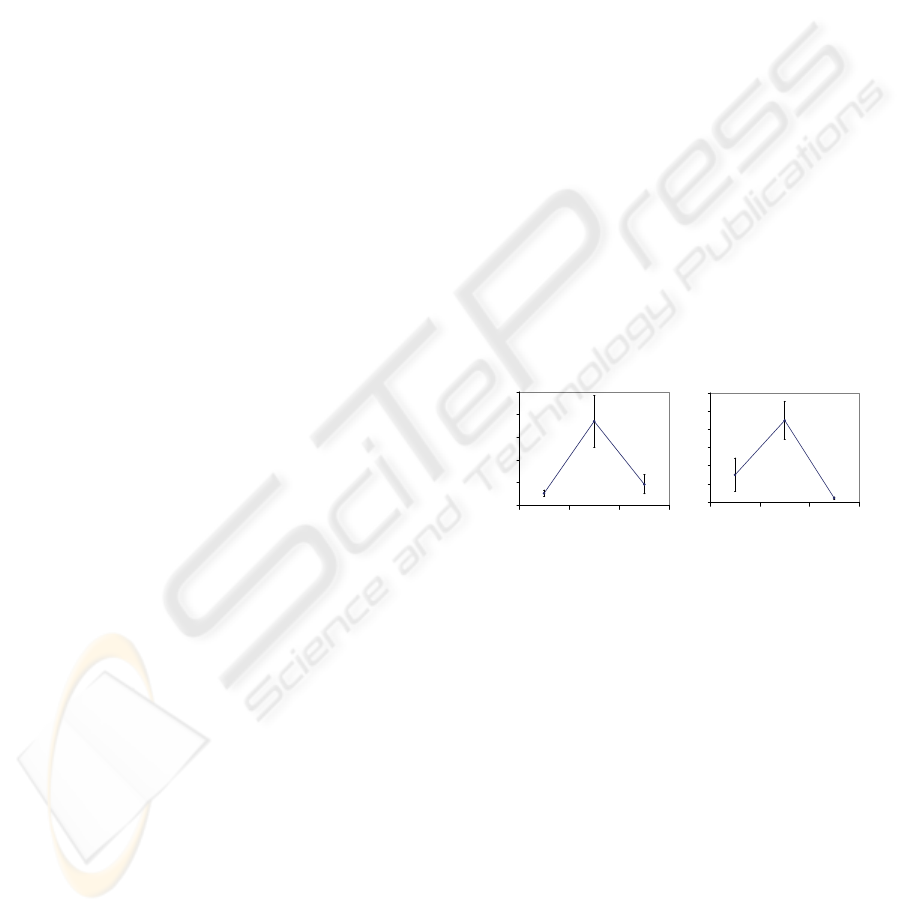
0.05 Hz (see Fig. 2). Nonetheless, average HR
reaction to the 0.1 Hz muscle tension task was
significantly lower than to 0.1 Hz breathing. In
contrast, average VT reaction to the 0.1 Hz muscle
tension task was significantly higher than to 0.1 Hz
breathing.
0
50000
100000
150000
200000
250000
0.05 Hz 0.1 Hz 0.2 Hz
RRI [ms
2
/H]
0
2000
4000
6000
8000
10000
12000
0.05 Hz 0.1 Hz 0.2 Hz
PTT [ms
2
/Hz]
Figure 2: Heart rate (RRI) and vascular tone (PTT)
reactions to rhythmical muscle tension at 0.05, 0.1, and 0.2
Hz. Y-Axis: power of the spectra at tested frequencies.
Data are presented as the mean ± 2 standard error bars.
Discussion: Rhythmically stimulating the CVS at its
resonance frequency with muscle tension and
breathing elicited significantly higher oscillations
than at other frequencies. These oscillations were
robust in both the HR and VT spectra. The efficacy
of the muscle tension task to stimulate HR
oscillations was lower than the efficacy of the
breathing task, but higher for VT oscillations. This
may be related to the physical load of muscle
tension, which increased mean HR and consequently
depressed HRV. These findings parallel those
reported by Lehrer et al. (2009), who noted that the
amplitude of oscillations in BP and VT caused by
0.1 Hz muscle tension stimulation was relatively
higher than those in HR. This suggests that increased
BP and VT oscillatory activity acts as a
RESONANCES IN THE CARDIOVASCULAR SYSTEM - Investigation and Clinical Applications
23

compensatory reaction to dampened HRV.
Conclusion: Results confirmed that rhythmical 0.1
Hz muscle tension tasks can trigger resonance in the
CVS. The ability to produce high amplitude
oscillations at this resonance frequency makes the
rhythmical muscle tension techniques potentially
valuable for developing clinical applications. In fact,
France, France, & Patterson (2006) have developed
the Rhythmical Skeletal Muscle Tension (RSMT)
technique to lower risk of a vasovagal reaction
(fainting
). RMST has been successfully employed to
avert fainting episodes that often occur during blood
collection procedures and may discourage people
from donating blood. It has also been successfully
used to treat patients with blood and injury phobias.
In their study, healthy young adults performed the
RSMT task at frequency of 0.1 Hz and demonstrated
significant increases in HR, systolic and diastolic
BP, and cerebral oxygen. High amplitude 0.1 Hz
oscillation in HR, BP, and VT were also observed.
The mechanisms by which the 0.1 Hz RSMT
procedures prevent vasovagal reactions have not
been examined. It may be due to resonance in the
CVS or simply to the increased sympathetic arousal
caused by the muscle tension. We propose that the
effect is due to the high amplitude oscillations that
activate the regulatory processes that balance
autonomic functioning and modulate the inhibitory
processes in the brain that buffer the body from
stress. Accordingly, we speculate that other kinds of
0.1 Hz RSMT procedures can be exploited by
researchers and clinicians for the development of
novel approaches to correct abnormal autonomic
regulation. France’s RSMT technique was originally
intended to be a single session performed
immediately prior to an event that could induce a
negative physiological reaction. Systematic, every
day use of this technique, however, may produce a
cumulative and longer lasting effect and, in this way,
be practical in the same way as the HRV
biofeedback procedure. In fact, such procedures may
prove especially useful in the rehabilitation process
of patients following a cerebral stroke or myocardial
infarction, in treatments where physical exercises are
prescribed (Buch, Coote, & Townend, 2002), or in
sport medicine.
2.3 0.1 Hz Resonance in the CVS
Caused by Emotional Pictures Cues
The CVS actively participates in emotional
regulation, and emotions strongly affect
cardiovascular function. Picture cues that elicit
emotional reactions have been reported to produce
the common triphasic HR response. Moreover, the
magnitude of this triphasic response, particularly the
accelerative leg, appears to accurately discriminate
picture valence (Gatchel & Lang, 1973; Lang et al.,
1993). In accordance with our model, instigating
rhythmical emotional reactions at 0.1 Hz should
produce resonance oscillation in HR, and the
oscillation amplitude should discriminate the degree
of emotional arousal. To test this, emotionally
arousing picture cues were presented at frequency of
0.1 Hz to trigger CVS resonance. In addition, the
ability of the resonance amplitude to discriminate
the degree of emotional arousal caused by block of
picture cues was assessed.
Method: Seventy-six healthy participants, between
21 and 24 years old, were individually tested. Each
participant viewed six categories of picture blocks
(negative emotional, positive emotional, and neutral,
as well as alcohol, marijuana, and ecstasy) with 30
pictures in each. Pictures were presented for 5
seconds with a 5-second inter-picture interval,
resulting in a 0.1-Hz picture presentation frequency.
The interval between each picture block was 30
seconds. Pictures were presented on a 75-cm LCD
TV (View Sonic N3000W). ECG and finger pulse
were recorded during all tasks. Beat-to-beat RRI and
pulse transit time (PTT) and their Fourier spectra
were calculated for each picture cue exposure task.
Reaction of the CVS to the picture cue block was
estimated by the power of RRI and VT spectra at
frequency of 0.1 Hz (i.e., the 0.1-Hz HR index and
the 0.1-Hz VT index, respectively). To evaluate the
sensitivity of the 0.1-Hz HR index for estimating
emotion valence, common HRV indices (total HRV,
high frequency (HF) HRV, and low frequency (LF)
HRV) were also calculated.
Results: In most cases, pictures presentation at a
frequency of 0.1 Hz caused high amplitude HR and
VT oscillations at the resonance frequency of the
CVS. The resonance in VT, however, was less
prominent than in HR. Participant’s average 0.1-Hz
HR index response to neutral picture cues was
significantly less than for any other cue block.
Conversely, the average 0.1-Hz HR index response
to negative picture cues was significantly higher
than for other picture cue blocks. Averaged 0.1-Hz
HR index responses to positive, alcohol, marijuana,
and ecstasy picture cues did not differ significantly
from one another, but there were individual
differences in the response patterns of 0.1-Hz HR
indices across picture cue blocks. For example, one
participant strongly reacted to negative and alcohol
stimuli but weakly reacted to positive and marijuana
cues; another participant showed high 0.1-Hz HR
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
24

index responses to ecstasy cues, a moderate response
to negative and positive cues, and a weak response
to all other cue blocks (see Fig. 3).
The 0.1-Hz HR index detected individuals’
reactions to the picture cue blocks differently than
other, more commonly used HRV indices (total
HRV, HF HRV, LF HRV). For example, the 0.1-Hz
HRV index more sensitively differentiated reactions
to negative versus neutral picture cue blocks than
common HRV indices. Further, there was no
significant variability in individual reactions to
different cue blocks detected with the common HRV
indices.
Figure 3: One participant’s RRI spectra for all 0.1 Hz
picture cue tasks. The power of spectra at 0.1 Hz reflects
the strength of emotions caused by picture cue blocks.
Discussion: Emotions modulated by rhythmical
picture cue exposure at frequency of 0.1 Hz imposed
high amplitude resonance oscillation on HR and
other cardiovascular functions. We hypothesize that
the amplitude of the oscillations depends on the
degree of emotional arousal elicited by the paced
visual stimulation, but were not able to directly
assess this hypothesis. Nevertheless, the fact that
some individuals reacted more strongly to emotional
picture cues whereas others reacted more strongly to
drug-related picture cues suggests that the salience
of the stimuli has a significant impact on the
amplitude of resonance oscillations.
It is unlikely that picture cues presented at the
resonance frequency caused greater emotional
arousal than picture cues presented at others
frequencies; rather, we hypothesize that the
amplified reactivity we observe is directly related to
the presentation of emotional cues at the CVS
resonance frequency.
Our approach of using the CVS’ 0.1 Hz
resonance frequency to assess degree of emotional
arousal in response to stimuli is similar to the
engineering approach of measuring weak oscillatory
signals. An engineer tunes a measurement device to
the main frequency of the weak signal. Tuning to the
resonance frequency does not change the value of
the signal, but rather enhances the sensitivity of the
measurement device.
Conclusion: The method we have developed for
estimating emotional arousal may be useful in
psychophysiological research, particularly, for the
diagnosis of psycho-emotional disorders. In
addition, the use of paced visual stimulation may
prove useful for the treatment and rehabilitation of a
variety of disorders because visual cues may cause
high-amplitude HR oscillation similar to those
caused by paced breathing or paced muscle tension.
An advantage of picture cue stimulation is that the
content of the stimuli can be easily manipulated;
thus, it is conceivable that rhythmical visual
stimulation using cues with specific cognitive
content (e.g., emotional cues to induce altered mood
states or drug-related cues to induce craving) may
open new doors to treatment applications in the
mental health and addictions field.
3 0.03 HZ RESONANCE IN THE
CVS
The VT baroreflex is one of two interconnected
branches of the baroreflex, but is much less studied
than its counterpart, the HR baroreflex. Like the HR
baroreflex, the VT baroreflex controls BP and
participates in modulating the coordinated actions of
the central and autonomic nervous systems;
however, it does so by modulating the stretch of
blood vessel walls and operates in a lower frequency
range (Aljuri, Marini, & Cohen, 2004). Based on
prior research (Vaschillo et al., 2002; Vaschillo et
al., 1983), we hypothesized that a resonance
frequency of ~0.03 Hz would be found for the VT
baroreflex.
Based on the utility of the 0.1 Hz resonance in
developing novel medical applications for treating
various physical and mental disorders, we expected
that exploration of the 0.03 Hz resonance could
prove valuable in much the same way. However,
little is known about this resonance frequency and
thus additional basic experimental investigations are
necessary prior to the assessment of its clinical
value. Accordingly, we performed two studies to
define whether external stimuli can elicit a 0.03-Hz
oscillatory HR response (the 30 second
triphasic
RESONANCES IN THE CARDIOVASCULAR SYSTEM - Investigation and Clinical Applications
25
waveform response) in addition to a 0.1 Hz
response. Very strong stimuli were used to
effectively induce a 30 second triphasic response.
The first study
investigated the HR response to
highly unpleasant sounds.
Method: Seventeen adult participants were exposed
to 8 synthetic sounds which were chosen as the most
unpleasant from 362 sounds created in the lab.
Participants sat in a room and listened to each sound
at 82 dB (A) or 92 dB (A) for 2 minutes (with a 30
second inter-stimulus interval) from four equidistant
speakers. ECG was collected and beat-to-beat HR
curves were calculated (see Fig. 4).
ID: Theta
82dB
ID:24
82dB
ID:26
92dB
H
R
[
b
p
m
]
Sound
0
30
60
90
Heart Rate
Time [s]
0 200 400 600
70
80
90
100
S
o
u
n
d
[
m
V
]
Figure 4: Heart Rate during aversive sound exposure.
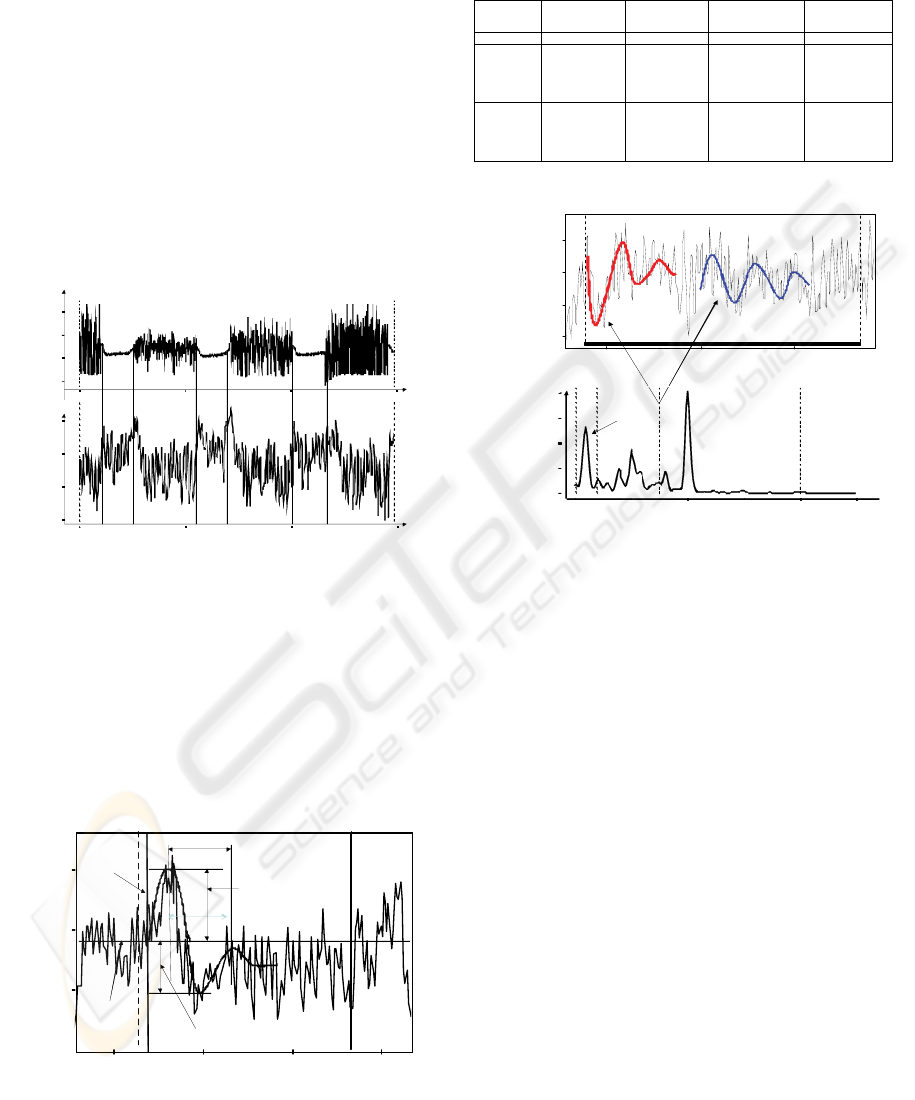
Results: Onset of unpleasant sounds usually caused
a long-duration triphasic HR response, which
appeared to contain overlapping 10-second triphasic
waveforms that lasted for about 30 seconds (see Fig.
5) Sound offset occasionally caused the same
response. Two types (Tab. 1) of 30-second triphasic
responses were found: one with an initial HR
deceleration and one with an initial HR acceleration.
Heart Rat e
Ti me [ s ]
HR [bpm]
100 15 0 200 25 0
60
70
80
Soun d
Onset
Sound
Offset
Extremum1
(13. 5bpm)
Extremum2
(8.6bpm)
33 s
Mean HR
before sound
onset
Figure 5: An example of ~0.03 Hz oscillatory HR reaction
to aversive sound (30-s triphasic waveform HR response).
Table 1: The two types of 30-s triphasic waveform HR
responses averaged across all participants and sounds
characteristics.
R R I
Time [s]
RRI [ms]
700 80 0 90 0
70 0
80 0
90 0
1000
Ng
0.027 Hz
RRI Spectrum
Frequency [Hz]
P
S
D
[
1
0
5
m
s
2
/
H
z
]
0.0 0. 1 0.2 0. 3 0.4 0.5
0.00
0.25
0.50
0.75
1.00
Time [s]
R
R
I
[
m
s
]
RRI
Figure 6: An example of ~0.03 Hz oscillatory HR reaction
to aversive picture cues.
The second study investigated the HR response
to highly negative pictures.
Method: See section 2.3.
Results: We found that very negative pictures (e.g.,
plane crashes, blood, violence) sometimes caused a
strong ~0.03 Hz oscillatory HR response. This HR
response usually demonstrated a significantly higher
amplitude than the 0.1 HR response (see Fig 6).
Discussion: Strong stimuli from various modalities
elicited oscillatory HR responses at a frequency of
about 0.03 Hz, which overlapped with the 0.1 Hz
response. This effect may be the result of resonance
in the VT baroreflex closed-loop. In these studies,
we employed very strong stimuli and clearly
observed 0.03 Hz oscillatory responses. Less
aversive stimuli should also be capable of eliciting
these slower oscillations; however, these oscillations
may be masked by those associated with HRV.
Future studies are needed to assess the malleability
of the 0.03 Hz resonance and its utility in clinical
applications.
Conclusion: To be value for clinical application, it
is necessary to develop experimental methods that
HEART
RATE
RESPONSE
EXTREMUM
1
(M ± STD ERR)
EXTREMUM
2
(M ± STD ERR)
OSCILLATORY
PERIOD
(M ± STD ERR)
OSCILLATORY
FREQUENCY
(M ± STD ERR)
UNITE [ BPM ] [ BPM ] [ S ] [ HZ ]
TYPE 2
7.86±0.58
-5.03±0.79
28.62 ±1.23
0.035±0.006
TYPE 2
- 8.34±0.78
4.14±1.1
26.74 ±1.86
0.037±0.007
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
26

can reliably and easily produce stable high
amplitude oscillation in the CVS. The use of very
strong negative stimuli, while capable of producing
such oscillations, may not prove clinically useful
because of the possibility of negative psychological
side effects. Exploration of paced breathing or
rhythmical muscle tension techniques at ~0.03 Hz
warrants further study as these procedures may also
be capable of triggering therapeutic oscillation in the
same way as 0.1 Hz stimulation triggers them.
Elements of such stimulation can be found in eastern
health procedures (e.g., Yoga, Tai Chi).
4 DISCUSSION
Two resonance frequencies, at 0.1 and 0.03 Hz, have
been identified in the CVS. These resonances are
thought to reflect two interdependent closed-loop
systems with delays, namely the HR and VT
baroreflex systems and contribute independently to
the overall resonance properties for the CVS.
Typically, the baroreflex system is touted for its
ability to buffer perturbations in BP (Just et al.,
1994; Jones, Christou, Jordan, & Seals, 2003; Jordan
et al., 2002; Magosso, Biavati, and Ursino, 2001;
van de Vooren et al., 2007); however, because it has
a very narrow operating frequency range (~0.01-0.5
Hz) with 2 resonance frequencies inside this range,
the ability of the baroreflex system to stabilize the
CVS is limited. The high frequency boundary is
defined by inertia of blood mass and slow changes in
vessel tone, while the low frequency boundary is
defined by differentiative property of the
baroreceptors (i.e., they react only to the speed of
BP changes).
The long term aim of investigating the dynamic
properties of the HR and VT baroreflexes is to
develop new therapeutic methods for treating
diseases associated with the dysregulation of the
autonomic and central nervous systems. HRV
biofeedback is capable of harnessing the resonances
within the CVS and promoting health benefits.
These therapeutic effects have been linked to the
generation of generalized high-amplitude
oscillations in autonomic functions that are elicited
by the biofeedback procedure (Chernigovskaya et
al., 1990; Lehrer et al., 2003, 2004) and act to retrain
autonomic reflexes. The systematic retraining of
autonomic reflexes normalizes and improves
autonomic regulation. Our studies show that
breathing, visual cues, and muscle tension
management of the 0.1 Hz resonance is useful for
the treatment of various unhealthy physical and
mental conditions. We believe that novel therapeutic
interventions involving the VT baroreflex and its
resonance at 0.03 Hz through passive or active tasks
may also be beneficial, but additional investigations
are needed.
5 CONCLUSIONS
Classical control system theory applied to the
investigation of physiological systems can be a
useful tool for the medical practice. An engineering
approach offers the opportunity to create simple,
clinically-useful stimulation procedures which may
be used to enhance an individual’s regulatory
capacity and thus open new doors to treatment
applications in the mental health and addictions
field.
ACKNOWLEDGEMENTS
This research was supported by grants from the
National Institute of Alcohol Abuse and Alcoholism
(R01 AA015248 and K02 AA00325) and the
National Institute of Drug Abuse (P20 DA017552).
REFERENCES
Aljuri, N., Marini, R. J. R. & Cohen, R. J., 2004, Test of
dynamic closed-loop baroreflex and autoregulatory
control of total peripheral resistance in intact and
conscious sheep, Am J Physiol Heart Circ Physiol
287: H2274-H2286.
Angelone, A. & Coulter, N. A. Jr., 1964, Respiratory sinus
arrhythmia: A frequency depended phenomenon,
Journal of Applied Physiology, 19, 479–82.
Bertram, D., Barres, C., Cuisinaud, G., & Julián, C., 1998.
The arterial baroreceptor reflex of the rat exhibits
positive feedback properties at the frequency of Mayer
waves, J Physiol 513: 251–261.
Borst, C. & Karemaker. J. M., 1983, Time delays in the
human baroreceptor reflex, J Auton Nerv Syst 9: 399–
409.
Bradley, M. M. & Lang, P. J., 2000, Affective reactions to
acoustic stimuli, Psychophysiology, 37, 204-215.
Buch, A. N., Coote, J. H., & Townend, J. N., 2002.
Mortality, cardiac vagal control and physical
training—what’s the link? Exp Physiol 87: 423–435.
Burgess, D. E., Hundley, J. C., Li, S. G., Randall, D. C., &
Brown, D. R., 1997, First-order differential-delay
equation for the baroreflex predicts the 0.4-Hz blood
pressure rhythm in rats, Am J Physiol Regul Integr
Comp Physiol 273: R1878–R1884.
RESONANCES IN THE CARDIOVASCULAR SYSTEM - Investigation and Clinical Applications
27

Chapuis, B., Vidal-Petio, E., Orea, V., Barres, C., &
Julien, C., 2004, Linear modelling analysis of
baroreflex control of arterial pressure variability in
rats, J Physiol 559: 639–649.
Chernigovskaya, N. V., Vaschillo, E. G., Rusanovsky, V.
V., & Kashkarova, O. E., 1990, Instrumental
autotraining of mechanisms for cardiovascular
function regulation in treatment of neurotics, The SS
Korsakov's Journal of Neuropathology and Psychiatry,
90: 24–28.
Clynes, M., 1960, Respiratory sinus arrhythmia: laws
derived from computer simulation, Journal of Applied
Physiology,15(5): 863-874.
Codispoti, M., Bradley, M. M., & Lang, P., 2001, Affective
reactions to briefly presented pictures,
Psychophysiology, 38, 474-678.
Dworkin, B. R., Elbert, T., Rau, H., Birbaumer, N., Pauli,
P., Droste, C., & Brunia, C. H., 1994, Central effects
of baroreceptor activation in humans: attenuation of
skeletal reflexes and pain perception, Proc Natl Acad
Sci U S A, 91, 6329-33.
France, C. R., France, J. L., & Patterson, S. M., 2006,
Blood pressure and cerebral oxygenation responses to
skeletal muscle tension: a comparison of two physical
maneuvers to prevent vasovagal reactions, Clinical
Physiology and Functional Imaging, 26, 21–25.
Hassett, A. L, Radvanski, D. C., Vaschillo, E., Vaschillo,
B., Sigal, L., Karavidas, M., Buyske, S., & Lehrer, P.
M., 2007, A pilot study of the efficacy of heart rate
variability biofeedback in patients with fibromyalgia
syndrome, Appl Psychophysiol Biofeedback, 32, 1-10.
Jones, P. P., Christou, D. D., Jordan, J., & Seals, D. R.,
2003, Baroreflex buffering is reduced with age in
healthy men, Circulation, 107, 1770–1774.
Jordan, J., Tank. J., Shannon, J. R., Diedrich, A., Lipp, A.,
Schroder, C., Arnold, G., Sharma, A. M., Biaggioni, I.,
Robertson, D., & Luft, F. C., 2002, Baroreflex
buffering and susceptibility to vasoactive drugs,
Circulation, 105, 1459–1464.
Julien, C., 2006, The enigma of Mayer waves: Facts and
models, Cardiovasc Res, 70, 12–21.
Just, A., Wittmann, U., Nafz, B., Wagner, C. D., Ehmke,
H., Kirchheim, H. R, & Persson, P. B., 1994, The
blood pressure buffering capacity of nitric oxide by
comparison to the baroreceptor reflex, Am J Physiol
Heart Circ Physiol 267: H521–H527.
Karavidas, M. K., Lehrer, P. M., Vaschillo, E., Vaschillo,
B., Marin, H., Buyske, S., Radvanski, D., & Hasset,
A., 2007, Preliminary results of an open label study of
heart rate variability for the treatment of major
depression, Appl Psychophysiol Biofeedback, 32, 19-
30.
Lang, P. J., Greenwald, M. K., & Bradley, M. M., 1993.
Looking at pictures: Affective, facial, visceral, and
behavioral reactions, Psychophysiology, 30, 261-273.
Lehrer, P. M., Vaschillo, E., & Vaschillo, B., 2000,
Resonant Frequency Biofeedback Training to Increase
Cardiac Variability: Rationale and Manual for
Training, Appl Psychophysiol Biofeedback, 25, 181-
192.
Lehrer, P. M., Vaschillo, E., Vaschillo, B., Lu, S. E.,
Eckberg, D. L., Edelberg, R., Shih, W. J., Lin, Y.,
Kuusela, T. A., Tahvanainen, K. U. O., & Hamer, R.,
2003, Heart rate variability biofeedback increases
baroreflex gain and peak expiratory flow,
Psychosomatic Medicine, 65, 796–805.
Lehrer, P., Vaschillo, E., Vaschillo, B., Lu, S., Scardella,
A., Siddique, M., & Habib, R.., 2004, Biofeedback
treatment for asthma, Chest, 126, 352–361.
Magosso, E., Biavati, V., & Ursino, N., 2001, Analysis of
cardiovascular instability by mathematical model of
baroreflex control, Proceedings of the 23
rd
Annual
EMBS International Conference, October 25-28,
Istanbul, Turkey. 596-599.
McCraty, R., Atkinson, M., & Tomasino, D., 2003, Impact
of a workplace stress reduction program on blood
pressure and emotional health in hypertensive
employees, J Altern Complement Med, 9, 355–369.
Nyklicek, I., Wijnen, V., & Rau, H., 2005, Effects of
baroreceptor stimulation and opioids on the auditory
startle reflex, Psychophysiology, 42, 213-22.
van de Vooren, H., Gademan, M. G. J., Swenne, C. A.,
TenVoorde, B. J., Schalij, N. J., & Van der Wall, E.
E., 2007, Baroreflex sensitivity, blood pressure
buffering, and resonance: what are the links?
Computer simulation of healthy subjects and heart
failure patients, J Appl Physiol 102: 1348-1356.
Vaschillo, E. G., Bates, M. E., Vaschillo, B., Lehrer, P.,
Udo, T., Mun, E. Y., & Ray, S., 2008, Heart rate
variability response to alcohol, placebo, and
emotional picture cue challenges: Effects of 0.1 Hz
stimulation, Psychophysiology, 45, 847-858.
Vaschillo, E., Vaschillo, B., & Lehrer, P., 2006,
Characteristics of resonance in heart rate variability
stimulated by biofeedback, Appl Psychophysiol
Biofeedback, 31, 129-142.
Vaschillo, E., Lehrer, P., Rishe, N., & Konstantinov, M.,
2002, Heart rate variability biofeedback as a method
for assessing baroreflex function: a preliminary study
of resonance in the cardiovascular system, Appl
Psychophysiol Biofeedback, 27, 1–27.
Vashchillo, E. G., Zingerman, A. M., Konstantinov, M.
A., & Menitsky, D. N., 1983, An investigation of the
resonance characteristics of the cardiovascular
system, Human Physiology, 9, 257-265.
Vaschillo, E. G. & Vaschillo
, B., 2009, Transfer function
of the heart rate control system with respiratory input.
The classical engineering approach, BIOSIGNALS
2009, 233-238.
Ursino, M. & Magosso, E., 2003, Role of short-term
cardiovascular regulation in heart period variability:
a modelling study, Am J Physiol – Heart and
Circulatory Physiology, 284, H1479-H1493.
Yasumasu, T., Reyes del Paso, G., Takahara, K., &
Nakashima, Y., 2006, Reduced baroreflex cardiac
sensitivity predicts increased cognitive performance,
Psychophysiology 43: 41-45.
BIOSIGNALS 2010 - International Conference on Bio-inspired Systems and Signal Processing
28
